Abstract
Background
Metabolic Syndrome (MetS) mainly comprises hyperglycemia, hypertension and dyslipidemia, and has been proven to increase the risk for type 2 diabetes mellitus (T2DM) and cardiovascular disease. Studies have suggested that many factors may be involved in the pathogenesis of MetS, but tumor necrosis factor alpha (TNF- α) may play a strong role as its gene polymorphism was associated with insulin resistance and obesity. The aim of this study was to evaluate the possible association of TNF-α-308 G > A (rs1800629) polymorphism with susceptibility of metabolic syndrome.
Methods
a case-control study was conducted upon 128 participants recruited from Suez Canal University Hospital (Ismailia, Egypt), divided into the MetS group (n = 64) and the control group (n = 64). Genotyping of the TNF-α-308 G > A (rs1800629) polymorphism was performed by restriction fragment length polymorphism (PCR-RFLP).
Results
The A allele was significantly higher among MetS patients (40%) than controls (11%) (p < 0.0001). A significant association was observed between the healthy and MetS groups under the influence of co-dominant, dominant and over-dominant genetic models (p < 0.05). Also, there were positive correlations between TNF-α-308 (G/A) polymorphism and risk factors of metabolic syndrome like body mass index (BMI); fasting blood sugar; cholesterol and low density lipoprotein (LDL) (p < 0.05). Regression analysis was done for predictors of MetS and the A allele was found to be a strong predictor (OR 2.752; 95% CI = 1.106 to 6.847; p = 0.03), as well as, BMI; triglyceride (TG); high density lipoprotein (HDL); LDL and cholesterol (p < 0.05).
Conclusions
TNF-α-308 G > A (rs1800629) polymorphism may be play an important role in the development of metabolic syndrome and A allele is a strong predictor in Egyptians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The metabolic syndrome refers to a range of clinical and biochemical abnormalities of a predominantly metabolic origin with insulin resistance and abdominal obesity as the main predisposing factors and it mainly comprises high blood glucose level, elevated blood pressure and atherogenic dyslipidemia [1].
The pathophysiology of the MetS is complex and involves an elaborate interaction between genetic susceptibility and environmental cues [2,3,4].
The obesity is associated with hypertrophy and hyperplasia of fat cells in adipose tissue. The blood supply to fat cells is reduced with a subsequent hypoxia which induce tissue death and macrophage infiltration of this tissue, that transforming it into a paracrine and endocrine organ which yields a range of biologically dynamic molecules known as adipokines [3, 4].
Adipokines include pro-inflammatory mediators like; (TNF-α) which may be responsible for the local inflammation of adipose tissue and the further systemic inflammation that may account for the range of morbidities comprising the MetS [2, 3].
The TNF-α paracrine properties include decreasing the insulin sensitivity of fat cells via diminish insulin-stimulated tyrosine phosphorylation of the insulin receptor substrate-1 and tyrosine kinase activity without affecting the number of receptors or their insulin-binding capacity which leads to inactivation of insulin receptors [3, 5, 6]. Serum TNFα levels have been found to be elevated in MetS patients of different racial origins [7, 8]. Thereby, the TNFα gene has been considered as a candidate gene for the MetS [9].
The TNF-α gene has been found within the human leucocyte antigen III region, placed on chromosome 6p21 and within the major histocompatibility complex in a position defined as 250 kb near the center of HLA-B locus and about 850 kb near the end part of HLA-DR locus [10]. Several SNPs have been identified in the promoter region of the TNFα and are believed to regulate its transcription [11]. A G/A substitution at position 308 upstream from the transcription initiation site has been identified and found to be associated with a higher transcription rate and elevated levels of TNFα which in turn play a role in the pathogenesis of metabolic syndrome [12].
