Abstract
A medium-entropy high-speed steel (ME-HSS) coating with the 76 at.% of Fe and multiple alloying elements was prepared by the wide-band laser cladding. Compared with the commercial W6Mo5Cr4V2 (M2) HSS coating which contains a large number of network lamellar M2C-type carbides along the grain boundaries, the presented ME-HSS coating has a high quantity of finer and more uniformly dispersed MC-type carbides; on the other hand, the coating has less retained austenite and much lower brittleness as well as similar secondary hardening effect and tempering hardness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The so-called medium-entropy alloys (MEAs) were provoked by the research boom in the high-entropy alloys (HEAs) and have been as a hot topic in the novel metallic materials. One-component designing strategy for MEAs is to prepare ternary or quaternary alloys with nearly equiatomic compositions. CrCoNi and CrCoFeNi MEAs are the well-known components and have been demonstrated to be of high strength, excellent ductility and cryogenic fracture resistance via the nano-twinning strengthening [1,2,3]. Another strategy is to improve the additive content of a single element exceeding 35 at.% in the as-designed multicomponent alloys with the configuration entropy between R and 1.5R [4,5,6,7,8,9,10]. Jose et al. [6] and Choi et al. [7] separately reported the Fe50Mn30Co10Cr10 and Fe50(CoCrMnNi)50 alloys with excellent strength and ductility by a conventional arc-melting technique. Zhang et al. prepared a non-equiatomic Fe6NiCoCrAlTiSi coating by laser cladding and proved that equimolarity was not a precondition for obtaining a single-phase solid solution in the HEAs [8]. Yao et al. [9] and Zhuang et al. [10] also, respectively, prepared the non-equiatomic Fe40Co5Cr2Mn27Ni26 and FeCo3NiCuAl alloys with simple solid-solution phases by a suck-casting method. Therefore, the concept of Fe-rich MEAs, which contains more than 50 at.% of Fe, is expected to greatly reduce the consumption of expensive elements like Co and Ni in the HEAs and thus to extend the potential application in industrial community.
In our previous work, a medium-entropy (ME) high-speed steel (HSS) coating with Fe content of 68 at.% by laser cladding was synthesized [11]. The coating possessed excellent oxidative hot wear resistance than the M2-HSS (W6Mo5Cr4V2) coating, because the former contains a high content of anti-oxidation elements, such as Cr, Co and Al, and hence leads to a more continuous and compact oxidation film on the worn surface after hot wear tests at 500 °C. Therefore, the ME-HSS component can be expected to partially replace the conventional HSS coating serving as iron-based thermal wear-resistant material, such as on the surface of hot working roll. However, industry application always pursues to reduce the cost and improves processing efficiency. Therefore, the current work aims to improve the iron content of ME-HSS coating from 68 to 76 at.% with the addition of more alloying elements like Cu and Ti, based on the previous works [11, 12]. The components of the newly proposed ME-HSS coating and a widely industrial used M2-HSS (W6Mo5Cr4V2) coating are comparably presented in Table 1. The weight percentage of W, Mo and C is controlled to be equal in the two alloys. Other elements are set to be 2.1 at.% in the ME-HSS component in order to improve the content of the alloying elements and lead to enhance the entropic effect. Furthermore, the wide-band laser cladding technique with spot size 15 mm × 2 mm was utilized to accelerate the coating forming efficiency, in comparison with laser spot of 4 mm diameters in our previous works [11, 12].
2 Experimental
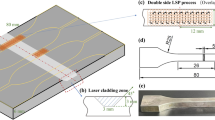
The ME-HSS powder was prepared by mechanically mixing the M2-HSS powder (Höganäs Company) with other required elemental powders according to the composition listed in Table 1. The motivation of choosing M2-HSS powder as a raw material was to improve the coating cladding properties, because the purchased powder prepared by gas atomization has a more suitable sphericity and particle size for laser cladding. A layer of ME-HSS powder bed with about 1.0 mm thickness was preset onto a steel substrate and cladded by the LDM 4000-100 diode laser. The specific laser parameters were 3 kW of power, 15 mm × 2 mm of rectangular beam spot and 300 mm min−1 of scanning speed. Argon flux was used to prevent the oxidation during the cladding process. Furthermore, a comparative M2-HSS coating was prepared under the same process. After the laser cladding, the coating was tempered three times with each hold time of 20 min in the 470–590 °C temperature range. The purpose of the triple tempering treatment was to strengthen the matrix by secondary hardening and eliminate the possibly existed retained austenite [13, 14].
