Abstract
The morphological evolution of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy has been investigated with various contents of Fe, Mn, and Cr. The results show that coarse Chinese script Fe-rich phases appear in the alloy with 0.6 wt% Fe combined with trace Mn, while the blocky Fe-rich phases appear combined with trace Cr. Under the coexistence of trace Mn and Cr, a large number of fine Chinese script Fe-rich phases could be visible in the low iron-bearing AlSi9Cu3Mg0.19(Fe) alloy (0.60 wt%). At high Fe level (1.30 wt%), numerous Fe-rich phases with hexagonal morphologies are observed with the trace Cr, while fish-bone and pentagonal morphologies of Fe-rich phases could be simultaneously observed with the addition of both Mn and Cr. The results reveal that the trace Mn and Cr promote the morphological evolution of Fe-rich phases. The morphology evolution mechanism of Fe-rich phases has been discussed by using the atom radius and electronegativity differences of Fe, Mn, and Cr.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Al–Si alloys are most extensively used in automotive and aerospace industry mainly due to their excellent castability, high strength, light weight, and good corrosion resistance [1–5]. As one of the major injurious impurities in commercial Al–Si alloys, iron is always present in the form of intermetallic compounds which are brittle and complex [6–10]. The essence of intermetallic compounds fully depends on other alloying elements present [11, 12]. Furthermore, a severe stress concentration is introduced to the matrix of Al alloys with a general reduction of the mechanical properties of the material because of the sharp edges of these needle-like Fe-rich phases [10, 13, 14]. At the same time, iron is a desirable and necessary element in die-casting alloys, which helps to prevent or alleviate the die soldering [12, 15]. For many years, various solutions have been developed to neutralize its negative effects. It has been shown that alloying elements such as Mn and Cr can modify the morphology of intermetallic phases to a less harmful and more compact morphology [12, 14, 16–23]. Generally, higher Mn concentration leads Fe-rich phases to Chinese script morphology [24–28]. And Cr element has a similar effect of Mn. Higher Cr addition results in the formation of Fe-rich phases with polyhedral, star-like, and blocky morphology [29–31]. Ferraro et al. [15] investigated the evolution of sludge particles in secondary die-cast Al alloys by using sludge factor (SF). The SF equation can predict whether or not the iron-rich particles formed in the Al alloy, which can be expressed as:
The results indicated that the number of primary iron-rich particles increased with increasing the Fe and Mn contents, while a great number of secondary iron-rich particles formed after adding Cr content. Coarser sludge with polyhedral and star-like morphologies formed in the Al alloy as long as the Fe, Mn, and Cr levels increased. Furthermore, the morphological evolution of iron-rich phases can be explained by the Gibbs–Wulff theory, which expatiates on the change of surface energy with crystallographic planes in the crystals [32–34]. Even though some attempts have been made to reveal the reaction mechanisms of microalloying on the morphology of Fe-rich phases, the morphology evolution mechanism of Fe-rich phases is not well established in the literature. This leads to the need for a systematic study of the detailed morphology evolution mechanism.
In this study, the morphological evolution of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy has been investigated over two levels (0.6, 1.3 wt%) of iron (Fe), and one level (0.1 wt%) of manganese (Mn) and chromium (Cr). The morphology evolution mechanism of Fe-rich phases has been discussed by using the atom radius and electronegativity differences of Fe, Mn, and Cr.
2 Experimental Procedure
Six different alloys were prepared by varying the Fe, Mn, and Cr contents according two levels (0.6, 1.3 wt%) of iron (Fe), and one level (0.1 wt%) of manganese (Mn) and chromium (Cr). The chemical composition of the experimental alloys is listed in Table 1. Additions of Si, Fe, Mn, and Cr were made to the melt in the form of commercial Al–21Si, Al–20Fe, Al–30Mn, and Al–20Cr master alloys. After addition, the molten metal was heated up to 740 ± 5 °C. Subsequently, refining treatment was performed at 720 ± 5 °C for 12 min. Then modification treatment was made at 730 ± 5 °C for 15 min. Before pouring, the temperature was decreased to 690 ± 5 °C. At last, the alloy melts were poured into a preheated (~ 200 °C) permanent mold to gain as-cast ingots with 23 mm in diameter and 200 mm in height.