Wilson et al; noticed that healthy carriers of the A allele have higher levels of TNFα than the carriers of G allele [10], in addition, Arbab et al; demonstrating that the presence of the high-production A allele of the TNFα gene increases the binding of a transcription factor to the promoter region thus altering its expression [13]. More than that, meta-analysis was done by Sookoian et al; illustrating that G-308A TNFα gene variant and phenotypes were positively correlated with the occurrence of obesity, hypertension and elevated insulin level. These results support the hypothesis that the TNFα gene can be involved in the pathogenesis of metabolic syndrome [14]. You et al; as well as, Lann and LeRoith were found a relationship between high TNFα levels and the presence of MetS [15, 16]. On the other hand, studies were done by Ranjith et al; and Pyrzak et al; along with a meta-analysis was done by Feng et al; denoting that there was no significant association between the TNF308 G/A polymorphism and risk for MetS [17,18,19]. Hand in hand with these studies, Voiculescu et al; found that the metabolic syndrome appeared to be independent of all five SNPs of TNFα genes in Romanian population with psoriasis [20].
Because of the fact that incidence of MetS is increasing at an alarming rate in developing countries [21] and despite the evidence of a genetic component for the MetS from early family studies [2, 22], the complexity of the MetS and the lack of a unifying pathogenetic pathway to explain its various components has made studying its genetic background challenging. As well as, genetic variability among different populations can be useful in understanding the course of the disease and susceptibility to its complications [23]. So, in this study we examined the TNFα 308G > A in a group of Egyptian MetS patients, to identify the distribution of the SNP variants as well as, their relationship and association with the individual components of the MetS.
Methods
Participants
This study was conducted upon 128 participants divided into two groups; the MetS group and the control group. The MetS group comprised 64 patients recruited from the Suez Canal University Hospital (Ismailia, Egypt). MetS was diagnosed according to the harmonized criteria proposed by Alberti et al. [24] and three of the following five criteria should be present in a patient for a diagnosis of MetS to be made:
-
WC: males ≥102 cm; females ≥88 cm
-
Serum HDL-cholesterol: males <40 mg/dl; females <50 mg/dl
-
Serum triglyceride: ≥150 mg/dl
-
Fasting blood glucose: ≥100 mg/dl
-
Blood pressure: ≥130/85 mmHg
Patients with co-existing type1 diabetes mellitus (T1DM), thyroid or hepatic disease, acute or chronic inflammation, acute infections, or autoimmune disease were excluded from the study. The control group consisted of 64 age and sex matched healthy individuals with normal fasting blood glucose levels.
Anthropometric measurements
Weight and height were measured to calculate BMI. Blood pressure readings were also acquired.
Biochemical analysis
All biochemical assays were performed by fully automated analyzer (Cobas® 6000 Auto-analyzer, Roche Diagnostics, Mannheim, Germany) using kits provided by the manufacturer. Measurement of serum TG was based on the enzymatic determination of glycerol using the enzyme glycerol phosphate oxidase after hydrolysis by lipoprotein lipase. Total cholesterol (TC) and HDL were evaluated by enzymatic colorimetric method, according to the manufacturer’s standards. LDL was estimated by the Friedewald’s formula: LDL = TC – HDL – (TG/5), if the TG was less than 400 mg/dL and it was measured by enzymatic colorimetric method, if the TG was more than or equal 400 mg/dL. Fasting blood glucose levels were estimated for all study participants by using hexokinase method.
Genotyping of the TNF-α-308 (G/A) polymorphism
DNA was extracted from peripheral blood leucocytes using the QIAamp®DNA Blood Mini Kit (Cat No. /ID: 51104, QIAGEN, Hilden, Germany). PCR-RFLP assay was used to genotype the TNF-α-308 (G/A) polymorphism. The following primers were used: 5’-AGGCAATAGGTTTTGAGGGCCAT-3′ (forward) and 5’-TCCTCCCTGCTCCGATTC CG-3′ (reverse). PCR was performed in a final reaction volume of 25 μl, and included 5 μl genomic DNA, 12.5 μl Maxima Hot Start Green PCR Master Mix (2X) (#K1062, Thermo Fisher Scientific, Massachusetts, USA), 5.5 μl RNase free water, and 1 μl of each primer. Amplification was carried out in Eppendorf® Mastercycler Personal (SIGMA-ALDRICH, Missouri, USA) for 40 cycles, each comprising an initial denaturation at 95 °C for 4 min, denaturation at 95 °C for 30 s, annealing at 50 °C for 30s and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 5 min.
