Abstract
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis that may be caused by an adverse drug reaction. We discuss the clinical presentation and outcomes of 52 cases of drug-induced PG reported to date in the literature. We conducted our literature search for case reports of drug-induced PG using keywords on PubMed and Medical Subject Heading (MeSH) terms on MEDLINE and EMBASE. To assess the probability that each case of PG was related to drug therapy, we used the Naranjo criteria. We identified 44 studies in the literature, with a total of 52 cases of drug-induced PG. The mean Naranjo score for cocaine-induced PG (n = 13) was 9.4, indicating a definite adverse drug reaction, while the mean Naranjo scores for isotretinoin (n = 5), propylthiouracil (n = 5), and sunitinib (n = 5) were 6.2, 6.8, and 7.4, respectively, indicating probable adverse drug reactions. Drugs should be considered as a possible triggering event whenever PG is diagnosed, and clinicians should particularly consider this in patients taking isotretinoin, propylthiouracil, or sunitinib, as well as in patients with a history of cocaine use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cocaine-induced pyoderma gangrenosum (PG) is a definite adverse drug reaction. |
PG associated with isotretinoin, propylthiouracil, and sunitinib is a probable adverse drug reaction. |
Onset can be years after a drug is started and there is no specific clinical or pathological pattern that suggests drug-induced PG. |
1 Introduction
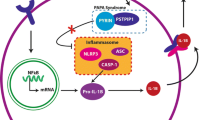
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis. It is characterized by painful, sterile ulcers, often occurring on the lower legs and trunk, and most cases occur in adults aged 30–50 years [1]. PG is polygenic and autoinflammatory in nature [2]. There is overexpression of interleukin (IL)-1β, IL-17, tumour necrosis factor (TNF)-α, and multiple chemokines, which supports the mechanism of neutrophil activation and migration [2, 3]. Genetic analysis in PG has shown mutations in several known autoinflammatory genes, including MEFV and PSTPIP1 [2].
In 50% of cases, PG is associated with underlying disease, most commonly inflammatory bowel disease (IBD), rheumatoid arthritis, myelodysplastic syndrome (MDS), or haematologic malignancies [1, 4]. There are four major clinical variants of PG: ulcerative, pustular, bullous, and vegetative, with ulcerative being the most common by far [4]. Treatment is focused on immunosuppression.
Drug-induced PG is a rare adverse cutaneous drug reaction and its diagnosis can be challenging. Drugs that have been implicated to date are propylthiouracil, granulocyte colony-stimulating factor (G-CSF), and sunitinib [4, 5]. In this review, we discuss the clinical presentation and outcomes of 52 cases of drug-induced PG reported to date in the literature.
2 Methods
We conducted our literature search for case reports of drug-induced PG using keywords on PubMed, and Medical Subject Heading (MeSH) terms on MEDLINE and EMBASE. We then used snowballing and forward citation tracking of selected articles to identify additional relevant studies.
We included articles where a diagnosis of PG was made either based on clinical suspicion alone or with the aid of biopsy. Articles were excluded if there was a high clinical suspicion of alternative diagnoses, such as Sweet’s syndrome, unspecified neutrophilic dermatoses, or autoimmune vasculitides. Two articles were not available in the English language and one article was not available through our institution’s library system [6,7,8]. In these three cases, we extracted data from abstracts.
To assess the probability that each case of PG was related to drug therapy, we used the Naranjo criteria (Table 1) [9]. Two reviewers each independently scored half of the cases and then checked each other’s scores. Any discrepancies were discussed with the primary investigator. For question 1, we scored ‘yes’ only if there were other conclusive reports of that particular drug implicated in PG, and not other drugs within the same class; for question 3, we scored ‘no’ if the lesions did not improve with discontinuation of the suspected drug or did not respond to systemic corticosteroids; for question 5, we scored ‘yes’ if any of the following associated diseases were present: MDS, any haematologic malignancy, rheumatoid arthritis, or IBD; and for question 7, we scored ‘yes’ if the drug was detected in body fluids in any concentration as there are presently no data to support dose dependence of drug-induced PG.
According to the Naranjo criteria, a score of 1–4 indicates a possible adverse drug reaction, a score of 5–8 indicates a probable adverse drug reaction, and a score of 9–13 indicates a definite adverse drug reaction.