The microstructure morphology was analyzed by using an optical microscope (OM, MA200) and a scanning electron microscope (SEM, Zeiss Auriga), equipped with an energy-dispersive spectrometer (EDS, Inca100) and a transmission electron microscope (TEM, JEM-2100). The samples for OM and SEM were ground, polished, and etched for 5–10 s in a solution of 0.5% hydrochloric acid. The samples for TEM were ground and polished into 50–70-μm-thick foils. Then several disks with 3 mm in diameter were punched out from the polished foils, followed by electropolishing in a solution of 10% perchloric acid at the temperature in the range of − 25 and 20 °C with the voltage of 20 V. Finally, ion-beam thinning was carried out for TEM observation. In addition, identification of each phase was also approached by X-ray diffraction (XRD, D/MAX-1200) measurements using Cu Kα radiation, operated at 40 kV and 20 mA. The diffraction patterns were obtained in the 2-theta degree range of 10°–90° with a step size of 0.01°. Furthermore, the volume fraction, size (length), and roundness of Fe-rich phases were analyzed by image analysis software. Microhardness measurements were carried out using Vickers microhardness tester with the load of 200 g and the holding time of 15 s.
3 Results and Discussion
3.1 Microstructural Analysis
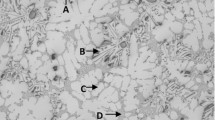
Figure 1 exhibits the optical micrographs of low iron-bearing (0.60 wt%) and high Fe level (1.30 wt%) AlSi9Cu3Mg0.19(Fe) alloys. Coarse Chinese script Fe-rich phases are recognized in the Alloy 1 (Fig. 1a), while the blocky Fe-rich phases appear in the Alloy 2 (Fig. 1b). Numerous Fe-rich phases with needle-like morphologies can be clearly observed in the both Alloy 1 and Alloy 2. As presented in Fig. 1c, a large number of Chinese script Fe-rich phases could be evidenced, which are finer than that of Alloy 1. Similar observations were also showed by Seifeddine et al. [26], who revealed that iron-rich phases with Chinese scripts, polyhedral and star-like iron-rich phases were detected in the Al alloy with the addition of 0.68 wt% Mn. However, at high Fe level (1.30 wt%), the Fe-rich phases appeared in the Alloy 4 (Fig. 1d) are almost needle-like morphology, the size of which is coarser compared to the needle-like Fe-rich phases of other AlSi9Cu3Mg0.19(Fe) alloys. More remarkably, a great number of Fe-rich phases with hexagonal morphologies are observed as shown in Fig. 1e. Meanwhile, hexagonal Fe-rich phases are uneven distributed in the Al matrix. As for the Alloy 6, fish-bone Fe-rich phases form as shown in Fig. 1f. In addition, the microstructure also shows the presence of Fe-rich phases with pentagonal morphologies, as better evidenced in Fig. 1g.
OM images of AlSi9Cu3Mg0.19(Fe) alloys with 0.6 wt% Fe and a 0.1 wt% Mn, b 0.1 wt% Cr, c 0.1 wt% Mn, 0.1 wt% Cr; with 1.3 wt% Fe and d 0.1 wt% Mn, e 0.1 wt% Cr, f, g 0.1 wt% Mn, 0.1 wt% Cr. The red arrows in a–g show the coarse Chinese script, blocky, fine Chinese script, needle-like, hexagonal morphologies, fish-bone, and pentagonal morphologies of Fe-rich phases, respectively
The SEM images with EDS results of all AlSi9Cu3Mg0.19(Fe) alloys with trace addition are showed in Fig. 2. As it is seen from Fig. 2a, the trace addition of Mn leads to the apparent fragmentation of needle-like Fe-rich phases (marked by the red arrows), which causes the Fe-rich phases with short rod-like. As a result, it could be Chinese script morphologies (Fig. 1a). The occasional observation of fragmented morphology has been reported before by Shabestari et al. [35]. It is interesting to note that a similar fragmented morphology is also observed in the Alloy 2 with the trace addition of Cr (marked by the red arrows, Fig. 2b), which could promote the formation of blocky Fe-rich phases (Fig. 1b). In the SEM image of Alloy 3 (Fig. 2c), the Chinese script Fe-rich phases are recognized. At high Fe level (1.30 wt%), innumerable needle-like Fe-rich phases are observed as shown in Fig. 2d. Furthermore, it is worthy to point out that Fe-rich phases with hexagonal morphologies are evidenced as shown in Fig. 2e. Moreover, Fig. 2h shows the fish-bone Fe-rich phases presented in the Alloy 6. Therefore, a schematic illustration of the evolution of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy with different contents of Fe, Mn, and Cr could be established to have a better insight of aforementioned observations (Fig. 3).