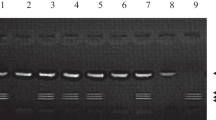
Next, PCR products were digested using FastDigest NcoI (FD0573, Thermo Fisher Scientific, Massachusetts, USA) and incubated for 1 h at 37 °C. The digested products were then separated by electrophoresis on 3% (w/v) agarose gel and the DNA bands were visualized under UV light and photographed using gel documentation system (Syngene, UK).
Comparing DNA bps by using DNA ladder (Figs. 1 and 2):
-
Homozygous GG: represented by the presence of a single band of 107 bp.
-
Homozygous AA: represented by the presence of two bands of 87 bp and 20 bp.
-
Heterozygous GA: represented by the presence of three bands of size 107, 87 and 20 bp.
Statistical analysis
We performed descriptive statistics that were represented by number, percentage (%), mean and standard deviation (SD). Analytical statistics included Student’s t test that was used to indicate the presence of any significant difference between two groups of quantitative variable, Chi-square test and Fisher’s exact test that were used to compare between two groups or more regarding one qualitative variable. Spearman correlation analysis was used to show strength and direction of association. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as a measure of the risk factors of MetS with the TNF-α-308 alleles. The regression analysis of genetic models also adjusted based on risk factors such as hypertension and BMI. Hardy-Weinberg Equilibrium for control group was calculated and (P value =0.15). Regression analysis was done to detect the predictors of MetS. P value, was considered significant difference if p < 0.05. All statistics were calculated by using the IBM SPSS for Windows version 16.0 (SPSS Armonk, NY: IBM Corp USA).
Results
In this study, we examined the TNF-α-308 G > A (rs1800629) polymorphism in 64 MetS patients and 64 matched apparently healthy controls. The mean age was 57.3 ± 8.5 years old. In the MetS group there were 20 males (31.25%) and 44 females (68.75%); which were matched with control group as the males were 18 (28.1%) and females were 46 (71.9%).
As shown in (Table 1) the BMI was significantly higher in the MetS group than in controls (p = 0.001). Also, the number of hypertensive persons; mean fasting blood sugar; cholesterol and LDL level in the MetS group were significantly higher than in controls (p < 0.001);as well as, the level of serum triglycerides (p = 0.001). The HDL level in MetS patients was significantly lower than in controls (p < 0.001).
As presented in (Table 2), TNF-α-308 G/A gene polymorphism had significant association with MetS under the influence of co-dominant, dominant: G/A-A/A vs G/G and over-dominant model (p < 0.05). The AA genotype were presented in 9.4% in MetS group while in the healthy control it was only 3.1% (p < 0.0001). Also, both the GA and AA genotypes were represented in 70.3% in MetS patients versus 18.7% in controls. On the other hand, the GG genotype was presented in 81.2% of controls and only 29.7% of MetS patients. Furthermore, there was a significant statistical difference between the MetS and control groups in the TNF-α-308 G/A allele frequencies as the A allele was significantly higher among MetS patients (40%) than healthy controls (11%) (p < 0.05) and people who carry the A allele are liable for developing metabolic syndrome 2.75 times more than those who carry G allele (adjusted OR 2.75; 95% CI = 1.106 to 6.847; adjusted p = 0.03).
The correlation between TNF-α-308 G/A gene polymorphism and the different studied variables was done as shown in (Table 3). There were positive correlations with BMI (p = 0.016); Fasting blood sugar (p < 0.0001); Cholesterol (p = 0.017) and LDL (p = 0.026) but there were no correlation with serum triglycerides or HDL (p > 0.05).
Univariate regression analysis was done for predictors of MetS by crude odds ratios in (Table 4), The A allele was found to be a predictor for MetS (OR 5.393; 95% CI = 2.792 to 10.417; p < 0.0001), as well as, BMI (OR 1.267; 95% CI = 1.119 to 1.435; p = 0.001), serum triglycerides (OR 1.030; 95% CI = 1.018 to 1.042; p < 0.0001), cholesterol (OR 1.049; 95% CI = 1.029 to 1.070; p < 0.0001),LDL (OR 1.044; 95% CI = 1.025 to 1.063; p = 0.001) and HDL (OR 0.906; 95% CI = 0.869 to 0.945; p = 0.001).
