Abstract
Herpes zoster (HZ) is a common cutaneous entity with protean clinical presentations, management options, complication rates, and prevention strategies, all of which are rife with dogma. During an inpatient consultation for HZ, have you ever been approached by a frantic staff or family member, worried that a pregnant, elderly, or infant contact will be ‘infected’ if they get too close? Have your patients ever asked you about their risk of having HZ twice, or claimed that they have frequent ‘recurrences’? In what timeline should antiviral therapy be employed? Is there evidence for prednisone or gabapentin in acute HZ treatment? Who should be vaccinated against HZ and what are the benefits and risks? In case-based form, these and other complex but common scenarios will be examined using clinical and viral mechanistic clues, along with updated treatment and prevention guidelines, to provide a modern HZ case management compendium, comprehensive of the diverse age and health populations now presenting with this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Herpes zoster (HZ) is common and may present at any age depending on complex interplays between immune modulation, intrinsic disease, and iatrogenic influences. |
Successful management of acute and chronic HZ symptoms, particularly pain, depend on expedient diagnosis and treatment with antivirals and various methods of analgesia in a case-by-case fashion, depending on location and the severity of pain, along with medication onset of action and adverse effect profiles. |
The prevention of HZ and post-herpetic neuralgia is optimally managed with Shingrix, an efficacious non-live adjuvanted HZ vaccine, and its use is even expanding to immunosuppressed populations. |
1 Introduction
Herpes zoster (HZ) presents significant clinical and fiscal morbidity for patients and providers. It is not only an infectious entity but also a prevalent neurologic entity, occurring annually for at least 1 million people in the US, and increasing in incidence across the globe over the past two decades. At least 20–30% of the population and up to 50% of those living until age 85 years will be affected by HZ, although this landscape may be shifting based on evolving vaccination trends [1,2,3]. From acute and chronic wound and pain manifestations, to vaccination strategies and infection control measures, the ramifications of HZ intertwine between many medical disciplines and governing bodies.
A recent study of healthcare economic burden noted that herpes (including HZ) is one of the top 10 most costly categories of skin disease [4]. Moreover, dermatologists differ in their approaches to its management. For instance, they waver in their recommendations for the treatment of acute pain and post-herpetic neuralgia (PHN), yet several studies have shed light on the effectiveness of anti-inflammatory, antiviral, and neuroleptic regimens [5,6,7,8,9]. Additionally, vaccination strategies for HZ are inconsistent despite distinct recommendations for vaccination by the US FDA and Centers for Disease Control and Prevention (CDC) via the Advisory Committee on Immunization Practices (ACIP), and very few surveys over the past two decades have evaluated true patient compliance with the two available vaccines. Furthermore, in current clinical settings, it is unclear as to whom should be taking the lead for vaccination stewardship (primary care vs. specialists) and the strategies to meet supply and demand parameters, particularly considering the most recent vaccine’s superior efficacy and durability profile [10]. In addition, healthcare setting infection control policies for patients and staff with or exposed to HZ are often misunderstood, outdated, or poorly outlined in institutional guidelines, not to mention that the negative gradient of infectivity between native varicella and subsequent HZ is often misinterpreted. A disease entity with such disparate management strategies should be clarified. By summarizing the clinical presentations, pathogenesis, and diagnosis of HZ outlining the utility and efficacy of its treatment and vaccination, and delineating best practices given recent literature, this case-based review will aim to illustrate the appropriate management and education of HZ patients and their care teams.
2 Cases and Review
A 72-year-old otherwise healthy male presented with painful pink papules along the right mid-back for 3 days. He believed he may have been bitten by a spider or a tick. When you determine clinically that the diagnosis is shingles, he wonders how he ‘caught it’.
A substantial practice gap in the management of HZ lies in the utilization of its true pathophysiology to aid in narrowing the clinical differential diagnosis and avoiding unnecessary testing, treatment, and perpetuation of trepidation when it comes to this infectious disease that is not classically infectious [11,12,13,14]. First, decoding HZ nomenclature is instructive. Zoster is the Ancient Greek term for ‘belt’ or ‘girdle’, and shingles comes from the Latin cingulum meaning ‘encircling the body’, both exemplifying the classic painful dermatomal papulovesicular thoracic distribution of more than half of HZ cases (see Fig. 1) [15, 16]. Furthermore, the herpes surname refers to the varicella-zoster virus (VZV), human herpesvirus type 3, classically responsible for HZ and primary varicella (chickenpox) infections and part of the α-herpesvirus subfamily that has the ability to remain latent in neural tissue. Finally, the etymology of chickenpox is unclear, but it may historically describe the eruption as a less dangerous (chicken) ‘pox’ than smallpox, or that the eruption itself resembles chicken ‘pecks’ on the skin [17].
All patients with HZ have incurred some version of primary VZV exposure, usually in childhood, either from a sick contact resulting in a true viremia, or from primary VZV vaccination. For the former, the initial VZV infection may have manifested as an upper respiratory infection-like prodrome, diffuse cutaneous self-limited papulovesicles, rarely systemic complications, or perhaps subtle findings never even recalled by the patient. After 1995, the current pediatric and young adult population may have instead received a live attenuated primary VZV vaccination based on pediatric standard guidelines [18,19,20]. Nearly all adults in the US have measurable immunity to VZV no matter which version of primary VZV infection or vaccination was at play, but even those with antibody-negative laboratory results are assumed to have been exposed for the purposes of HZ vaccination candidacy [21,22,23]. This creates confusion and worry for patients who may have remembered neither of these events, but presenting this initial pathophysiology to the patient clarifies their understanding of how they reactivated but did not ‘catch’ HZ.