SEM images and EDS results (insets) of AlSi9Cu3Mg0.19(Fe) alloys with 0.6 wt% Fe and a 0.1 wt% Mn, b 0.1 wt% Cr, c 0.1 wt% Mn, 0.1 wt% Cr; with 1.3 wt% Fe and d 0.1 wt% Mn, e 0.1 wt% Cr, h 0.1 wt% Mn, 0.1 wt% Cr. f and g are the linear scanning curves of elements on needle-like phase d, hexagonal phase e (marked by the red two-way arrows)
The insets in Fig. 2 are the EDS analysis of the corresponding region marked by the orange dot. The EDS results of phases present are summarized in Table 2. It was found that gradual changes in the chemical composition of iron-rich phases were recorded when the morphology underwent some changes [33]. EDS analysis of needle-like Fe-rich phases reveals that the phases contain more aluminum content than other elements because of the small size of needle-like Fe-rich phases (inset, Fig. 2d). EDS analysis of both the Chinese script (inset, Fig. 2c) and fish-bone Fe-rich phases (inset, Fig. 2h) suggests that silicon dramatically decreases in alloys with the trace addition of both Mn and Cr compared to other alloys with the trace addition of Mn. Additionally, at low Fe level, Cr causes the increase in silicon element, while decreases at high Fe level. Figure 2e shows the EDS results of Fe-rich phases with hexagonal morphologies. Compared to the EDS results of Fe-rich phases (Fig. 2b), the chromium level is much more than other elements, which may be due to chemical segregation as Fe is replaced by Cr during solidification. In addition, the level of iron is kept mostly constant as the iron concentration increases in the melt.
Figure 2f and g shows the linear scanning curves of elements on phases in the AlSi9Cu3Mg0.19(Fe) alloy (marked by the red arrows, Fig. 2d and e). And chemical composition profiles over the phases were recorded. As it is seen from Fig. 2f, the needle-like Fe-rich phases contain aluminum, iron, silicon, and manganese contents. And this kind of phase contains more iron content compared to the matrix of Al alloys. It was found that significant variations in the aluminum and silicon contents were observed over the needle-like iron-rich phases. The linear scanning curves in Fig. 2g suggest that the hexagonal Fe-rich phases contain aluminum, iron, silicon, and chromium contents. In addition, the level of these elements is kept mostly constant in the hexagonal Fe-rich phases, which indicates the homogeneous distribution of elements. It is worth noting that both needle-like Fe-rich phases and hexagonal Fe-rich phases contain less aluminum and more silicon contents compared to the Al matrix. That is to say, the increase in silicon content exists concurrently with the decrease in aluminum content in the two kinds of Fe-rich phases compared to the Al matrix. In conclusion,the addition of minor Mn and Cr could cause the alteration of chemical composition of iron-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy.
Figure 4 exhibits the TEM micrograph of the hexagonal Fe-rich phase in the Alloy 5 and its corresponding EDS results. The hexagonal Fe-rich phase exists in the Alloy 5 as shown in Fig. 4a. The corresponding EDS spectra indicate that this kind of phase contains Al, Si, Fe, Mn, and Cr contents (Fig. 4b). These results are consistent with the work of Wang et al. [13].
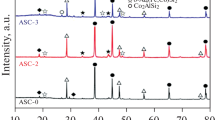
XRD analysis was carried out to confirm EDS results and to identify accurately the intermetallic phases in the cast samples (Fig. 5). However, there is mainly α-Al, Al–Si eutectic, and Al2Cu phases in all alloys. Meanwhile, no Fe-rich phases appear in all alloys.