Discussion
Metabolic syndrome is a complex disorder of metabolism the pathophysiology of which remains far from explained. A genetic component for MetS has long been recognized, but genetic studies have only been successful at demonstrating the genetic association with individual components of MetS rather than for MetS as a whole. TNFα is a pro-inflammatory cytokine that has been implicated in the pathogenesis of several diseases. The TNF-α-308 G > A (rs1800629) polymorphism is a single nucleotide substitution in the promoter region of the TNF-α gene which is believed to affect the TNF-α transcription levels. This polymorphism has 2 allelic forms, the common form is the G allele, while the rare allele is A. Although the A allele has been found to be associated with higher spontaneous or stimulated expression levels of TNF-α and individuals carrying the GA genotype have higher amounts of TNF-α mRNA, and serum protein levels, than individuals with the GG genotype [25], the effect of the A allele on TNF-α expression remains controversial. The TNF-α-308 G/A polymorphism has been found to be associated with obesity, insulin resistance and hypertension. However, some but not all studies have indicated the responsibility of TNF-α gene polymorphism in the pathogenesis of many components of metabolic syndrome and insulin resistance [6, 12, 25].
In the present study, significant results were obtained in the clinico-demographic characteristics among healthy and metabolic syndrome groups in all five MetS components according to the diagnostic criteria of the International Diabetes Federation (IDF) from 2009 [24] and that are matching hand in hand with Gupta et al; [12] and Szkup et al.; [26] who illustrated that control and study groups showed significant results in most of the anthropometric and biochemical characteristics. The genetic factors which responsible for the development of MetS like the multiple genes’ variants whose expression are responsible for MetS components such as obesity and high BMI; insulin resistance and carbohydrate metabolism disorders; as well as dyslipidemia are the main causes for the difference between the control and MetS group [27].
Also, our study indicated that frequency of AA genotype and A allele in MetS and control groups were significantly different (p < 0.05). In line with these results were Gupta et al; [12] who found that homozygous mutant genotype (AA) (p < 0.001: OR = 3.24: 95% CI = 2.15–4.89) and mutant allele (A) (p < 0.001: OR = 3.04: 95% CI = 2.08–4.43) were significantly less frequently presented in the control population as compared to MetS group. On the other hand, a study was done by Lee SC et al; [6] who found that TNF- α gene G-308A polymorphism was unlikely to play an important role in the increase of metabolic syndrome in Chinese population, as well as, Szkup et al.; [26] who illustrate the same result in a Poland population. Also, Kabita et al; [11] illustrated that heterozygote GA was found to be higher in hypertensive (7.82%) and MetS (8.87%) cases than the healthy group (6.37%); but the difference was not statistically significant and no mutant homozygotes of TNF-α 308G/A could be found among cases; however, 0.27% were observed among controls. Alternatively, Yong et al; [25] found that the genotypic and allelic frequency of TNF-α 308G/A did not show significant difference between asthmatic patients who have metabolic syndrome and healthy controls. However, the frequency of A allele was significantly higher in asthma group with Mets (22.36%) than in controls (15.71%) (P = 0.02; OR = 0.647; 95% CI = 0.447–0.936). Substitution of G allele by the A allele in the promoter or regulatory areas of TNF-α can act as probable immune regulators by directing the expression of it. There can be person to person discrepancies in TNF-α levels depending upon the genetic coding as well as different ethnicities resulting in dissimilar immune response [28].
