Abstract
Purpose
Identification of nutrient patterns (NPs) may be helpful to evaluate the overall quality of diet and its relation to metabolic syndrome (MetS). We investigated the association of major nutrient patterns with MetS and its components among apparently healthy adults living in Tehran.
Methods
We included 522 adults (24–83 years old) who referred to health centers in Tehran. Fasting blood samples were collected to measure glycemic indicators and lipid profile. Principal component analysis (PCA) based on the 26 nutrient items were applied. Metabolic syndrome was defined based on the guidelines of the National Cholesterol Education Program Adult Treatment Panel III (ATP III).
Results
Three major nutrient patterns were identified: “Mono and disaccharides pattern”, “Macronutrient pattern” and “Antioxidant pattern”. After controlling for potential confounders, serum HDL (p < 0.001) increased across tertiles of “Antioxidant pattern”. A significant inverse association was observed between adherence to “Antioxidant pattern” and odds of MetS (OR: 2.36, 95% CI: 1.51, 3.69) in crude model which was disappeared after adjusting for age, sex, education, physical activity, smoking, marital status, energy and BMI. Moreover, we found that adherence to “Antioxidant pattern” was inversely related to odds of reduced levels of serum high density lipoprotein (HDL) (OR: 0.09, 95% CI: 0.02, 0.35). No association was found between “Mono and disaccharides pattern”, “Macronutrient pattern”, MetS and its components.
Conclusions
In general, adherence to “Antioxidant pattern “resulted in lower level of HDL concentrations. More studies are needed to investigate the possible causal relationship of these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is recognized as the main epidemic of the twenty-first century [1]. Metabolic syndrome (MetS), which has also been referred to as “insulin resistance syndrome” [2], “syndrome X” [3], “hypertriglyceridemic waist” [4] and “the deadly quartet” [5], is characterized through a complex accumulation of metabolic abnormalities such as hyperinsulinism (impaired glucose tolerance), hypertension, low HDL cholesterol, and hypertriglyceridemia [6]. MetS is one of the most frequent metabolic disorders leading to several chronic diseases like cardiovascular disease (CVD), diabetes mellitus, cancer, renal disease and mental illness [7,8,9].
Nevertheless, the epidemic of metabolic syndrome occurs not just in developed countries and the rate of metabolic syndrome is also high in underdeveloped countries [10]. In 2015, 71% of deaths worldwide were caused by non-communicable diseases (NCDs) [11], among which MetS has been a real problem [12]. An increased prevalence of MetS in Asia has also been reported. This increase in Asia has been attributed to the adoption of Western lifestyles in recent decades [13]. The incidence of MetS in Iranian youths has been reported to be dramatically high, ranging from 4.8–24.5%. Remarkably, the prevalence of MetS in the elderly was significantly higher than in younger people, namely 49.5% compared to 17.5% [14, 15]. In Tehran, the capital of Iran, one-third of adults and one-tenth of the adolescents are affected with MetS [16,17,18].
Several lifestyle factors such as a high body mass index (BMI), smoking, consumption of alcohol, exercise as well as eating habits have been demonstrated to affect metabolic syndrome abnormalities. Among the dietary patterns,Mediterranean diet has been reported to have a protective impact on metabolic syndrome [19,20,21,22]. As a result, emerging evidence has indicated that dietary factors are also associated with MetS [23, 24].