A Rigaku X-ray diffractometer (XRD) (Cu Kα) was used to identify the phase structure in the coating. The microstructure was characterized by a field emission scanning electron microscope (SEM, Quanta 450). The elemental composition and distribution were determined by an energy-dispersive spectrometer (EDS) equipped with SEM. The Vickers hardness of the coatings was measured on a standard HMV-2T Vickers hardness tester. The test pressure and dwell time were fixed at 4.9 N and 20 s, respectively, and each sample was measured nine times to calculate the average hardness. The brittleness of the coatings was evaluated via the length of cracks provoked by Vickers indentation at the corners of Vickers pyramid under a large loading force of 294 N.
3 Results and Discussion
3.1 Coating Morphology and Structure
Figure 1a shows the smooth surface morphology with the width of ca. 15 mm in the single-track ME-HSS coating. The XRD patterns in Fig. 1b show that the coatings are mainly composed of martensite matrix, retained austenite and MC-type and M2C-type reinforcements after laser cladding. After the triple-tempering at 530 °C, the residual austenite completely disappears and transforms to martensite. This microstructure transition is almost same as most of the previous results on the traditional Fe–C–X HSS coating prepared by laser cladding [15]. The main difference in the controllable coatings is that the dominant carbide in the M2-HSS coating is M2C type, but the carbide transforms to MC in the ME-HSS coating. Therefore, it is convinced that the proposed medium-entropy alloy coating is reasonably classified to the HSS alloy system.
3.2 Microstructure and Hardness Evolution
Figure 2 shows the cross-sectional microstructure at the center of the two coatings. The microstructure of solidified M2-HSS coating (Fig. 2a) is characterized by an acicular martensitic matrix separated by the network mixture of the retained austenite and carbides. After the triple tempering at 530 °C, Fig. 2b shows that the retained austenite almost disappears and a high quantity of lamellar carbides can be observed at grain boundaries. The carbide network has the typical lamellar morphology as M2C-type carbide, which has been reported in the previous works [16, 17].
Figure 2c shows that the content of network mixture of retained austenite and carbides is much less in the ME-HSS-solidified coating than those in the M2-HSS coating. Furthermore, Fig. 2d shows that more carbides are uniformly dispersed in the martensitic matrix after the tempering treatment. The network carbide distribution is broken and almost completely disappeared. EDS analysis compares the chemical composition in the carbides (labeled as C) with the martensitic matrix (labeled as M) after the tempering treatment. It is found that the carbides are mainly enriched in W, Mo, Cr and V elements in the M2-HSS coating, while the main difference is that Ti greatly is involved in carbide phases in the ME-HSS coating.
Figure 3 shows the interface microstructure between the coating and substrate and the component distribution of main alloying elements along the EDS scanning line. The purple, green, red and blue lines refer to iron, molybdenum, chromium and tungsten elements, respectively. Component scanning lines of other additive elements, such as Al and Ni, were not presented here avoiding difficulty to be distinguished. The results indicate that the carbides are also well distributed near the interface of the coating. There is a clear interface for the elemental distribution between the coating and substrate. The component transition region, where the Cr, W and Mo alloying elements gradually increased from the interface to the coating, is very narrow with a thickness of about 5 µm, suggesting that the component dilution is very low in the ME-HSS coating.
Figure 4a shows the obvious secondary hardening both occurred in the compared coatings after triple tempering in the temperature range of 470–590 °C. The maximum tempering hardness in the ME-HSS and M2-HSS coatings reaches about 827 HV0.5 and 840 HV0.5, respectively, which both exceed the hardness of about 800 HV in the traditional casting prepared Fe–C–X HSS [14, 18, 19]. Figure 4b and c shows the comparison of the indentation brittleness of the two coatings in the highest tempering hardness state by the Vickers indentation method. This method can evaluate the brittleness for high-brittle and high-hardness materials (poor toughness and resistance to propagation) by the growth length of a crack initiated at the corners of the Vickers pyramid [20]. The M2-HSS coating has much higher brittleness with the cracks obviously observed at the corner of the indentation. Therefore, it is believed that the fine and well-dispersed carbides bring lower brittleness in the ME-HSS coating.