VZV from hematogenous or sensory nerve sources from the original exposure then becomes latent in the spinal root or cranial nerve ganglia, with latency driven by VZV-specific T cells and subsequent immunoglobulin (Ig) M then IgG responses. Throughout life, subclinical reactivations may then occur from community contact with the virus, which continues to boost immunity. For a typical immunocompetent patient, this balance is maintained throughout adulthood until HZ vaccination age is reached or if a threshold immunity level is not reached, and HZ ensues (Figs. 2, 3) [24]. The most common contributors to this type of waning immunity are age, intrinsic or pharmacologic immunosuppression, or muted original immune protection from the original VZV infection or vaccination [25,26,27].

(reproduced with permission from [24])
Schematic representation of the VZV life cycle. Ab antibody, IFN interferon, NK natural killer, VZV varicella-zoster virus

(reproduced with permission from [24])
Reactivation of latent VZV and new encounters with the virus maintain immunity. VZV varicella-zoster virus
A 30-year-old pregnant female (28 weeks) presented with an eruption consistent with acute primary varicella infection with diffuse vesicles all over the body. Her daughter was subsequently born at term with no complications, but at age 10 months, the infant developed a herpetic rash involving the T3 region.
Pediatric HZ may occur in several scenarios. If a pregnant female develops primary VZV (chickenpox) during pregnancy without expedient antiviral and immunoglobulin treatment, then the fetus is technically experiencing its first exposure to VZV at the same time. In the first two trimesters, the risk of congenital viral complications from varicella is high (at least 25%), including low birth rate, preterm birth, and congenital varicella syndrome, and later in pregnancy, maternal primary VZV exposure can cause neonatal varicella [28]. Furthermore, all primary VZV transmissions in pregnancy (in this vignette, presenting in the third trimester) or primary VZV infections in the first year of life carry a higher risk of early (under 4 years of age) HZ, based on the fetus and the infant’s fledgling cellular and humoral immunity and therefore muted immune response to the primary infection (Fig. 4) [29, 30].
HZ in children may also present because of true immunosuppression, such as malignancy (especially hematologic), immunomodulatory medications such as long-term systemic corticosteroids or azathioprine, surgery, trauma, and certain autoimmune and immunodeficiency disorders such as systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, asthma, human immunodeficiency virus (HIV) infection, and type 1 diabetes mellitus [27, 31]. Based on the patient’s medication list, review of systems, and family history, most of these etiologies would be swiftly identified. Of note, because only 3% of pediatric HZ cases occur concurrently with malignancy, unless there are worrisome symptoms, signs, or a particularly severe disseminated presentation of HZ in the child, routine malignancy work-up is not otherwise recommended [32, 33].
Generally, routine cases of pediatric HZ are rare, especially in the post-1995 vaccination era for primary VZV. Typically, the pediatric presentation of HZ is less symptomatic and carries less risk for PHN than adult cases [29]. Of note, vaccinated children are 72–79% less likely to present with HZ in childhood than unvaccinated children, and vaccinated children tend to present in the cervical or lumbar dermatome regions and unvaccinated children in the thoracic region, postulated in vaccinated children to correspond to the sites where their vaccination was administered [34, 35]. It has been postulated that over time, with fewer community wild-type primary VZV infections and assuming high pediatric vaccination rates in the community, vaccinated children will be adults with a reduced risk of HZ. However, in this watershed period where there is waning incidence of wild-type primary VZV in the community to provide immune boosting to adults who had prior chickenpox, current adult HZ risk remains steady [36, 37]. Improved efficacy of adult HZ vaccinations may be balancing this equation, but further study is required to predict the future dynamic landscape of generational HZ incidence.
A 24-year-old, ill-appearing African American male presented to the dermatology clinic, at the request of the urology department, with rapidly spreading tense vesicles all over the body for a few days. He was febrile and could not walk on his own due to painful lesions, particularly on the plantar and genital regions. He was admitted to the hospital and treated with intravenous aciclovir. Further work-up revealed a new diagnosis of concurrent HIV infection.
Some presentations of HZ suggest disseminated infection requiring not only expedient management with hospital admission and appropriate isolation but also reflexive work-up for immunosuppression when risk factors are identified. Disseminated HZ occurs in approximately 2% of HZ cases and is defined as at least 20 widespread vesiculobullous lesions outside of the primary and adjacent dermatomes, with evolving lesions 1–2 weeks beyond the primary presentation. These cases usually present with more prominent pain and other systemic signs mimicking primary VZV viremia, and carry a higher risk of lung, neurologic, and secondary bacterial infection complications [38, 39]. Patients with HIV infection and hematologic malignancy or its immunosuppressive therapies, as well as transplant recipients, are most at risk for dissemination because of insufficient VZV-specific immune response, and their presentations may vary or even evolve into a chronic illness with waxing and waning crops of vesiculobullous lesions or verrucal papular eruptions over time [40,41,42]. Of note, the incidence of HZ is decreasing in HIV patients as a result of evolving antiretroviral therapy, but because of the suppression of T cells immune to VZV in this condition, it still occurs three times more often, presents at a younger age, and displays more severe symptoms, including site-specific genital presentations, in patients with HIV infection compared with the general population, as exemplified by this case (Fig. 5) [43,44,45,46]. Furthermore, patients with HIV and other immunosuppressive conditions may be more likely to have aciclovir resistance than immunocompetent patients, and may also experience more severe PHN, particularly when HZ presents in the V1 dermatomal distribution [41].