In recent years, researchers have investigated the impact of certain foods and dietary patterns on MetS [25,26,27]. For instance, some findings suggest that consumption of fruits and vegetables is associated with a lower risk of MetS [28,29,30]. Vegetables and fruits are important sources of Vitamin A, Vitamin C, Magnesium and Potassium and can help protect health and potentially decrease the risk of chronic diseases [31]. It has also been shown that increasing dietary magnesium and zinc intake is related with a lower risk of MetS [32, 33]. In a recent published study from Iran, Khayatzadeh et al. [34] has reported that a nutrient pattern rich in dietary protein, carbohydrate, starch, glucose, fructose, sucrose, maltose was associated with a higher risk of MetS in both genders. In contrast, a pattern consist of copper, selenium, vitamin A, riboflavin, vitamin B12 was associated with greater odds of Mets, in women. Iran is a multi-ethnic country which has faced nutrition transition in recent years. Moreover, dietary patterns can vary depending on geographical environment, food culture, economic habits and dietary habits are substantially different between geographical regions of Iran. Additionally, recent dietary studies have progressively used dietary pattern approaches rather than the traditional focus on individual foods, nutrients or dietary components. Dietary pattern analysis is useful because it captures the complex nature of dietary intake, explores its correlation with disease and may be more predictive of chronic disease risk than the intake of nutrients or foods [35]. Dietary pattern analysis can assess the overall features of diet composition and help identify complex relationships, potential interactions, as well as antagonistic or synergistic relationship between nutrient intake and disease.
Few studies have been conducted on the association between dietary nutrients and MetS in Iranian populations. In this study, the association between adherence to dietary nutrients and the development of MetS was explored in adult population of Tehran.
Methods
Study population
Using two-stage cluster sampling from 25 health centers in Tehran, a total of 522 eligible individuals were selected for this cross-sectional study. 522 subjects, including 232 men and 290 women with a mean age of 52.64 ± 14.09, were eventually recruited. Sampling was done by dividing the health centers of Tehran into five regions: north, south, east, west, and center. A list of health centers in each area was prepared and 25 health centers were selected (due to budget and time constraints) considering the number of centers in each region with a split ratio and specifying the number of health centers under survey in each area. After selecting the health centers, the eligible centers were randomly chosen. Then, the samples (n = 522) were divided according to total health centers (n = 25), and the number of samples in each health center was obtained.
The collected data included participants’ demographics (age, gender, education, income), lifestyle such as smoking and physical activity, medical history, anthropometric as well as dietary assessment via face-to-face interview with subjects. The ethical issues of this project were approved by Tehran University of Medical Sciences (ethic Number: IR.TUMS.VCR.REC.1396.2307) and written informed consent was obtained from all the participants.
Anthropometric measurements
The height and weight of subjects were measured by nutritionists. The weight was recorded to the nearest 100 g with subjects minimally clad and barefoot when standing on digital scales (Seca, Germany). The height was measured using a stadiometer and recorded to the nearest 0.5 cm. By dividing weight in kilograms by square of height (m2), body mass index was calculated (kg m−2). Weight circumference (WC) was recorded at the umbilical site by an outstretched tape meter without pressure to body surfaces and was recorded to the nearest 0.1 cm. The blood pressure of each participant was twice measured using a standard mercury sphygmomanometer after 15 min of rest.
Dietary intake assessment
Dietary intake was collected using a validated semiquantitative FFQ, which contained 147 food items [36]. Nutritional information was recorded by trained nutritionists through interviews. The participants reported their frequency of consumption for each food item during the previous year on a daily, weekly, monthly or yearly basis. For our analysis, daily intake of all food items from FFQ was calculated and portion sizes of consumed foods reported in household measures were then converted to grams. Each food and beverage item was then coded according to the protocol and analyzed for energy and nutrient content using Nutrition IV Software (First Database, San Bruno, CA), which was designed for Iranian foods.
Dietary pattern assessment
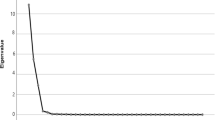
Factor analysis with orthogonal transformation (varimax method) was conducted to extract nutrient patterns based on 26 nutrients and bioactive compounds. Eigenvalues, the scree plot test, and interpretability were evaluated to retain factors (>1.5) for further analysis. Food groups with absolute factor loading≥0.3 were considered as significantly contributing to the pattern. We labeled the dietary patterns according to the food groups positively and negatively correlated to the identified patterns as well as based on previous studies that found similar dietary patterns in adults. We calculated the factor score for each nutrient pattern by summing up the intakes of nutrients weighted by their factor loadings [37]. Each participant received a factor score for each identified pattern and the subjects were grouped based on quintiles of nutrient pattern scores.