In the current study we found that there were positive correlations between TNFα 308G/A polymorphism and BMI (p = 0.016); fasting blood sugar (p < 0.0001); cholesterol (p = 0.017) and LDL (p = 0.026), while there were no correlation between the gene polymorphism and both TG and HDL (p > 0.05). Kabita et al; [11] found that TNFα 308G/A polymorphism was associated with high TC, high very low density lipoprotein (VLDL) and TG in overall population and only with high VLDL and TG among the hypertensive cases, but there was no correlation with HDL like our study. On the other hand, Yong et al; [25] found that the level of LDL was significantly higher in the asthmatic patients with Mets who carrying the GA and AA genotypes than in the carriers of GG genotype (P = 0.029, P = 0.022, P = 0.043) respectively, but there was no correlation between the gene polymorphism and TG; and these results were matching with our results. The study conducted by Pausov et al; on rats suggests that TNFα (−308 (gene has an impact on obesity, high glucose level, serum leptin levels, and elevated blood pressure, but only when the rats are given a riches fatty diet [29]. Also, de Luis et al; found that patients with the A allele had a higher BMI than the carriers of the G allele who also showed a healthier metabolic reaction than those with the A allele [30]. The findings in our study and the similar studies as well; may be due to the presence of the mutant A allele which enhance the promoter region in chromosome 6 to increase the transcription of TNF α and subsequent increase in serum TNFα level in these populations which may plays a role in the pathogenesis of MetS as mentioned earlier [2, 3] and therefore A allele has also been associated with MetS components like obesity, T2DM, insulin resistance as well as coronary artery diseases and elevated serum CRP [31].
On calculating the crude Odds ratio for TNF-α-308 A allele, about 5.4 folds increased risk was observed with metabolic syndrome and it was a strong predictor (P < 0.0001) OR = 5.393 (95% CI: 2.792–10.417). Hand in hand with our results, Yong et al; [29] identified that TNF-α-308 A allele was the risk factor for asthmatic patients with MetS in Hebei population, China. As well as, Gupta et al; [12] who found that A allele has about 3 folds increased risk in the MetS group (P < 0.001) OR = 3.04 (2.08–4.43). On the other hands, although Kabita et al; [11] found that about one fold increased risk was observed with metabolic syndrome OR = 1.37 (95% CI: 0.65–2.87) but statistically it was not significant (P > 0.05), as well as, Zafar et al; who found that no significant difference in the A allele between MetS and healthy group (P = 0.357). The difference in the response after TNF-α-308 gene mutation between the Egyptians in the current study and the other populations in the other studies maybe due to different ethnicities and genetic considerations as that different origins; Caucasians, Asians or Africans; might exert considerable effects on between-study heterogeneity [32].
Conclusion
Our results suggest that the G-308A polymorphism of the TNF- α gene is associated with metabolic syndrome and A allele is a strong predictor in Egyptian population.
References
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52.
Stančáková A, Laakso M. Genetics of metabolic syndrome. Rev Endocr Metab Disord. 2014;15(4):243–52.
Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36(1):14–20.
Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031–41.
Lee SC, Pu YB, Thomas GN, Lee ZS, Tomlinson B, Cockram CS, et al. Tumor necrosis factor alpha gene G-308A polymorphism in the metabolic syndrome. Metab Clin Exp. 2000;49(8):1021–4.
Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west Virginian population. Int J Med Sci. 2016;13(1):25–38.
Mohammadi M, Gozashti MH, Aghadavood M, Mehdizadeh MR, Hayatbakhsh MM. Clinical significance of serum IL-6 and TNF-α levels in patients with metabolic syndrome. Rep Biochem Mol Biol. 2017;6(1):74–9.
Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16.
Wilson AG, de Vries N, Pociot F, di Giovine FS, van der Putte LB, Duff GW. An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med. 1993;177(2):557–60.
Kabita S, Garg PR, Newmei MK, Saraswathy KN, Singh HSJJotPoCS. Tumor necrosis factor-alpha gene polymorphisms and complex disorders: A study among mendelian population with East Asian Ancestry 2019;5(1):35.
Gupta V, Gupta A, Jafar T, Gupta V, Agrawal S, Srivastava N, et al. Association of TNF-α promoter gene G-308A polymorphism with metabolic syndrome, insulin resistance, serum TNF-α and leptin levels in Indian adult women. Cytokine. 2012;57(1):32–6.
Arbab M, Tahir S, Niazi MK, Ishaq M, Hussain A, Siddique PM, et al. TNF-α genetic predisposition and higher expression of inflammatory pathway components in Keratoconus. Invest Ophthalmol Vis Sci. 2017;58(9):3481–7.
Sookoian SC, González C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res. 2005;13(12):2122–31.
You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol Ser A Biol Sci Med Sci. 2008;63(4):414–9.
Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91(6):1063–77 viii.