An 83-year-old female patient presented with rapidly-evolving painful, pruritic, crusted pink papules on the right cheek for a few days. She was unsure of her medication list.
The clinical differential diagnosis of HZ is broad depending on the patient’s original symptoms, particularly if classic dermatomal papulovesicles are not acutely present. Prodromal pain can mimic a myocardial event, stroke, pulmonary embolus, renal colic, appendicitis, cholecystitis, acute angle-closure glaucoma, and costochondritis, to name a few. When possible, full skin examination to evaluate for cutaneous eruptions at the initial acute visit should be performed to eliminate the necessity for extensive testing; however, zoster sine herpete (lacking skin involvement) is often a diagnosis of exclusion when the aforementioned morbid conditions are part of the differential diagnosis [39].
Cutaneous mimickers of HZ include other herpetic eruptions such as herpesvirus types 1 or 2 (HSV-1, HSV-2), superinfection with HSV presenting as eczema herpeticum, or breakthrough VZV after childhood vaccination, and other viral illnesses such as hand, foot, and mouth disease. Non-infectious etiologies include allergic or irritant contact dermatitis, arthropod assault, autoimmune blistering disease, or, as in this case, a medication reaction [47]. A common confounder for many cutaneous eruptions is the use of topical 5-fluorouracil for actinic damage because if a patient does not include or consider this to be part of their medication list, the obvious culprit of the focal inflammation where the topical agent was applied may be muddied [48]. Clues to rule out this broad differential involve, in particular, noting dermatomal distribution, as in this case, as the patient’s eruption crosses V1 and V2 dermatomes in a haphazard and geometric pattern based on where she applied the 5-fluorouracil cream. Although HZ in a healthy patient can involve aberrant papules in nearby dermatomes, the shape of the HZ eruption should primarily follow a representative dermatome (Fig. 6).
Diagnostically, HZ clinical presentation is paramount, but when signs and symptoms are ambiguous, the unroofed base of an active lesion can be sent for viral polymerase chain reaction (PCR) for VZV DNA, direct fluorescent antibody (DFA) to the VZV antigen, Tzanck preparation, culture, or a set of lesions in their entirety may be collected for skin biopsy for histopathologic review. Average sensitivity/specificity parameters for cutaneous samples are as follows: PCR 98%/99%; DFA 88%/94%; and viral culture 46%/99% [49]. Routine wound culture and skin biopsy do not provide expedient results (minimum turnover is several days) compared with PCR and DFA, which can be performed in most laboratories in under 24 h. Furthermore, viral culture is not particularly sensitive in HZ where the viral burden is typically small, and skin biopsy and Tzanck preparation do not speciate which herpesvirus is causing the viral cytopathic change. PCR and DFA therefore remain the preferred diagnostic tests when needed, with selection between them usually depending on a laboratory-specific contract. Of note, serologic testing, such as enzyme-linked immunosorbent assay (ELISA) for vial antibody titers, is not particularly sensitive or practical for acute HZ, but its utility lies in detecting immunity to VZV for employment, pregnancy, and other population screenings [50].
A 62-year-old male presented to his primary care physician with HZ in the V1 distribution and was treated with oral antivirals. He had been a patient in dermatology for yearly skin examinations and sometimes called to ask if he should be doing anything else to help the HZ lesions to heal more quickly and for pain. He was a college engineering professor and had been reading about prednisone and gabapentin.
The decision of whether and how to treat HZ with antivirals and other concurrent medications can be complex. In standard cases, 1 week of antiviral therapy (e.g. oral aciclovir, valaciclovir, or famciclovir) should be employed within 72 h of the primary rash, although often this can be difficult to quantify by patient history. Therefore, treatment is particularly initiated if the patient is in significant pain, continuing to develop new skin lesions, or is at risk for complications, for instance in elderly or immunosuppressed patients [5, 7]. Intravenous antiviral therapy, namely aciclovir or foscarnet for aciclovir-resistant cases, is indicated for disseminated disease, notable ophthalmologic involvement, severe neurologic symptoms, or other toxic signs for hospital admission [51, 52].
Primary HZ treatment with oral antivirals such as aciclovir, valaciclovir, or famciclovir supports significantly reduced time to lesion resolution, viral shedding, and pain control. In particular, duration of acute pain has been quantified in various studies, outlining, in immunocompetent patients, an average time to acute HZ-associated pain resolution of 119 days with placebo compared with 38 days with valaciclovir, 51 days with aciclovir, and 63 days with famciclovir therapy [5, 7, 53, 54]. However, antivirals do not prevent the incidence of PHN [55]. Antiviral choice may depend on insurance coverage and patient’s preference with the dosing schedule rather than efficacy, particularly due to the frequency of aciclovir administration. Of note, oral bioavailability and time to response are higher for valaciclovir and famciclovir compared with aciclovir, and valaciclovir is more cost-effective than famciclovir [56,57,58]. For children at higher risk for complications, antiviral therapy is advisable, and, for this indication, aciclovir is the only FDA-approved therapy, although FDA approval for valaciclovir exists for the management of HSV in children and may be considered for HZ [29]. Additionally, in populations such as HIV and post-transplant patients, or patients who may not be candidates for an HZ vaccine, low-dose aciclovir may be employed on a long-term preventative basis [59]. There is no evidence or indication for topical antiviral therapy in the management of cutaneous or mucosal HZ.