Biochemical assessment
Blood samples were drawn from each participant after fasting for at least 12 h in accordance with a standard protocol. Serum TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glucose and FBG were measured using an automatic analyzer (Eppendorf, Germany).
Metabolic syndrome
MetS was diagnosed if the patient had three or more of the following risk factors as established by NCEP-ATP III: large waist circumference (WC > 102 in men and WC > 88 in women), high blood pressure (BP > 130/80), high triglyceride (TG > 150), high glucose (FBG > 110), and low HDL (HDL < 40 in men and HDL < 50 in women) [38].
Statistical analysis
The subjects were divided based on the quartiles of dietary patterns. We used one-way ANOVA for quantitative variables and chi-square test for qualitative variables to compare general characteristics among quartiles of nutrition pattern scores. Analysis of covariance (ANCOVA) test was performed to evaluate the association between major nutrient patterns and MetS after adjusting for potential confounders. We used binary logistic regression to determine the association between nutrient patterns and MetS. Data were analyzed using the statistical software package SPSS version 22 with a significance level of 5%.
Results
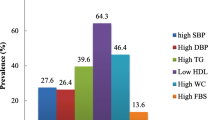
General characteristics of study participants are shown in Table 1. The number of participants were 522 that 57.6% of participants were female and 42.4% were male, also the mean of ages was (53.62 ± 14.09). Compared to females, men had higher WC, waist to hip ratio (WHR), height and lower BMI, and were also older.
The nutrient grouping used in the factor analysis and factor loading matrix for the identified nutrient patterns were presented in Table 2. Three major nutrient patterns were identified (Fig. 1) by factor analysis which explained 34.77% of the total variance in nutrient intakes amount. The first factor (NP1) showed the highest factor loadings for glucose, sucrose, fructose, lactose and galactose so was labeled as the “Mono and disaccharides pattern”. The second factor (NP2) demonstrated the highest factor loading for protein, carbohydrate, fat and saturated fatty acid that was labeled as the “Macronutrient pattern”. The third one (NP3) showed the highest factor loading for omega3, sodium, potassium and lycopene that labeled as the “Antioxidant pattern”.
Table 3 shows the multivariate adjusted means for TG, SBP, DBP, FBS, HDL and WC according to tertiles of major nutrient patterns. Higher adherence to NP1 (“Mono and disaccharides pattern”) SBP (p < 0.001) and HDL (p < 0.001) significantly increased however after controlling for potential confounders this relationship did not remain statistically significant. Moreover, there was a negative significant association between tertiles of NP2 (“Macronutrient pattern”) and HDL (p < 0.01) which disappeared after controlling for confounders (p = 0.26). Adherence to NP3 (“Antioxidant pattern”) resulted in a reduction in SBP (p < 0.001), DBP (p < 0.001), FBS (p < 0.001) and WC (p < 0.01) and an increase in HDL (p < 0.001). After adjusting for confounders significant association remained for HDL (p < 0.001).
Multivariate adjusted ORs and corresponding 95% confidence intervals (CI) for MetS and its components across tertiles of NPs are presented in Table 4. There was no significant association between higher adherence of NP1 and MetS (p = 0.76), hyper DBP (p = 0.38), hyper FBS (p = 0.99) in the crude model even after adjusting for confounders. However, significant associations between higher adherence of NP1 and hyper TG (p = 0.76), hyper SBP (p < 0.001), hyper HDL (p < 0.001) and high WC (p < 0.01) was found in the crude model which were disappeared after controlling the confounders. There was also no significant relationship between adherence to NP2 and MetS (p = 0.48), hyper DBP (p = 0.77), hyper FBS (p = 0.30) ،hyper TG (p = 0.52), hyper SBP (p < 0.10), hyper HDL (p < 0.28) and high WC (p < 0.58). The odds of MetS (p < 0.001), hyper DBP (p < 0.001), hyper SBP (p < 0.001), and high WC (p < 0.001) was different across tertiles of NP3. However, controlling for resulted in no significant association between adherence to NP3 and MetS (p = 0.13), hyper DBP (p = 0.93), hyper SBP (p = 0.43), and high WC (p = 0.72).