3 Results
We identified 44 case reports (or case series) in the literature, with a total of 52 cases of drug-induced PG (Table 2). The majority of investigators used biopsy to support their diagnosis or to exclude other causes. In only five reports, diagnosis was made based on clinical examination and history alone [10,11,12,13,14]. Su et al. developed guidelines for the diagnosis of ulcerative PG, in which two major criteria and two of four minor criteria must be met [15]. The two major criteria are morphology consistent with PG and exclusion of other causes, while the four minor criteria are pathergy or cribriform scarring, systemic disease associated with PG, histopathologic findings, and response to immunosuppression [15]. Of the 50 cases of ulcerative PG, 35 met these criteria, 10 did not [10, 11, 13, 14, 16,17,18,19,20,21], and there were insufficient diagnostic data in 5 cases [6,7,8, 22, 23]. Two cases were bullous PG [24, 25].
For all drugs, with the exception of cocaine, none of the investigators attempted to administer placebo, or to measure serum or urine drug levels; therefore, there was a ceiling Naranjo score of 11 for all prescription therapeutic drugs.
Our study identified only two drugs reported in the literature indicating definite adverse drug reactions (mean Naranjo score 9–13), i.e. cocaine and imatinib, and 13 drugs indicating probable adverse drug reactions (mean Naranjo score 5–8), i.e. propylthiouracil, isotretinoin, interferon, sunitinib, gefitinib, enoxaparin, erythropoietin, pazopanib, etanercept, ipilimumab, azacitidine, alitretinoin, and ciprofloxacin. Five drugs indicated possible adverse drug reactions (mean Naranjo score 1–4), i.e. G-CSF, infliximab, adalimumab, lenalidomide, and red tattoo dye.
3.1 Isotretinoin
All five patients were being treated for severe acne vulgaris, at a dose of 0.5–1 mg/kg/day [10, 12, 26,27,28]. In four patients, PG onset was 2–6 weeks after initiation of isotretinoin [10, 12, 26, 28]. In the last patient, PG onset was 3 years later [27], however this patient was later confirmed to have MDS. All five patients presented with lesions on the trunk, and two patients also had limb lesions. All patients experienced ulcer healing with discontinuation of isotretinoin and systemic immunosuppressive therapy (corticosteroids, dapsone, or mycophenolate mofetil). The mean Naranjo score for all cases was 6.2.
3.2 Propylthiouracil
All five reported patients were females being treated for hyperthyroidism [6, 29,30,31,32]. Four patients presented with PG on the limbs [6, 29,30,31] and one presented with PG on the trunk [32]. Lesions appeared between 1.5 and 6 years after propylthiouracil was initiated. In all cases, ulcer healing was observed with discontinuation of propylthiouracil and initiation of systemic corticosteroid therapy. Time to re-epithelialization, reported in four cases, was 2–8 weeks [6, 29,30,31]. The mean Naranjo score for all cases was 6.8.
3.3 Granulocyte Colony-Stimulating Factor and Other Subcutaneous Injection Medications
Drug-induced PG secondary to G-CSF was reported in five patients [24, 33,34,35], four of whom had concurrent underlying illnesses that were significant risk factors: MDS (n = 2) [24, 34] and Hodgkin’s lymphoma (n = 2) [33, 35]. The location of lesions was variable between patients and occurred at injection sites in only one patient [35]. In all cases, ulcers gradually improved with discontinuation of G-CSF and systemic corticosteroid therapy. One patient relapsed 7 months after stopping G-CSF and required cyclosporine 600 mg/day for control [24]. The mean Naranjo score for all cases was 4.2.
Interferon was implicated in drug-induced PG in three cases [8, 36, 37], two of which presented with lesions at injection sites [36, 37]. Onset of PG varied from 1 month to 5 years. All three patients experienced wound healing with discontinuation of interferon and initiation of immunosuppressive therapy (corticosteroids, or cyclosporine). The mean Naranjo score for all cases was 5.3.
One patient developed PG at the injection site of enoxaparin (Naranjo = 5) [14], while another patient developed PG at the injection site of erythropoietin (Naranjo = 5) [38].
3.4 Tyrosine Kinase Inhibitors
There were five reports of patients developing PG while taking sunitinib, all of whom were being treated for malignant solid tumours [16, 17, 39,40,41]. All five patients presented with lesions restricted to the lower limbs. The range of time to onset of PG, reported in four cases, was 1–18 months [17, 39,40,41]. Two patients were on a dosing regimen of 4 weeks on and 2 weeks off [39, 40]; one of these patients experienced transient improvement in the ulcers during every 2-weeks-off period [39]. Four patients responded well to systemic corticosteroids and discontinuation of sunitinib [16, 17, 39, 41], while in the fifth patient, sunitinib was continued, despite the occurrence of PG [40]. This patient responded to systemic prednisolone initially but relapsed 7 months later. At this time, sunitinib was discontinued and the lesions resolved within 4 weeks. The mean Naranjo score for all cases was 7.4.