Mild cases of HZ may only require localized wound care and perhaps over-the-counter analgesic agents such as ibuprofen or acetaminophen. For more pronounced presenting symptoms, the addition of systemic corticosteroids has secured a nuanced role in HZ management. These agents may indeed reduce acute HZ pain and improve daily function; they particularly improve quality of life for patients with severe initial pain at any site or site-specific neurologic (for instance, Bell palsy-like symptoms) or ophthalmologic involvement [5, 6, 8]. It remains unclear if systemic corticosteroids decrease time to complete lesion healing, however they do not prevent PHN [60, 61]. Opioids may not only reduce severe pain but also do not prevent PHN [62]. As for neuroleptics such as gabapentin and tricyclic antidepressant agents (TCAs), their usefulness in acute pain management in HZ is unsupported, particularly given their slow (often weeks to months) onset of action, and, despite evidence as below of their usefulness for PHN symptoms, they do not prevent PHN [5, 9, 62, 63]. Furthermore, the broad adverse effect profiles of these classes of all these anti-inflammatory, analgesic, and neuroleptic medications dictate patient-specific dosing and duration of therapy and militate careful titration and clinical follow-up for safe symptom management.
The first branch of the trigeminal nerve (V1) is affected by HZ in approximately 10–15% of HZ cases and may initially only present as Hutchinson sign on the tip of the nose. Acute antiviral and possible systemic corticosteroid therapy, as above, are indicated, as is expedient ophthalmologic referral to diagnose and prevent a wide range of ocular complications [52, 64].
An 80-year-old female with a history of HZ 5 years ago presented to dermatology with what her primary care physician has deemed ‘recurrent zoster’ periodically since her original HZ diagnosis. She is actively scratching and rubbing her forehead during the visit.
This case describes marked dysesthesia often associated with PHN after HZ, which can sometimes be mistaken for chronic or recurrent HZ. Its incidence in HZ cases is 9–34%, and, as opposed to acute pain and lesions associated with the original HZ presentation, PHN is instead broadly defined as clinically relevant pain or altered sensation persisting in the regions affected by HZ at least 3 months beyond the initial HZ eruption [65, 66]. This may be due to sensory nerve disruption caused by the replication of VZV, with associated inflammation and various proposed further mechanisms of tissue damage in and around the dorsal root ganglion or cranial nerve root corresponding to the affected dermatome [67, 68]. Clinically, as in this case, the patient’s skin may display chronic signs of trauma, bacterial superinfection, and scarring from the healing HZ eruption and any self-manipulation of the region due to dysesthesia and/or pain, building the erroneous case for the patient, and perhaps the provider, that an active viral eruption still remains (Fig. 7). In 30–50% of PHN cases, symptoms may persist for over 1 year [69].
The only evidence-driven intervention to prevent PHN is HZ vaccination. Short of that, there are countless strategies that may aid in subsequent PHN pain control, with no particular consensus on optimal agents, initiation window, or dosing schedules. For example, gabapentin may improve PHN but, as aforementioned, does not prevent PHN. It has a slow onset of action and a myriad of dosage options and therefore protean adverse effect tendencies [9, 63, 70, 71]. Some opioids and TCAs also carry evidence for PHN pain control, although gabapentin is more widely prescribed by dermatologists for this and other indications [70]. Considering each agent’s onset of action and adverse effect profile for a particular patient, one of these medication classes should be considered to decrease PHN pain intensity in those particularly at higher risk for debilitating chronic symptoms, for instance in elderly patients with severe initial HZ eruptions and poorer baseline health [72, 73]. There is some evidence for topical analgesics such as lidocaine for short-term relief of PHN symptoms, with less evidence for topical therapies containing capsaicin. Logistically, particularly for severe head and neck or large surface area dermatome involvement of HZ, their practicality is limited [74, 75]. Referral to pain management specialists or neurosurgery for potential interventional options, such as intrathecal corticosteroids, may be recommended in recalcitrant PHN cases [76].
A patient’s wife was with him during his dermatology visit and was taking adalimumab for psoriatic arthritis. She was wondering if she and her husband should be getting the ‘shingles vaccine’. She was 52 years of age and he was 61 years of age. He had the ‘old vaccine’ 2 years ago but is wondering about the new version.
Methods for the prevention of HZ have undergone a revolution in recent decades as HZ vaccination, compared with other infectious disease processes, is a relatively modern concept. Vaccination for HZ aims to boost VZV-specific cell-mediated immunity. Zostavax live-attenuated single-dose subcutaneous HZ vaccine was the first ‘shingles vaccine’ in the US, approved by the FDA in 2006 for adults aged 60 years and older [77]. Although in 2011 the FDA lowered the minimum approved age to 50 years, the CDC/ACIP did not endorse this change, likely because of supply and demand and long-term efficacy concerns, leading to confusion in the public as to whom would be appropriate candidates for vaccination, as in this case [78]. Based on serial CDC surveys, the utilization of this vaccine in indicated populations was only 15.8% in 2011 and 33% in 2016 [79, 80].