Discussion
In this cross-sectional study, there was no significant relationship between any of three nutrient patterns and metabolic syndrome and its components after controlling for potential confounders, but a significant positive association between the third dietary nutrient pattern and HDL levels was found.
The NP1 which representative of glucose, sucrose, fructose, lactose, and galactose is likely found in sugars, sweets, and sugary/fruit beverages. Some publications have reported that high consumption of sweets and also beverages rich in simple sugar is correlated with increased insulin resistance and central obesity [39, 40]. Further, the role of sugary beverages intake in weight gain and increasing the risk of having metabolic syndrome and type 2 diabetes have been observed [41].
The relationship of our second nutrient pattern which named as macronutrient pattern with MetS is previously evaluated in Paniagua et al. [42] study. In that study patients were randomly assigned to four groups: two high fat diets, one high in saturated fatty acid (HSFA) and the other high in monounsaturated fatty acid (HMUFA), and two low fat diets, one high in complex carbohydrates (LFHCC) supplemented with 1.24 g/day of long-chain n-3 polyunsaturated fatty acids (LFHCC n-3) and the other LFHCC diet with placebo (LFHCC). They resulted the reduced chance of increased waist circumference, high blood pressure and hypertriglyceridemia thorough following the LFHCC n-3 diet. Thus, the prevalence of MetS lowered by 20.5% after LFHCC n-3 diet compared with the HSFA (10.6%), HMUFA (12%) diet or LFHCC (10.4%) diets. In contrast, in some publications that compared high-carbohydrate with high-MUFA diets, no changes in the blood pressure response or even concerned with MUFA diets was demonstrated [43, 44].
The NP3 labeled as antioxidant pattern is somehow similar to Mediterranean dietary pattern. Mediterranean diet may have some beneficial influences to reduce the development of the metabolic syndrome via its antioxidant and anti- inflammatory effects [45,46,47]. In the Nureta PREDIMED [48] cross-sectional study, subjects with the highest adherence vs. the lowest adherence to the Mediterranean diet had 56% lower chance of having the metabolic syndrome. In contrast to Nureta PREDIMED study and also in agreement with our result, in a cross-sectional study conducted by Leon et al. [49], there was no significant association between adherence to the Mediterranean diet and odds of the metabolic syndrome.
The results of the ZINCAGE project (n = 957 healthy subjects from 5 European countries) were in line with our study. There exists a positive association between higher adherence to the Mediterranean diet and higher HDL concentrations in women [50]. Besides, in the Catalan Nutritional Survey [51] in Spain, the higher HDL levels was observed in the highest adherences to the Mediterranean diet compared with the lowest one. In addition, in a randomized clinical trial in Scotland with a 6-month intervention period and a 3-month follow-up duration, patients under Mediterranean diet in intervention group had an increased HDL concentrations in comparison to control group [52]. In contrast, no significant associations between adherence to the Mediterranean diet and HDL levels in the ATTICA Study was seen [53]. A randomized trial that assigned 60 subjects to either a high saturated fat diet, a high MUFA diet or a Mediterranean diet for 8 week did not found significant rise in HDL concentrations after the Mediterranean diet intervention [54].