Imatinib and gefitinib were reported to be associated with PG in one patient each [13, 21]. In both cases, the tyrosine kinase inhibitor (TKI) was reintroduced at a lower dose. The patient taking imatinib developed recurrence of PG at the same site [21], while the patient taking gefitinib did not develop recurrence [13]. One case of pazopanib-induced PG on the glans penis was reported [42]. This patient had previously developed similar ulcerative lesions diagnosed as PG while taking sunitinib. The mean Naranjo score for non-sunitinib TKIs was 7.0.
3.5 Tumour Necros Factor-α Inhibitors
TNFα inhibitors have been successfully used to treat PG, but we also identified five reports in the literature where these drugs may have been implicated in causing PG [22, 23, 43,44,45]. Three patients developed PG while taking infliximab [43,44,45], two of whom had known diseases that predispose to PG—one patient had ulcerative colitis, and the other had rheumatoid arthritis. In the patient with ulcerative colitis, infliximab was continued for disease control and systemic corticosteroids were started [43]. Slow improvement of lesions was observed. In the patient with rheumatoid arthritis, infliximab was stopped [44]. Etanercept was started for disease control and minocycline was added for PG control. Ulcers healed and no relapses were observed on etanercept therapy. In the last patient, ulcers resolved within 4 weeks of discontinuing infliximab, without additional corticosteroid therapy [45].
One patient developed PG while taking adalimumab for rheumatoid arthritis [39], however lesions did not improve with discontinuation of adalimumab, and improvement was only seen once systemic corticosteroids were initiated. The mean Naranjo score for all cases of TNFα inhibitors was 4.6.
3.6 Cocaine and Levamisole
Thirteen patients in five studies were reported to have developed PG in association with chronic cocaine use [7, 20, 46,47,48]. Eleven patients presented with limb lesions [20, 46, 47] and one patient developed a Wegener’s granulomatosis-like syndrome at the same time as PG [20]. All patients experienced relapse or worsening of PG that was temporally correlated with repeated cocaine use, as confirmed by urine drug testing. Ulcers tended to improve with cocaine abstinence and systemic immunosuppressive therapy (corticosteroids, cyclosporine, cyclophosphamide, infliximab, or mycophenolate mofetil). Three authors strongly suspect the culprit agent to be levamisole rather than cocaine [46,47,48]. In their geographical locations, a large proportion of cocaine is contaminated with levamisole, although no formal laboratory testing was conducted for confirmation. In their eight cases of cocaine-induced PG, Jeong et al. report serologic findings that are similar to patients with levamisole-associated vasculitis, namely elevated P-ANCA, antimyeloperoxidase antibodies, and antiphospholipid antibodies. The mean Naranjo score among all studies was 9.4.
4 Discussion
Similar to classic PG, cases classified as possible or probable drug-induced PG according to the Naranjo criteria typically began as painful nodules or pustules, which then progress to ulcers over the course of days to weeks. The lesions tend to have boggy, necrotic, sometimes purulent bases, with ragged, undermined borders and surrounding erythema. Histologically, drug-induced PG, like classic PG, presents as neutrophilic infiltration into the dermis, with or without leukocytoclastic vasculitis. The size of the ulcerations is variable, although they may progressively become larger without appropriate treatment. Once healed, permanent cribriform scarring often remains.
Pathergy (characterized by the development of PG at the site of trauma) has been reported in 20–30% of patients with PG. In cases where PG lesions develop after the injection, pathergy is suspected to play a role.
The age and sex distribution of patients who develop drug-induced PG is comparable with the age and sex distribution of patients receiving the suspected drugs (i.e. females taking propylthiouracil for Grave’s disease, and young adult males taking isotretinoin for acne vulgaris) (Table 2). Based on the sample size available, there appears to be no demographic risk for drug-induced PG.
Eleven patients (22%) in our sample had a concurrent underlying disease that predisposed them to developing PG: MDS (n = 4), haematologic malignancy (n = 4), rheumatoid arthritis (n = 2), or IBD (n = 1). This is appreciably lower than the reported incidence of 50% of all PG patients having underlying disease [1, 4]. This difference suggests that the use of drugs discussed in this paper is a risk factor for PG.