In most recent studies (with up to 7-year follow-up), Zostavax prevents HZ in 42–54.7% of CDC-eligible recipients, with older age groups and those with longer duration since vaccination representing lesser and waning efficacy, respectively [77, 81]. It reduces time to acute HZ lesion healing and symptom severity if a vaccinated patient does develop HZ, and its prevention rate for PHN in eligible vaccinated patients who develop HZ is 47.4–77% with consistent efficacy initially across all age groups, but less preventative effect with longer duration from initial vaccination. Nonetheless, if PHN from a case of HZ ensues after vaccination, it typically presents with less severe symptomatology [77]. The vaccine purports few adverse effects, notably occasional injection site reaction and headache, but because it is a live vaccine, immunosuppressed populations, such as those with active or recent hematologic malignancy, those receiving cytotoxic chemotherapy, biologic medications, or other higher dose immunosuppressive agents, or those with HIV, may not be candidates for its use. Additionally, it does contain preservatives and is contraindicated in patients with anaphylaxis to neomycin or gelatin [77].
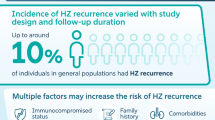
In 2017, Shingrix, a subunit (non-live) recombinant vaccine with adjuvant, was approved by the FDA and supported by the CDC and ACIP for age 50 years and older [22]. It is an intramuscular two-dose vaccine that should be administered 2–6 months apart and may be administered in patients who have already received Zostavax if at least 2 months have passed between vaccines. Furthermore, neither vaccine should be administered during an acute outbreak of HZ or while a patient is taking antiviral medications for any indication. Although the risk of recurrent HZ in immunocompetent patients is small, namely 5.7% over 8 years from the initial HZ episode, both vaccines are well tolerated in patients who have already experienced HZ [2, 26]. An episode of HZ in an immunocompetent patient is theoretically akin to receiving the vaccine; therefore, supply, demand, and stewardship for appropriate use in the highest-risk populations should be considered.
Importantly, Shingrix efficacy exceeds that of the prior vaccine, namely 84.7–97.4% prevention of HZ and 88.8–91.2% prevention of PHN, with less variability or waning efficacy with age, and otherwise eliciting less severe presentations of HZ and PHN in breakthrough cases. In ongoing follow-up studies, this effect persists for at least 4 years. Its adverse effect profile is more pronounced, namely localized pain and flu-like symptoms, compared with Zostavax because of the two-dose schedule, intramuscular administration, and added adjuvant [22, 23].
Shingrix is not live and does not contain preservatives, therefore the only true contraindications to its use are prior anaphylaxis to the initial vaccine injection and acute illness [22]. This vaccine’s usefulness in special populations, namely those with significant sources of immunosuppression, including patients excluded from Zostavax vaccination, is still under study. Some promising reports, particularly in post-transplant, current chemotherapy, and HIV-infected patients, reveal significant efficacy and safety, but the CDC and ACIP have not secured official recommendations in this arena of use. However, compared with the true contraindication to Zostavax in patients with moderate to severe immunosuppression, Shingrix does not exclude this population [82,83,84,85,86,87]. In fact, particularly germane to dermatology, a recent review by the Medical Board of the National Psoriasis Foundation recommended that all psoriasis and psoriatic arthritis patients age 50 years and older and patients younger than 50 years taking tofacitinib, systemic corticosteroids, or combination systemic therapy (including biologic and conventional synthetic disease-modifying antirheumatic drugs) should receive Shingrix [88]. Therefore, in this case vignette of an otherwise vaccine-eligible patient receiving a biologic medication, the patient should be offered Shingrix. Those patients with chronic illness, particularly chronic renal failure, diabetes mellitus, rheumatoid and psoriatic arthritis, and systemic lupus erythematosus, are at risk for severe cases of HZ, and although patients taking an immunosuppressive medication might, in theory, mount a muted immune response to the vaccine, the overriding benefits of HZ prevention and morbidity are gaining support [89, 90]. Additionally, because these patient populations are often receiving antimicrobial prophylaxis for opportunistic infections, it is imperative that they discontinue antivirals before vaccination so that, in theory, their optimal immune responses to the true vaccine concentration are elicited [39, 45].
Because of its superior efficacy and potential applicability to a wider age range and health status range of patients, the CDC and ACIP prefer Shingrix to Zostavax vaccination [86]. However, this guideline has influenced the accessibility of this vaccine as abrupt demand in the past 2 years has resulted in shortages and missed second doses of the vaccine. Of note, if the second dose window is exceeded, it is recommended that the second injection be nonetheless administered without restarting the vaccine series [23]. Since the advent of these vaccines, although data reflecting the Shingrix effect on these trends are fledgling, the burden of HZ disease in the US has begun to dissipate. That being said, true HZ incidence may still be underreported and underdiagnosed, and the concomitant effect of an aging population and the aforementioned childhood VZV vaccine population all create a complex and unpredictable epidemiologic spectrum and trajectory [25, 36, 86].
An otherwise healthy 80-year-old female called the office because just last week she had been treated by her primary care physician for HZ on the left upper arm, and, while finishing up a course of aciclovir, wanted to know if she could babysit her infant great-granddaughter. She was also wondering if she could ‘catch’ shingles from the vaccine like she said she ‘caught’ the flu after her last flu shot?
Isolation guidelines for HZ reflect the location of the eruption, age, and immune status of each patient and their contacts. In immunocompetent patients with classic dermatomal presentation, standard precautions (hand hygiene and respiratory etiquette) apply, and the lesions should be dressed or otherwise covered until they are dry and crusted over. There is debate as to whether patients with mucosal, particularly trigeminal, HZ distribution cases shed more VZV than other dermatomes, but the same guidelines apply [90]. In this vignette, the patient’s eruption was localized to the upper arm and she was otherwise healthy, therefore the aforementioned standard precautions would be prudent. Because her great-granddaughter is an infant, she would not yet have received either dose of childhood VZV vaccination (age 12–16 months and 4–6 years), therefore, in theory, the infant would be at risk for primary VZV infection from a HZ patient who is actively shedding the virus [18]. However, considering that the patient is not immunosuppressed and had been receiving oral antiviral treatment for the past week, and that the area of HZ eruption is easily covered and bandaged, the risk of transmission of VZV is negligible [91]. Conversely, in immunosuppressed patients, VZV may shed more readily, as it would in primary VZV infection, and perhaps for extended periods of time. Acutely, when these patients are hospitalized, standard (see above), airborne (private negative pressure room, patient standard mask, and staff respirator mask), and contact (single-use staff gloves, gown, and equipment) precautions apply until HZ lesions are dry and crusted [45, 91].