one of the important component of factor 3 nutrient pattern, the ω-3 and ω-6 FA (PUFAs), were inversely associated with MetS prevalence in females [55]. The more, in a systematic review [56], total PUFA and its types (LA or ALA) intake were negatively associated with hypertension and positively associated with abdominal obesity. Some evidence from observational and intervention studies is in agreement with our results that states the benefits of both ω-3 and ω-6 PUFA in decreasing the odds of MetS [57,58,59,60]. However, the controversies also are documented [61,62,63]. Increased eicosapentaenoic acid (EPA) intake can significantly decrease IL-6 and other adipokine levels. The nuclear factor-κB (NF-κB), a pro-inflammatory transcription factor, can be suppressed by EPA in compared to control. Besides, EPA diminished TNF-α and further reduced its secretion in the existence of an NF-κB inhibitor. This feature expresses anti-inflammatory influences of ω-3 PUFAs and beneficial effects in adipocyte inflammation and metabolic disorders, such as metabolic syndrome [64].
Lycopene, as one of the carotenes, is another component of our third NP3. In a study [65], intakes of dietary α-carotene, β-carotene, and lycopene can have favorable effects on glucose metabolism in individuals at high risk for type 2 diabetes mellitus. Because of its impression on glucose metabolism, it presumed that lycopene would be inversely associated with odds of MetS.
One of the strengths of our study is the application of a validated FFQ and controlling for possible confounders in the analyses. We used a semi quantitative food frequency questionnaire (FFQ) validated for Iranian foods while, in similar studies such as khayyatzadeh study [34], the data was collected by a 24-h recall questionnaire. We believe that FFQ is suitable for our work because it can properly reflect the long-term and usual intake. Another one is the enrollment of trained dieticians for the interviews to gather the food frequency data; it could be expected this tactic (as compared with self-administration) decreased any possible misclassification error. The third is that in our manuscript metabolic syndrome was defined based on the guidelines of the ATP III which its validity is proved by numerous previous studies before [66, 67]. fHowever, some limitations exist. The cross-sectional design; thereby, the causal inferences cannot be concluded. Although the factor analysis method is identified to represent real-world dietary behaviors [68], this method is founded on some subjective judgments such as naming nutrient patterns, method of rotation and selection of food groups which can resulting in an overall evaluation error.
Conclusion
In general, a significant positive association between the NP3 and HDL concentrations was observed among adults living in Tehran, Iran. More studies are needed to investigate the possible causal relationship of this finding.
Data availability
The datasets generated or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Nestel P, Lyu R, Low LP, Sheu WH-H, Nitiyanant W, Saito I, et al. Metabolic syndrome: recent prevalence in East and Southeast Asian populations. Asia Pacific journal of clinical nutrition. 2007;16(2).
Lecture RGBJD. Role of insulin resistance in human disease. Nutrition. 1988;37(12):1595–607.
DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94.
Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–84.
Kaplan NM. The deadly quartet: upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149(7):1514–20.
Reaven GMJD. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607.
Borena W, Edlinger M, Bjørge T, Häggström C, Lindkvist B, Nagel G, et al. A prospective study on metabolic risk factors and gallbladder cancer in the metabolic syndrome and cancer (Me-Can) collaborative study. Metabolic Syndrome and Gallbladder Cancer. 2014;9(2):e89368.
Maleki A, Montazeri M, Rashidi N, Montazeri M, Yousefi-Abdolmaleki EJJorimstojoIUoMS. Metabolic syndrome and its components associated with chronic kidney disease. J Res Med Sci. 2015;20(5):465.
Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35(5):1171–80.
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB. Willett WCJTAjocn. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85(3):910–8.
Organization WH. NCD mortality and morbidity: World Health Organization; 2016 [cited 2020. Available from: https://www.who.int/gho/ncd/mortality_morbidity/en/.
Saklayen MGJC. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12.
Cheung BM. The cardiovascular continuum in Asia-a new paradigm for the metabolic syndrome. LWW; 2005.
Sarrafzadegan N, Gharipour M, Sadeghi M, Khosravi AR, Tavassoli AAJA. Metabolic syndrome in Iranian elderly. ARYA Atherosclerosis. 2012;7(4):157.