3.3 Formation Reasons of Uniform Carbide Dispersion in the ME-HSS Coating
The hardness of the HSS mainly depends on the type of the carbides, in which the higher hardness and the finer and more uniformly distributed microstructure are much pursued [14, 21, 22]. It is already known that the hard MC carbides are proeutectoid phase and can be uniformly dispersed inside grains [21], while other carbides like M2C, M6C and M7C3 come from the eutectic reaction and mainly distributed at the grain boundaries [14, 16]. The reported Vickers hardness of the different carbides was MC (2100–2800 HV), M2C (1800–2250 HV), M6C (1800–2250 HV) and M7C3 (1890–2060 HV) [22]. Therefore, MC is the most favorably hardest carbide and can be uniformly distributed. However, the obtained carbides are more likely to be M2C and M6C in the traditional casting prepared Fe–C–X HSS [21,22,23]. It is rarely reported that MC can be obtained as main carbide reinforcement and the network carbide formation can be completely avoided.
In this work, uniformly dispersed M2C and MC carbides were obtained in the ME-HSS coating. The high content of MC obtained in the ME-HSS coating should be attributed to the medium-entropy effect which suppresses the nucleation of the carbides causing a lower content of the carbides in the ME-HSS coating than that in the M2-HSS coating. According to the mixing enthalpy in the different types of carbides, MC carbide is firstly precipitated from the liquid and more stable than other carbides in the HSS system [14, 17]. Therefore, the inhibition for MC nucleation is the relatively weak and MC becomes the dominant reinforcement in the ME-HSS coating. Furthermore, it is known that TiC has strong formation free energy [24]. As a consequence, the additive element of Ti is another reason to promote the MC nucleation in the ME-HSS coating. This analysis is primarily supported by a high Ti content in the MC carbide, according to EDS results (Table 2). Alternatively, adding Ti alloying element is a main difference between the current work and our previous works [11, 12]. High-quantity and uniformly distributed MC carbides were not clearly observed previously, suggesting that Ti is responsible for the formation of a high quantity of MC in this work.
It is well known that solidification rate strongly affects the carbide distribution in the HSS coatings [14, 25]. Figure 5 displays the microstructure evolution in the ME-HSS coatings with thicker powder bed prepared under the same laser processing parameters. It is demonstrated that a thinner powder bed thickness generally refers to a higher solidification rate under the same laser energy input [26]. Figure 5a validates that the networks of carbide phase are still existed in the thick coatings with powder bed of 2 mm. Figure 5b shows the network is gradually broken up in the coating with powder bed of ca. 1.5 mm, but the uniformity of carbide distribution is not as good as that of in the 1-mm-thick coating, as compared with Fig. 2d. The circles marked MC carbides are still mainly distributed at the grain boundaries (Fig. 5b). This phenomenon indicates that the higher solidification rate favors the more uniform distribution of carbide in the ME-HSS coating. Because rapid solidification causes a high quantity of alloying elements to accumulate at the front of the solid–liquid interface, the enrichment of Mo, Co, Ni and Al in the residual liquid is all believed to promote the formation of M2C (instead, W is beneficial to the formation of M6C and V facilitate the formation of MC) [14, 27]. Therefore, a fast solidification rate is required for the preparation of the ME-HSS coating. The thickness of about 1 mm with uniform carbide dispersion obtained in the current work can satisfy the industrial requirements for the wear-resistant coating thickness.
4 Conclusions
-
1.
Uniformly dispersed MC and M2C carbides with the martensitic matrix are obtained in a newly designed ME-HSS coating by wide-band laser cladding.
-
2.
The ME-HSS coating has lower brittleness and similar maximum tempering hardness of about 827HV, compared with the M2-HSS coating prepared under the same condition.
-
3.
The formation reasons of uniform carbide dispersion in the ME-HSS coating are attributed to rapid solidification and a high content of alloying elements, particularly Ti.