Vaccinated patients often enquire, as in this case, about the infectivity of HZ vaccination itself. In cases where live vaccine (Zostavax) was administered, within a 42-day window, there have been reported ‘zosteriform’ eruptions in non-injection sites, consistent with incidental wild-type HZ eruption, with the incidence of HZ being similar in both placebo and vaccinated groups in this time period [77]. Injection site reactions, including swelling, urticaria, or papulovesicular lesions, have been acutely reported after vaccination, but they have not been specifically characterized as HZ or infectious in nature. There are isolated cases of the Zostavax vaccine-strain Oka virus as the source of HZ in immunocompetent patients, just as there are pediatric HZ cases of Oka strain HZ from childhood VZV vaccination, but these present outside of the 1- to 2-month acute window. In other words, this is after the HZ strain returns to a dormant state in the dorsal root or cranial nerve ganglion, and is not attributable to an acute reaction to the vaccine itself [77, 92,93,94]. Furthermore, to date, there are rare reported cases of Oka strain from HZ or VZV vaccination transmitted to a vaccinated patient contact, and if in fact a prospective case is suspicious for this sequence, laboratory confirmation by PCR must be performed by the CDC [77, 95,96,97]. The above discussion is an epidemiological exercise that may become moot as Shingrix utilization outweighs that of Zostavax, as an adjuvanted subunit vaccine would not undergo neurologic dormancy or reactivation like a live vaccine would.
A 32-year-old otherwise healthy pregnant female (32 weeks, first pregnancy) notes ‘ripping’ pain across the right abdomen for 2 days, followed by a cluster of pink papulovesicles across the right lateral abdomen and right upper back. She was a healthcare worker in an outpatient clinic. Her sister also had an eruption like this in pregnancy.
Only 1 in 20,000 pregnancies may include an eruption of HZ, but steadily, over several decades, the incidence of HZ has risen in all adults, for explainable reasons, perhaps because of the increasing average lifespan and more sources for immunosuppressed living, and unexplainable reasons in kind; pregnancy remains in this area of uncertainty [2, 98]. In theory, a healthy pregnant female’s immune responses may be less brisk because of shared maternal cellular immunity with the fetus, therefore HZ may ensue more frequently than in non-pregnant counterparts [99, 100]. Furthermore, differently than the true viremia of primary VZV infection, which is now rarer in pregnancy in the US because of routine prenatal screenings for VZV, HZ does not cause viremia with transplacental infection, and maternal protective VZV-specific IgG antibodies from either appropriate childhood vaccination or prior primary VZV infection pass between mother and fetus. This would also, in theory, protect the newborn if the pregnant women developed HZ around the time of delivery, but the aforementioned standard precautions and wound care would still be indicated [98, 100,101,102].
In this case of a pregnant healthcare worker who developed HZ, fear of transmission to the fetus would be particularly allayed as she would have undergone employment screening for VZV immunity, particularly by either documentation of childhood VZV, childhood VZV vaccination, or laboratory immunity, for example by one of the aforementioned ELISA assays (Fig. 8). Of note, if the healthcare worker’s environment includes patients not immune to VZV, particularly infants or the immunosuppressed, scrupulous diligence with standard precautions would be prudent. In this case, since the involved HZ area is a constantly clothed region of the trunk in the workplace, and if she indeed undergoes antiviral treatment in the appropriate window, viral shedding and infectivity risk to other patients would be negligible [50, 90].
Antiviral therapy for HZ in pregnancy is considered safe, and expedient initiation of such in the indicated treatment window militates against severe morbidity, including pain and wound complications, in pregnancy that could adversely affect the well-being of her fetus [98, 99, 103]. Pain control measures would be at the discretion of the patient’s obstetrical team, depending on her pain severity, comorbidities, and stage of pregnancy [99, 104].
In this case, the patient’s sister had also developed HZ in pregnancy, which may reflect a strong genetic predisposition to HZ along maternal ancestry. This information may become relevant for future HZ vaccine timing in younger childbearing populations as surveillance studies continue [105]. Currently, Zostavax is contraindicated in pregnancy, first because it is a live attenuated vaccine in which the maternal protective and immune responses may not be predictable and safety for the fetus cannot be determined, and practically because Zostavax is only indicated per the CDC for adults aged 60 years and older, which excludes the typical pregnancy age range. Alternatively, for Shingrix, there are no data to exclude pregnant females from receiving the vaccine, and, in theory, its age indication of 50 years and older may include a minority of pregnant females. Additionally, lactation contact risk for an infant of postpartum women receiving HZ vaccination is unknown for both vaccines [22, 76].
3 Conclusions
These vignettes and practical approaches to the diagnosis, management, and prevention of HZ sample the complicated nuances that characterize dermatologic and coordinated care team frameworks for this disease. A reactivation of a virus behaves differently than a primary infection, and understanding the pathophysiology of its virulence, immune response, and reactions to therapy, in addition to applying evidence-based clinical management and vaccination guidelines to each case, create the opportunity for a calmer environment for prudent, expedient, and effective HZ care.