Sarrafzadegan N, Talaei M, Sadeghi M, Kelishadi R, Oveisgharan S, Mohammadifard N, et al. The Isfahan cohort study: rationale, methods and main findings. Human Hypertension. 2011;25(9):545–53.
Azizi F, Salehi P, Etemadi A, Zahedi-Asl SJDr, practice c. Prevalence of metabolic syndrome in an urban population: Tehran lipid and glucose study. Diabetes Res Clin Pract. 2003;61(1):29–37.
Fakhrzadeh H, Ebrahimpour P, Pourebrahim R, Heshmat R. Larijani BJMs, disorders r. metabolic syndrome and its associated risk factors in healthy adults: A population-based study in Iran. Metab Syndr Relat Disord. 2006;4(1):28–34.
Esmaillzadeh A, Mirmiran P, Azadbakht L, Etemadi A, Azizi FJO. High prevalence of the metabolic syndrome in Iranian adolescents. Obesity. 2006;14(3):377–82.
Potenza MV, Mechanick JIJNCP. The metabolic syndrome: definition, global impact, and pathophysiology. Nutrition in Clinical Practice. 2009;24(5):560–77.
Rumawas ME, Meigs JB, Dwyer JT, McKeown NM, Jacques PFJTA. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham offspring cohort. Am J Clin Nutr. 2009;90(6):1608–14.
Shin A, Lim S-Y, Sung J, Shin H-R, Kim JJJADA. Dietary intake, eating habits, and metabolic syndrome in Korean men. J Am Diet Assoc. 2009;109(4):633–40.
Park HS, Oh SW, Cho S-I, Choi WH, Kim YSJ. The metabolic syndrome and associated lifestyle factors among south Korean adults. Int J Epidemiol. 2004;33(2):328–36.
Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi FJTA. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82(3):523–30.
Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu SJD. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care. 2005;28(6):1438–44.
He Y, Li Y, Lai J, Wang D, Zhang J, Fu P, et al. Dietary patterns as compared with physical activity in relation to metabolic syndrome among Chinese adults. Nutr Metab Cardiovasc Dis. 2013;23(10):920–8.
Hong S, Song Y, Lee KH, Lee HS, Lee M, Jee SH, et al. A fruit and dairy dietary pattern is associated with a reduced risk of metabolic syndrome. Metabolism. 2012;61(6):883–90.
Guo H, Niu K, Monma H, Kobayashi Y, Guan L, Sato M, et al. Association of Japanese dietary pattern with serum adiponectin concentration in Japanese adult men. Nutr Metab Cardiovasc Dis. 2012;22(3):277–84.
Castanho GKF, Marsola FC, Mclellan KCP, Nicola M, Moreto F, Burini RCJC, et al. Consumption of fruit and vegetables associated with the metabolic syndrome and its components in an adult population sample. Cien Saude Colet. 2013;18(2):385–92.
Huang H-Y, Korivi M, Tsai C-H, Yang J-H, Tsai Y-CJE-BC, Medicine A. Supplementation of lactobacillus plantarum K68 and fruit-vegetable ferment along with high fat-fructose diet attenuates metabolic syndrome in rats with insulin resistance. Evid Based Complement Alternat Med. 2013;2013:1–12.
Ali A, Yazaki Y, Njike VY, Ma Y, Katz DLJN. Effect of fruit and vegetable concentrates on endothelial function in metabolic syndrome: a randomized controlled trial. Nutr J. 2011;10(1):72.
Fulgoni VL, Dreher M, Davenport AJJNj. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. 2013;12(1):1.
Li Y, Guo H, Wu M, Liu MJAPJoCN. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac J Clin Nutr. 2013;22(1):60.
Huang J-H, Lu Y-F, Cheng F-C, Lee JN-Y, L-CJNj T. Correlation of magnesium intake with metabolic parameters, depression and physical activity in elderly type 2 diabetes patients: a cross-sectional study. Nutr J. 2012;11(1):41.