3.2 Morphology Characterization of Fe-rich Phases
Figure 6 shows the volume fraction and size (length) of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloys, which were analyzed by image analysis software. At low Fe level, compared to the alloy with the trace addition of Mn or Cr, the volume fraction and size (length) of Fe-rich phases are larger (10.3%, 9.69 μm, respectively) in the alloy with the trace addition of both Mn and Cr, which are caused by the formation of Chinese script Fe-rich phases (Fig. 6a). At high Fe level (1.30 wt%), the volume fraction and size (length) of Fe-rich phases in the alloy with the trace addition of Cr are 9.7%, 10.23 μm, respectively, which are usually resulted from the formation of hexagonal Fe-rich phases (Fig. 6b). The combination of Mn and Cr could promote the formation of fish-bone Fe-rich phases, which leads to the larger volume fraction (9.6%, Fig. 6b). The volume fraction and size (length) of Fe-rich phases in the alloy with 1.30 wt% Fe and 0.10 wt% Mn are larger (8.8%, 10.91 μm, respectively) than that of alloy with 0.60 wt% Fe and 0.10 wt% Mn (6.6%, 8.5 μm, respectively). This is because many needle-like Fe-rich phases are observed in the alloy with 1.30 wt% Fe and 0.10 wt% Mn. A small amount of blocky Fe-rich phases appears in the alloy with 0.6 wt% Fe combined with trace Cr. This produces a smaller volume fraction and size (length) of Fe-rich phases (5%, 8.66 μm, respectively, Fig. 6a) compared to that of alloy with 1.30 wt% Fe combined with trace Cr. However, under the coexistence of trace Mn and Cr, the volume fraction and size (length) of Fe-rich phases have no great difference between the low iron-bearing (10.3%, 9.69 μm, respectively, Fig. 6a) and high Fe level AlSi9Cu3Mg0.19(Fe) alloy (9.6%, 10.7 μm, respectively, Fig. 6b).
The volume fraction and size (length) of Fe-rich phases are reported in Fig. 7 as function of the SF. In order to analyze the influence of each single element (Fe, Mn, and Cr) on the Fe-rich phases, the further analysis and demonstration are necessary based on Fig. 6. It is clear that the variation of Fe content in the AlSi9Cu3Mg0.19(Fe) alloy produces a progressive increase in the volume fraction of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy with trace Mn or trace Cr (33, 94%, respectively). At the same time, the volume fraction of Fe-rich phases slightly decreases by 7% in the alloy with trace Mn combined with trace Cr. On the other hand, at low Fe level, the volume fraction of Fe-rich phases significantly increases by 106% with the increase in Mn content, while the volume fraction of Fe-rich phases slightly decreases by 1% at high Fe level. In addition, the increase in Cr element level causes a gradual increase in the volume fraction of Fe-rich phases, 56% at low Fe level, 9% at high Fe level, respectively. Hwang et al. [36] examined that the Mn addition seems to lead to the linearly increase in total volume fraction of intermetallic phases in alloys by means of quantitative stereology.
Besides the volume fraction, the size (length) of Fe-rich phases slightly increases as the iron or manganese content increases in alloys. At low Fe level, the size (length) of Fe-rich phases increases with the increase in Cr levels, while slightly decreases at high Fe level.
Combining the volume fraction with optical micrographs of iron-rich phases, we can find that the Mn and Cr additions produce the formation of many fine Chinese script Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy with 0.6 wt% Fe, which has the largest volume fraction. Namely, the addition of Mn and Cr leads to the maximum reduction in the content of Fe in the AlSi9Cu3Mg0.19(Fe) alloy with 0.6 wt% Fe. Therefore, we may conclude that the solid solubility of Fe in the AlSi9Cu3Mg0.19(Fe) alloy is decreased after adding trace alloying element,which will be further demonstrated by the following theoretical analysis at Sect. 3.4.
Furthermore, the influence of Mn and Cr additions on the morphological evolution of Fe-rich phases was studied by using the roundness of iron-rich phases, which was also analyzed by image analysis software. Figure 8 shows the roundness of Fe-rich phases as a function of the SF. The value of roundness of Fe-rich phases in AlSi9Cu3Mg0.19(Fe) alloys is reported in Table 3, which indicates a morphological change of Fe-rich phases. The roundness of needle-like Fe-rich phases is largest (2.00), while the Fe-rich phases with blocky shape contain the smallest roundness (1.58). Therefore, the roundness of Fe-rich phases is consistent with the morphology of iron-phases.
3.3 Microhardness
Figure 9 gives the microhardness profiles over the iron-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy. As shown in Fig. 9a, the microhardness of the needle-like iron-rich phase (point A) is 161.6 HV, which is much higher than others. And the microhardness of the hexagonal iron-rich phase is 95.1 HV as shown in Fig. 9b (point B). Moreover, another hexagonal iron-rich phase displayed the maximum microhardness (212.8 HV, point C), which could be attributed to the chemical segregation. And this microhardness analysis is consistent with the aforementioned results. Therefore, the altered chemical composition profiles of iron-rich phase produce the change of microhardness profiles over the iron-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy.