The strongest evidence for drug-induced PG concerns cocaine and levamisole. Because patients often continued cocaine use after the onset of PG, we have evidence of temporal correlation between cocaine use and relapse or worsening of ulcers. The Naranjo scores for these cases are 9 or 10, suggesting that the occurrence of PG is definitely linked to cocaine use. Therefore, if PG is suspected, it is important to ascertain cocaine use in the patient’s history. Because levamisole is known to cause other immune-mediated reactions, such as vasculitis, there is a clinical suspicion of levamisole being the cause of PG in these cases; however, no laboratory testing was performed to confirm the presence of serum or urine levamisole in these patients.
Three drugs, i.e. propylthiouracil (n = 5), isotretinoin (n = 5), and sunitinib (n = 5), demonstrated consistently high (5 or greater) Naranjo scores in multiple case reports, suggesting that for these drugs, PG was a probable adverse drug reaction.
TNFα inhibitors have been shown to effectively treat refractory PG; however, there were five reports of this class of drugs causing PG. All five of these patients had systemic inflammatory disease, for which TNFα inhibitors were indicated. Therefore, it is suspected that these drugs did not in fact cause PG, but rather failed to suppress it [23]. Alternatively, these may represent paradoxical reactions due to a shift towards T helper-17 polarization.
Drug-induced PG is frequently initially misdiagnosed as bacterial infection. Although wounds may become secondarily infected, systemic antibiotics provide little to no relief. As is the case with all severe adverse drug reactions, the mainstay of therapy is discontinuation of the suspected drug. Systemic corticosteroids and immunosuppressive agents can accelerate healing time but are not curative if the causative drug is continued.
Ultimately, drug-induced PG poses a diagnostic challenge. Due to the severity of the reaction, giving placebo or rechallenging the drug is usually unacceptable. Therefore, correlation between drug administration and PG may be difficult to establish.
4.1 Limitations
Drug-induced PG is a very rare adverse event with no standard for diagnosis. Therefore, it is possible that some of these cases were misdiagnosed and were not in fact drug-induced PG. This is particularly an issue in the case of cocaine as some cases may present similar to, or concurrently with, Wegener’s granulomatosis [20].
5 Conclusions
Drug-induced PG is a rare but serious adverse drug reaction. Drugs should be considered as a possible triggering event whenever PG is suspected or diagnosed. Where there is clinical suspicion of this reaction, the suspected drug should be discontinued immediately. It is important to recognize that the onset of PG can be years after a therapy is started and that there is no specific clinical or pathological pattern that suggests drug-induced PG. Clinicians should particularly consider drug causes for PG in patients taking propylthiouracil, isotretinoin, or sunitinib, and in patients with a history of cocaine use.
References
Crowson AN, Mihm MC Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol. 2003;30(2):97–107.
Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2016. doi:10.1111/bjd.15226.
Marzano AV, Fanoni D, Antiga E, et al. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin Exp Immunol. 2014;178(1):48–56.
Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;15(2):19.
Wu BC, Patel ED, Ortega-Loayza AG. Drug-induced pyoderma gangrenosum: a model to understand the pathogenesis of pyoderma gangrenosum. Br J Dermatol. 2016. doi:10.1111/bjd.15193.
Boulenger-Vazel A, Kupfer-Bessaguet I, Gouedard C, et al. Neutrophilic dermatosis associated with propylthiouracil-induced p-ANCA (p-antineutrophil cytoplasmic antibodies). Ann Dermatol Venereol. 2005;132(1):27–31.
Roche E, Martínez-Menchón T, Sánchez-Carazo JL, et al. Two cases of eruptive pyoderma gangrenosum associated with cocaine use. Actas Dermosifiliogr. 2008;99(9):727–30.
Yurci A, Guven K, Torun E, et al. Pyoderma gangrenosum and exacerbation of psoriasis resulting from pegylated interferon alpha and ribavirin treatment of chronic hepatitis C. Eur J Gastroenterol Hepatol. 2007;19(9):811–5.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Exner JH, Dahod S, Pochi PE. Pyogenic granuloma-like acne lesions during isotretinoin therapy. Arch Dermatol. 1983;119(10):808–11.
Dasanu CA, Bockorny B, Alexandrescu DT. Pyoderma gangrenosum due to lenalidomide use for multiple myeloma. J Oncol Pharm Pract. 2015;21(6):471–3.