Khayyatzadeh SS, Moohebati M, Mazidi M, Avan A, Tayefi M, Parizadeh SMR, et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur J Clin Investig. 2016;46(10):840–52.
Song Y, Joung HJN. Metabolism, diseases C. a traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr Metab Cardiovasc Dis. 2012;22(5):456–62.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–62.
Stanhope KLJArom. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. 2012;63:329–43.
Grundy SM, Brewer Jr HB, Cleeman JI, Smith Jr SC, Lenfant CJC. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition 2004;109(3):433–438.
Liao C-H, Shaw H-M, Chao P-MJN. Impairment of glucose metabolism in mice induced by dietary oxidized frying oil is different from that induced by conjugated linoleic acid 2008;24(7–8):744–752.
Wirfält E, Hedblad B, Gullberg B, Mattisson I, Andrén C, Rosander U, et al. Food patterns and components of the metabolic syndrome in men and women: a cross-sectional study within the Malmö Diet and Cancer cohort 2001;154(12):1150–1159.
Malik VS, Schulze MB, Hu FBJTAjocn. Intake of sugar-sweetened beverages and weight gain: a systematic review 2006;84(2):274–288.
Paniagua J, Perez-Martinez P, Gjelstad IM, Tierney AC, Delgado-Lista J, Defoort C, et al. A low-fat high-carbohydrate diet supplemented with long-chain n-3 PUFA reduces the risk of the metabolic syndrome. 2011;218(2):443–50.
Mensink RP, Janssen M-C, Katan MBJTAjocn. Effect on blood pressure of two diets differing in total fat but not in saturated and polyunsaturated fatty acids in healthy volunteers 1988;47(6):976–980.
Shah M, Adams-Huet B, Bantle JP, Henry RR, Griver KA, Raatz SK, et al. Effect of a high-carbohydrate versus a high—cis-monounsaturated fat diet on blood pressure in patients with type 2 Diabetes. 2005;28(11):2607–12.
Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, et al. Association between adherence to the Mediterranean diet and oxidative stress 2008;88(5):1364–1370.
Giugliano D, Esposito KJCoil. Mediterranean diet and metabolic diseases 2008;19(1):63–68.
Pitsavos C, Panagiotakos DB, Tzima N, Chrysohoou C, Economou M, Zampelas A, et al. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: the ATTICA study– 2005;82(3):694–699.
Babio N, Bullo M, Basora J, Martinez-Gonzalez M, Fernández-Ballart J, Márquez-Sandoval F, et al. Adherence to the Mediterranean diet and risk of metabolic syndrome and its components 2009;19(8):563–570.
León EA, Henriquez P, Serra-Majem LJPhn. Mediterranean diet and metabolic syndrome: a cross-sectional study in the Canary Islands 2006;9(8A):1089–1098.
Dedoussis GV, Kanoni S, Mariani E, Cattini L, Herbein G, Fulop T, et al. Mediterranean diet and plasma concentration of inflammatory markers in old and very old subjects in the ZINCAGE population study 2008;46(7):990–996.
Bach-Faig A, Geleva D, Carrasco J, Ribas-Barba L, Serra-Majem LJPhn. Evaluating associations between Mediterranean diet adherence indexes and biomarkers of diet and disease 2006;9(8A):1110–1117.
Papadaki A, Scott JAJPe, counseling. Follow-up of a web-based tailored intervention promoting the Mediterranean diet in Scotland 2008;73(2):256–263.
Tzima N, Pitsavos C, Panagiotakos DB, Skoumas J, Zampelas A, Chrysohoou C, et al. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. 2007;6(1):22.
Bos M, de Vries J, Feskens E, Van Dijk S, Hoelen D, Siebelink E, et al. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity 2010;20(8):591–598.