References
Brisson M, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127(2):305–14.
Yawn BP, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–9.
Kawai K, et al. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63(2):221–6.
Lim HW, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958–72.e2.
Dworkin RH, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26.
Wood MJ, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896–900.
McKendrick MW, et al. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed). 1986;293:1529–32.
Whitley RJ, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–83.
Zhang M, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin in the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int. 2018;2018:7474207.
Feli KH, et al. Update in herpes zoster prevention and the role of dermatologists. J Drugs Dermatol. 2019;18(1):18–22.
Nair PA. Herpes zoster: mistaken for radiculopathy and back pain. J Indian Med Assoc. 2012;110(6):399.
Böer A, et al. Refining criteria for diagnosis of cutaneous infections caused by herpes viruses through correlation of morphology with molecular pathology. Indian J Dermatol Venereol Leprol. 2006;72(4):270–5.
Morgan R, King D. Characteristics of patients with shingles admitted to a district general hospital. Postgrad Med J. 1998;74(868):101–3.
Brănişteanu DE, et al. Clinical-epidemiological trends of herpes zoster: a 5-year study. Rev Med Chir Soc Med Nat Iasi. 2014;118(3):817–22.
Aggarwal S, Radhakrishnan S. A clinico-epidemiological study of herpes zoster. Med J Armed Forces India. 2016;72(2):175–7.
Abdul E, Pavithran K. Herpes zoster: a clinical study in 205 patients. Ind J Dermatol. 2011;56:529–32.
Shors T. Herpesviruses: Varicella Zoster Virus (VZV). In: Shors T, editor. Understanding viruses. 2nd ed. Burlington: Jones & Bartlett; 2011. p. 459.
CDC Varicella Vaccine Recommendations. https://www.cdc.gov/vaccines/vpd/varicella/hcp/recommendations.html. Accessed 28 Aug 2019.
ProQuad package insert. https://www.merck.com/product/usa/pi_circulars/p/proquad/proquad_pi_4171.pdf. Accessed 28 Aug 2019.
Varivax package insert. https://www.merck.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf. Accessed 28 Aug 2019.
Reynolds MA, et al. Varicella seroprevalence in the US: data from the National Health and Nutrition Examination Survey, 1999–2004. Public Health Rep. 2010;125(6):860–9.
Shingrix package insert. https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Shingrix/pdf/SHINGRIX.PDF. Accessed 28 Aug 2019.
CDC Shingrix recommendations. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/recommendations.html. Accessed 28 Aug 2019.
Freer G, Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41(2):95–105.
Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2019;69(2):341–4.
Ragozzino MW, et al. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982;61:310–6.
Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–21.
Lamont RF, et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG. 2011;118(10):1155–62.
Feder HM, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451–7.
David TJ, Williams ML. Herpes zoster in infancy. Scand J Infect Dis. 1979;11:185–6.
Forbes H, et al. Quantification of risk factors for herpes zoster: population-based case–control study. BMJ. 2014;348:g2911.
Prabhu S, et al. Childhood herpes zoster: a clustering of ten cases. Indian J Dermatol. 2009;54:62–4.
Katakam BK, et al. A prospective study of herpes zoster in children. Indian J Dermatol. 2016;61:534–9.
Weinmann S, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis. 2013;208:1859–68.
Weinmann S, et al. Incidence of herpes zoster among children: 2003–2014. Pediatrics. 2019;144(1):e20182917.
Harpaz R. Do varicella vaccination programs change the epidemiology of herpes zoster? A comprehensive review, with focus on the United States. Expert Rev Vaccines. 2019;18(8):793–811.
Wutzler P, et al. Herpes zoster in the context of varicella vaccination—an equation with several variables. Vaccine. 2018;36(46):7072–82.
Lewis DJ, et al. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100(5):321, 324, 330.
Schmader K. Herpes zoster. Ann Intern Med. 2018;169:ITC19–31.
Tilley DH, et al. Disseminated verrucous varicella zoster with exclusive follicular involvement. Arch Dermatol. 2012;148(3):405–7.
Wauters O, et al. Chronic mucocutaneous herpes simplex virus and varicella zoster virus infections. J Am Acad Dermatol. 2012;66(6):e217–27.
Prelog M, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420–6.
Gilbert L, et al. Herpes zoster rates continue to decline in people living with human immunodeficiency virus but remain higher than rates reported in the general US population. Clin Infect Dis. 2019;69(1):155–8.
Insinga RP, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–53.
Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–57.
Ljubojević HS, et al. Genital herpes zoster as possible indicator of HIV infection. Acta Dermatovenerol Croat. 2018;26(4):337–8.
Dayan RR, Peleg R. Herpes zoster—typical and atypical presentations. Postgrad Med. 2017;129(6):567–71.
Chughtai K, et al. Topical 5-fluorouracil associated skin reaction. Oxf Med Case Rep. 2017;2017(8):omx043.
Wilson DA, et al. Should varicella-zoster virus culture be eliminated? A comparison of direct immunofluorescence antigen detection, culture, and PCR, with a historical review. J Clin Microbiol. 2012;50(12):4120–2.
Dobec M, et al. Serology and serum DNA detection in shingles. Swiss Med Wkly. 2008;138(3–4):47–51.
Balfour HH, et al. Acyclovir halts progression of herpes zoster in immunocompromised patients. N Engl J Med. 1983;308:1448–53.
Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–12.
Tyring S, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:89–96.
Beutner KR, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53.
Chen N, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2:CD006866.
Stein GE. Pharmacology of new antiherpes agents: famciclovir and valacyclovir. J Am Pharm Assoc (Wash). 1997;37(2):157–63.
Ormrod D, Goa K. Valaciclovir: a review of its use in the management of herpes zoster. Drugs. 2000;59(6):1317–40.
Madkan VK, et al. Open-label study of valacyclovir 1.5 g twice daily for the treatment of uncomplicated herpes zoster in immunocompetent patients 18 years of age or older. J Cutan Med Surg. 2007;11(3):89–98.
Barnabas RV, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213(4):551–5.
Chen N, et al. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2010;12:CD005582.
Han Y, Zhang J, Chen N, He L, Zhou M, Zhu C. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2013;3:CD005582.
Dworkin RH, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain. 2009;142(3):209–17.
Bulilete O, et al. Efficacy of gabapentin for the prevention of postherpetic neuralgia in patients with acute herpes zoster: a double blind, randomized controlled trial. PLoS One. 2019;14(6):e0217335.
Ghaznawi N, et al. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology. 2011;118(11):2242–50.
Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67(2–3):241–51.
Thyregod HG, et al. Natural history of pain following herpes zoster. Pain. 2007;128(1–2):148–56.
Zhu SM, et al. Influence of systemic immune and cytokine responses during the acute phase of zoster on the development of postherpetic neuralgia. J Zhejiang Univ Sci B. 2009;10(8):625–30.
Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92:139–45.
Kawai K, et al. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833.
Hempenstall K, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
Johnson P, et al. Real-world treatment of post-herpetic neuralgia with gabapentin or pregabalin. Clin Drug Investig. 2013;33(1):35–44.
Nagasako EM, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46(6):834–9.
Drolet M, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11(11):1211–21.
Argoff CE. Review of current guidelines on the care of postherpetic neuralgia. Postgrad Med. 2011;123(5):134–42.
Galer BS, et al. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain. 1999;80:533–8.
Lin CS, et al. Interventional treatments for postherpetic neuralgia: a systematic review. Pain Physician. 2019;22(3):209–28.
Zostavax package insert. https://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed 28 Aug 2019.
CDC Zostavax recommendations. https://www.cdc.gov/vaccines/vpd/shingles/hcp/zostavax/recommendations.html. Accessed 28 Aug 2019.
CDC National Health Interview Survey. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html. Accessed 28 Aug 2019.
Opstelten W, et al. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine. 2009;27(2):192–6.
Schmader KE, Shingles Prevention Study Group, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55(10):1320–8.
Vink P et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase III, randomized clinical trial. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz177.
Vink P, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12.
Berkowitz EM, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–87.
Bastidas A, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–33.
Dooling KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–8.
Dagnew AF, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000.
Baumrin E, et al. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2019;81(1):102–10.
Failla V, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224(3):251–6.
Zaal MJ, et al. Longitudinal analysis of varicella-zoster virus DNA on the ocular surface associated with herpes zoster ophthalmicus. Am J Ophthalmol. 2001;131:25–9.
CDC Preventing VZV Transmission in Healthcare Settings. 2019. https://www.cdc.gov/shingles/hcp/hc-settings.html. Accessed 28 Aug 2019.
Tseng HF, et al. Herpes zoster caused by vaccine-strain varicella zoster virus in an immunocompetent recipient of zoster vaccine. Clin Infect Dis. 2014;58(8):1125–8.
Guffey DJ. Herpes zoster following varicella vaccination in children. Cutis. 2017;99(3):207–11.
Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94–5.
Lopez AS, et al. Transmission of a newly characterized strain of varicella-zoster virus from a patient with herpes zoster in a long-term-care facility, West Virginia, 2004. J Infect Dis. 2008;197(5):646–53.
Marin M, Güris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices, CDC. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1–40.
Goulleret N, et al. Safety profile of live varicella virus vaccine (Oka/Merck): 5-year results of the European Varicella Zoster Virus Identification Program (EU VZVIP). Vaccine. 2010;28(36):5878–82.
Pryde PG. Varicella zoster virus in pregnancy. In: Craigo SD, Baker ER, editors. Medical Complications in Pregnancy. NewYork: McGraw-Hill; 2005. p. 383–98.
Müllegger RR, et al. Skin infections in pregnancy. Clin Dermatol. 2016;34(3):368–77.
Hayward K, et al. Management of herpes zoster (shingles) during pregnancy. J Obstet Gynaecol. 2018;38(7):887–94.
Sauerbrei A, Wutzler P. Placental boost to varicella-zoster antibodies in the newborn. J Perinat Med. 2002;30(4):345–8.
Casanova RG, et al. Herpes zoster in immunocompetent pregnant women and their perinatal outcome. Ginecol Obstet Mex. 2004;72:63–7.
Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304(8):859–66.
Schafer R, et al. Herpes zoster in pregnancy. J Midwifery Womens Health. 2019;64(2):230–5.
Hernandez PO, et al. Family history and herpes zoster risk in the era of shingles vaccination. J Clin Virol. 2011;52(4):344–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Dr Rosamilia has no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Rosamilia, L.L. Herpes Zoster Presentation, Management, and Prevention: A Modern Case-Based Review. Am J Clin Dermatol 21, 97–107 (2020). https://doi.org/10.1007/s40257-019-00483-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-019-00483-1