Gangaram HB, Tan LP, Gan AT, et al. Pyoderma gangrenosum following treatment with isotretinoin. Br J Dermatol. 1997;136(4):636–7.
Sagara R, Kitami A, Nakada T, Iijima M. Adverse reactions to gefitinib (Iressa): revealing sycosis- and pyoderma gangrenosum-like lesions. Int J Dermatol. 2006;45(8):1002–3.
Sobieszczańska M, Tubek S, Kura I. Pyoderma gangrenosum-like skin changes after subcutaneous administration of low molecular weight heparin. Hum Vaccin Immunother. 2014;10(4):968–9.
Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800.
Akanay-Diesel S, Hoff NP, Kürle S, et al. Sunitinib induced pyoderma gangrenosum-like ulcerations. Eur J Med Res. 2011;16(11):491–4.
Dean SM, Zirwas M. A second case of sunitinib-associated pyoderma gangrenosum. J Clin Aesthet Dermatol. 2010;3(8):34–5.
Levy JM, Richler D, Mahmood MN, Brassard A. Pyoderma gangrenosum associated with alitretinoin therapy. JAAD Case Rep. 2016;2(2):135–7.
Litvinov IV, Sasseville D. Pyoderma gangrenosum triggered by red tattoo dye. CMAJ. 2014;186(12):935.
Jiménez-Gallo D, Albarrán-Planelles C, Linares-Barrios M, et al. Pyoderma gangrenosum and Wegener granulomatosis-like syndrome induced by cocaine. Clin Exp Dermatol. 2013;38(8):878–82.
Pinato DJ, Sharma R. Imatinib induced pyoderma gangrenosum. J Postgrad Med. 2013;59(3):244–5.
Kowalzick L, Bertolini J, Baumann C, et al. Paradoxical reaction to etanercept: development of pyoderma gangraenosum during therapy of psoriasis arthritis. J Dtsch Dermatol Ges. 2013;11(5):447–9.
Stichenwirth M, Riedl E, Pehamberger H, Tappeiner G. Pyoderma gangrenosum in a patient with seronegative rheumatoid arthritis during therapy with adalimumab: toxic effects of adalimumab or failure of adalimumab to prevent the onset of this phenomenon? Arch Dermatol. 2008;144(6):817–8.
Lewerin C, Mobacken H, Nilsson-Ehle H, Swolin B. Bullous pyoderma gangrenosum in a patient with myelodysplastic syndrome during granulocyte colony-stimulating factor therapy. Leuk Lymphoma. 1997;26(5–6):629–32.
Ross HJ, Moy LA, Kaplan R, Figlin RA. Bullous pyoderma gangrenosum after granulocyte colony-stimulating factor treatment. Cancer. 1991;68(2):441–3.
Freiman A, Brassard A. Pyoderma gangrenosum associated with isotretinoin therapy. J Am Acad Dermatol. 2006;55(5 Suppl):S107–8.
Hughes BR, Cunliffe WJ. Development of folliculitis and pyoderma gangrenosum in association with abdominal pain in a patient following treatment with isotretinoin. Br J Dermatol. 1990;122(5):683–7.
Tinoco MP, Tamler C, Maciel G, et al. Pyoderma gangrenosum following isotretinoin therapy for acne nodulocystic. Int J Dermatol. 2008;47(9):953–6.
Darben T, Savige J, Prentice R, et al. Pyoderma gangrenosum with secondary pyarthrosis following propylthiouracil. Australas J Dermatol. 1999;40(3):144–6.
Gungor K, Gonen S, Kisakol G, et al. ANCA positive propylthiouracil induced pyoderma gangrenosum. J Endocrinol Invest. 2006;29(6):575–6.
Hong SB, Lee MH. A case of propylthiouracil-induced pyoderma gangrenosum associated with antineutrophil cytoplasmic antibody. Dermatology. 2004;208(4):339–41.
Seo JW, Son HH, Choi JH, et al. A case of p-ANCA-positive propylthiouracil-induced pyoderma gangrenosum. Ann Dermatol. 2010;22(1):48–50.
Miall FM, Harman K, Kennedy B, Dyer MJ. Pyoderma gangrenosum complicating pegylated granulocyte colony-stimulating factor in Hodgkin lymphoma. Br J Haematol. 2006;132(1):115–6.
Takagi S, Ohsaka A, Taguchi H, et al. Pyoderma gangrenosum following cytosine arabinoside, aclarubicin and granulocyte colony-stimulating factor combination therapy in myelodysplastic syndrome. Intern Med. 1998;37(3):316–9.