References
H. Yang, X.L. Shang, L.L. Wang, Z. Wang, J.C. Wang, X. Lin, Acta Metall. Sin. 54, 905 (2018)
B. Gludovatz, A. Hohenwarter, K.V.S. Thurston, H. Bei, Z. Wu, E.P. George, R.O. Ritchie, Nat. Commun. 7, 10602 (2016)
H. Feng, H.B. Li, P.C. Lu, C.T. Yang, Z.H. Jiang, X.L. Wu, Acta Metall. Sin. 55, 1457 (2019)
H. Zhang, H. Tang, W.H. Li, J.L. Wu, X.C. Zhong, Mater. Sci. Technol.-Lond. 34, 1 (2017)
F. He, Z. Wang, M. Zhu, J. Li, Y. Dang, J. Wang, Mater. Des. 85, 1 (2015)
Y.A. Jose, V.U. Alejandro, Z.M. Dario, P.H. Rodrigo, Mater. Sci. Eng. A 748, 244 (2019)
M. Choi, I. Ondicho, N. Park, N. Tsuji, J. Alloys Compd. 780, 959 (2019)
H. Zhang, Y. Pan, Y. He, H. Jiao, Appl. Surf. Sci. 257, 2259 (2011)
M.J. Yao, K.G. Pradeep, C.C. Tasan, D. Raabe, Scr. Mater. 72–73, 5 (2014)
Y.X. Zhuang, W.J. Liu, P.F. Xing, F. Wang, J.C. He, Acta Metall. Sin. (Engl. Lett.) 25, 124 (2012)
H. Tang, H. Zhang, L. Chen, S. Guo, J. Alloys Compd. 772, 719 (2019)
H. Zhang, B. Dou, H. Tang, Y.Z. He, S. Guo, Mater. Des. 159, 224 (2018)
X.U. Bojun, G.U. Nanju, Y. Dianran, Acta Metall. Sin. (Engl. Lett.) 3, 116 (1990)
M. Boccalini, H. Goldenstein, Int. Mater. Rev. 46, 92 (2001)
W. Darmawan, J. Quesada, F. Rossi, R. Marchal, F. Machi, H. Usuki, J. Laser Appl. 21, 176 (2009)
X. Zhou, D. Liu, W. Zhu, F. Fang, Y. Tu, J. Jiang, J. Iron. Steel Res. Int. 24, 43 (2017)
F. Pan, W. Wang, A. Tang, L. Wu, T. Liu, R. Cheng, Prog. Nat. Sci. Mater. 21, 180 (2011)
S. Wei, J. Zhu, L. Xu, Mater. Sci. Eng. A 404, 138 (2005)
V. Trabadelo, S. Giménez, I. Iturriza, Mater. Sci. Eng. A 499, 360 (2009)
V. Keryvin, V.H. Hoang, J. Shen, Intermetallics 17, 211 (2009)
G. Herranz, A. Romero, V. de Castro, G.P. Rodríguez, Mater. Des. 54, 934 (2014)
H.W. Zhang, K. Nakajima, M. Su, H. Shibata, P. Hedström, W. Wang, H. Lei, Q. Wang, P.G. Jönsson, J. He, Steel Res. Int. 89, 1800172 (2018)
X.F. Zhou, F. Fang, Y.Y. Tu, J.Q. Jiang, H.X. Xu, W.L. Zhu, Acta Metall. Sin. 50, 769 (2014)
H. Liu, J. Liu, P. Chen, H. Yang, Opt. Laser Technol. 118, 140 (2019)
Y. Ji, W. Zhang, X. Chen, J. Li, Acta Metall. Sin. (Engl. Lett.) 29, 382 (2016)
H.J. Niu, I.T.H. Chang, Microstructural evolution during laser cladding of M2 high-speed steel. Metall. Mater. Trans. A 31, 2615 (2000)
C.K. Kim, J.I. Park, S. Lee, Y.C. Kim, N.J. Kim, J.S. Yang, Metall. Mater. Trans. A 36, 87 (2005)
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 51971001, U1560105 and 51601050) and the Open Fund from State Key Laboratory of Solid Lubricating (No. LSL-1714).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195
Rights and permissions
About this article
Cite this article
Dou, B., Zhang, H., Zhu, JH. et al. Uniformly Dispersed Carbide Reinforcements in the Medium-Entropy High-Speed Steel Coatings by Wide-Band Laser Cladding. Acta Metall. Sin. (Engl. Lett.) 33, 1145–1150 (2020). https://doi.org/10.1007/s40195-019-00993-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-019-00993-1