Park S, Ahn J, Kim N-S, Lee B-KJIjofs, nutrition. High carbohydrate diets are positively associated with the risk of metabolic syndrome irrespective to fatty acid composition in women: The KNHANES 2007–2014. 2017;68(4):479–87.
Tortosa-Caparrós E, Navas-Carrillo D, Marín F, Orenes-Piñero EJCrifs, nutrition. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. 2017;57(16):3421–9.
Babio N, Toledo E, Estruch R, Ros E, Martínez-González MA, Castañer O, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial 2014;186(17):E649-EE57.
Baik I, Abbott RD, Curb JD, Shin CJJotADA. Intake of fish and n-3 fatty acids and future risk of metabolic syndrome. 2010;110(7):1018–26.
Chan T-F, Lin W-T, Huang H-L, Lee C-Y, Wu P-W, Chiu Y-W, et al. Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents 2014;6(5):2088–2103.
Shab-Bidar S, Hosseini-Esfahani F, Mirmiran P, Hosseinpour-Niazi S, Azizi FJJohn, dietetics. Metabolic syndrome profiles, obesity measures and intake of dietary fatty acids in adults: T ehran L ipid and G lucose S tudy 2014;27:98–108.
Ahola AJ, Harjutsalo V, Thorn LM, Freese R, Forsblom C, Mäkimattila S, et al. The association between macronutrient intake and the metabolic syndrome and its components in type 1 diabetes. 2017;117(3):450–6.
Ebbesson SO, Tejero ME, Nobmann ED, Lopez-Alvarenga JC, Ebbesson L, Romenesko T, et al. Fatty acid consumption and metabolic syndrome components: the GOCADAN study 2007;2(4):244–249.
Lai YL, Petrone AB, Pankow JS, Arnett DK, North KE, Ellison RC, et al. Association of dietary omega-3 fatty acids with prevalence of metabolic syndrome: the National Heart, Lung, and Blood Institute Family Heart Study 2013;32(6):966–969.
Siriwardhana N, Kalupahana NS, Fletcher S, Xin W, Claycombe KJ, Quignard-Boulange A, et al. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-κB-dependent mechanisms. 2012;23(12):1661–7.
Ylönen K, Alfthan G, Groop L, Saloranta C, Aro A, Virtanen SM, et al. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: the Botnia Dietary Study. 2003;77(6):1434–41.
Bahar A, Kashi Z, Kheradmand M, Hedayatizadeh-Omran A, Moradinazar M, Ramezani F, et al. Prevalence of metabolic syndrome using international diabetes federation, National Cholesterol Education Panel-Adult Treatment Panel III and Iranian criteria: results of Tabari cohort study. Journal of Diabetes & Metabolic Disorders. 2020:1–7.
Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. 2007;77(2):251–7.
Moeller SM, Reedy J, Millen AE, Dixon LB, Newby P, Tucker KL, et al. Dietary patterns: challenges and opportunities in dietary patterns research: an Experimental Biology workshop, April 1, 2006. 2007;107(7):1233–9.
Acknowledgements
The authors wish to thank the men and women who participated in the study.
Financial support
This manuscript has been granted by Tehran University of Medical Sciences (Grant No: 36721).
Authorship
SS-b have made the conception and design of the study. HSH, FDj, participated in acquisition of data. HSH and SS-b analyzed and interpreted the results. HSH and ZA and FDj drafted the article. SS-b a revised the manuscript critically for important intellectual content. All of authors approved final version the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical standards of the Tehran University of Medical Sciences (Ethic Number: ethic Number: IR.TUMS.VCR.REC.1396.2307), who approved the protocol and informed consent form. All participants signed a written informed consent prior to the start of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahinfar, H., Akbarzade, Z., Djafari, F. et al. Association of nutrient patterns and metabolic syndrome and its components in adults living in Tehran, Iran. J Diabetes Metab Disord 19, 1071–1079 (2020). https://doi.org/10.1007/s40200-020-00607-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00607-z