White LE, Villa MT, Petronic-Rosic V, et al. Pyoderma gangrenosum related to a new granulocyte colony-stimulating factor. Skinmed. 2006;5(2):96–8.
Mir-Bonafé JM, Blanco-Barrios S, Romo-Melgar A, et al. Photoletter to the editor: Localized pyoderma gangrenosum after interferon-alpha2b injections. J Dermatol Case Rep. 2012;6(3):98–9.
Montoto S, Bosch F, Estrach T, et al. Pyoderma gangrenosum triggered by alpha2b-interferon in a patient with chronic granulocytic leukaemia. Leuk Lymphoma. 1998;30(1–2):199–202.
Park CW, Shin YS, Shin MJ, et al. Pyoderma gangrenosum and spinal epidural abscess after subcutaneous administration of recombinant human erythropoietin. Nephrol Dial Transplant. 1997;12:1506–8.
Nadauld LD, Miller MB, Srinivas S. Pyoderma gangrenosum with the use of sunitinib. J Clin Oncol. 2011;29(10):e266–7.
ten Freyhaus K, Homey B, Bieber T, Wilsmann-Theis D. Pyoderma gangrenosum: another cutaneous side-effect of sunitinib? Br J Dermatol. 2008;159(1):242–3.
Ueharaguchi Y, Kabashima K, Sasahashi M, et al. A case of pyoderma gangrenosum possibly associated with sunitinib treatment. Int J Dermatol. 2013;52(5):634–6.
Usui S, Otsuka A, Kaku Y, et al. Pyoderma gangrenosum of the penis possibly associated with pazopanib treatment. J Eur Acad Dermatol Venereol. 2016;30(7):1222–3.
Jaimes-López N, Molina V, Arroyave JE, et al. Developement of pyoderma gangraenosum during therapy with infliximab. J Dermatol Case Rep. 2009;3:20–3.
Vandevyvere K, Luyten FP, Verschueren P, et al. Pyoderma gangrenosum developing during therapy with TNF-alpha antagonists in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26(12):2205–6.
Vestita M, Guida S, Mazzoccoli S, et al. Late paradoxical development of pyoderma gangrenosum in a psoriasis patient treated with infliximab. Eur J Dermatol. 2015;25(3):272–3.
Baliu-Piqué C, Mascaró JM Jr. Multifocal and refractory pyoderma gangrenosum: possible role of cocaine abuse. Australas J Dermatol. 2016. doi:10.1111/ajd.12498.
Jeong HS, Layher H, Cao L, et al. Pyoderma gangrenosum (PG) associated with levamisole-adulterated cocaine: clinical, serologic, and histopathologic findings in a cohort of patients. J Am Acad Dermatol. 2016;74(5):892–8.
Keith PJ, Joyce JC, Wilson BD. Pyoderma gangrenosum: a possible cutaneous complication of levamisole-tainted cocaine abuse. Int J Dermatol. 2015;54(9):1075–7.
Tseng E, Alhusayen R, Sade S, et al. Pyoderma gangrenosum secondary to azacitidine in myelodysplastic syndrome. Br J Haematol. 2015;169(4):461.
Kumaresan M, Rai R, Sekar S, Natarajan K. Ciprofloxacin induced pyoderma gangrenosum. Indian Dermatol Online J. 2011;2(2):122–4.
Rudolph BM, Staib F, Von Stebut E, et al. Neutrophilic disease of the skin and intestines after ipilimumab treatment for malignant melanoma—simultaneous occurrence of pyoderma gangrenosum and colitis. Eur J Dermatol. 2014;24(2):268–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
Jane Y. Wang, Lars E. French, Neil H. Shear, and Afkham Amiri have no conflicts of interest to declare. Afsaneh Alavi has received payment for professional services, including consulting, lecturing and participation in clinical trials, from AbbVie, Galderma, Janssen, LEO, Novartis, Valeant, Boehringer-Ingelheim, Glenmark, Merck Serono, Novartis, Regeneron-Sanofi, Roche, and Xenon; however, these payments do not affect her opinions or conclusions with regard to this submission.
Rights and permissions
About this article
Cite this article
Wang, J.Y., French, L.E., Shear, N.H. et al. Drug-Induced Pyoderma Gangrenosum: A Review. Am J Clin Dermatol 19, 67–77 (2018). https://doi.org/10.1007/s40257-017-0308-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-017-0308-7