Pyrzak B, Wiśniewska A, Rymkiewicz-Kluczyńska B. Tumor necrosis factor alpha (TNF-alpha) gene G-308A polymorphism relationship to insulin resistance and lipid abnormalities in children with obesity. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2006;12(3):171–4.
Ranjith N, Pegoraro RJ, Naidoo DP, Shanmugam R, Rom L. Genetic variants associated with insulin resistance and metabolic syndrome in young Asian Indians with myocardial infarction. Metab Syndr Relat Disord. 2008;6(3):209–14.
Feng RN, Zhao C, Sun CH, Li Y. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS One. 2011;6(4):e18480.
Voiculescu VM, Solomon I, Popa A, Draghici CC, Dobre M, Giurcaneanu C, et al. Gene polymorphisms of TNF-238G/A, TNF-308G/A, IL10-1082G/A, TNFAIP3, and MC4R and comorbidity occurrence in a Romanian population with psoriasis. J Med Life. 2018;11(1):69–74.
Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–25.
Brown AE, Walker M. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. 2016;18(8):75.
Banerjee M, Saxena M. Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World J Diabetes. 2014;5(4):493–504.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5.
Yang Y-H, Liu Y-Q, Zhang L, Li H, Li X-B, Ouyang Q, et al. Genetic polymorphisms of the TNF-α-308G/A are associated with metabolic syndrome in asthmatic patients from Hebei province, China. Int J Clin Exp Pathol. 2015;8(10):13739–46.
Szkup M, Chełmecka E, Lubkowska A, Owczarek AJ, Grochans E. The influence of the TNFα rs1800629 polymorphism on some inflammatory biomarkers in 45-60-year-old women with metabolic syndrome. Aging (Albany NY). 2018;10(10):2935–43.
Stein CM, Song Y, Elston RC, Jun G, Tiwari HK, Iyengar SK. Structural equation model-based genome scan for the metabolic syndrome. BMC Genet. 2003;4(Suppl 1):S99.
Peng Y, Li LJ. TNF-α-308G/A polymorphism associated with TNF-α protein expression in patients with diabetic nephropathy. Int J Clin Exp Pathol. 2015;8(3):3127–31.
Pausova Z, Sedova L, Berube J, Hamet P, Tremblay J, Dumont M, et al. Segment of rat chromosome 20 regulates diet-induced augmentations in adiposity, glucose intolerance, and blood pressure. Hypertension. 2003;41(5):1047–55.
de Luis DA, Aller R, Izaola O, González Sagrado M, Conde R, Romero E. Influence of G308A polymorphism of tumor necrosis factor alpha gene on insulin resistance in obese patients after weight loss. Med Clin (Barc). 2007;129(11):401–4.
Li Y-yJPO. Tumor necrosis factor-alpha g308α gene polymorphism and essential hypertension: a meta-analysis involving 2244 participants. 2012;7(4):e35408.
Zafar U, Khaliq S, Ahmad HU, Lone KP. Serum profile of cytokines and their genetic variants in metabolic syndrome and healthy subjects: a comparative study. Biosci Rep. 2019;39(2).
Funding
This work was done by own author funding.
Author information
Authors and Affiliations
Contributions
All authors have participated in the study.
Corresponding author
Ethics declarations
Author disclosures
The authors declare that they have no conflict of interest.
Consent to participate
All the patients were informed about the research and verbal informed consent was obtained prior to the interview.
Ethical approval
This research has been approved by the Medical Research Ethics Committee of the Suez Canal University Faculty of Medicine (Ismailia, Egypt) and the study has been conducted according to the principles expressed in the Helsinki Declaration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key messages
• There is an evidence indicates that TNF-α 308 (G/A) polymorphism may participate in development of metabolic syndrome.
• The A allele was found to be a strong predictor as well as, BMI; TG; HDL; LDL and cholesterol for susceptibility of metabolic syndrome in Egyptian patients.
Rights and permissions
About this article
Cite this article
Ghareeb, D., Abdelazem, A.S., Hussein, E.M. et al. Association of TNF-α-308 G>A (rs1800629) polymorphism with susceptibility of metabolic syndrome. J Diabetes Metab Disord 20, 209–215 (2021). https://doi.org/10.1007/s40200-021-00732-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00732-3