Compared to the face-centered cubic (fcc) elements (such as Al), the body-centered cubic (bcc) elements (such as Mn and Cr) have a higher stacking fault energy (SFE) [37]. The SFE of iron-rich phases would increase with the addition of trace Mn or Cr. Furthermore, the low microhardness is consistent with the low SFE and slow rates of recovery [38]. In other words, the microhardness is related to SFE. Thus, the addition of trace Mn or Cr leads to the higher microhardness of iron-rich phases.
3.4 Morphology Evolution Mechanism of Fe-rich Phases
It was found that the Gibbs–Wulff theory can be used to illustrate the effect of microalloying on the morphological changes of Fe-rich phases in the AlSi9Cu3(Fe) alloys, which describes the alteration of surface energy with crystallographic planes in the crystals [14, 32, 33, 39]. The equilibrium shape of the crystals is related to the free energy of the crystallographic planes in the crystals, and the growth rate of the crystallographic planes is proportional to their surface energy. Gibbs proposed the principle of minimum surface energy of crystal growth on the base of thermodynamics, which is, at constant temperature and pressure a crystal will be in stable equilibrium if it has the lowest possible value of the total surface energy, and the corresponding morphology is the equilibrium shape of the crystal [40].
Zhong et al. [41] investigated the effect and mechanism of manganese and ultrasonic vibration of needle-like Fe-rich intermetallic compounds of Al alloys and pointed out that the mechanism of morphological changes was explained by a theory, which describes the property of Fe and Mn element. Mn atoms can replace the Fe atoms of needle-like Fe-rich phases due to the transition elements of Mn and Fe [42].
Fe-rich phases crystallize prior to primary α-Al during solidification because of the very little solid solubility of Fe in the Al alloy (0.05 wt%) [43]. The formation of needle-like Fe-rich phases depends on alloying elements and cooling rate [44, 45]. Hence Fe-rich phases will preferentially grow along the direction of [110] with the minimum surface energy under normal casting conditions, which leads to the formation of needle-like Fe-rich phases. Concerning the AlSi9Cu3Mg0.19(Fe) alloy with the addition of minor Mn and Cr, the lattice vacancies of Fe-rich phases will be occupied by the Mn or Cr element. The atom radius of Fe, Mn, and Cr are listed in Table 4. As adjacent transition elements, Fe, Mn, and Cr have similar atomic structure. Mn or Cr atoms could substitute the Fe atoms of needle-like Fe-rich phases. It further restrains the priority growth of the needle-like iron-rich phases. Thus, the trace Mn or Cr promotes the morphological evolution of iron-rich phases. Consequently, Fe-rich phases with Chinese script, blocky or hexagonal morphologies are formed in the AlSi9Cu3Mg0.19(Fe) alloys. The Fe-rich phases are almost needle-like morphology because of a small amount of Mn (Fig. 1d). In this case, Mn fails to restrain the priority growth of the needle-like iron-rich phases due to the depletion of Mn before its neutralization. At high concentrations of manganese compared to Fe level, the lattice vacancies of Fe-rich phases will be occupied by the Mn, which restrains the priority growth of the needle-like iron-rich phases. Therefore, the altered morphologies such as Chinese scripts are observed (Fig. 1a). Similarly, the trace Cr could restrain the priority growth of the needle-like iron-rich phases due to the replacement of Fe atoms caused by Cr atoms during solidification, which leads to the formation of blocky or hexagonal Fe-rich phases. Additionally, the trace Cr causes the different morphology of iron-rich phases in the low Fe and high Fe level AlSi9Cu3Mg0.19(Fe) alloys, which needs further study.
On the base of Darken–Gurry theory [46], Wang et al. [47] proposed that the stability of intermediate phase in alloys can be described by the interaction intensity (W), which was defined as:
where \(\varepsilon_\text{r }\) and \(N_\text{r}\) are relative size factor and relative electronegativity difference, respectively. The relative size factor can be calculated by equation below:
where \(r_{\text{A}}\) and \(r_{\text{B}}\) are the atom radius of solvent A and solute B, respectively. The relative electronegativity difference is given by:
where \(N_{\text{A}}\) and \(N_{\text{B}}\) are the electronegativity difference of solvent A and solute B, respectively. High W means good stability of intermediate phase. Stable intermediate phase leads to the lower solubility of solid solution in alloys [37, 47]. Namely, high W means the lower solubility of solid solution of intermediate phase in alloys. As shown in Table 5, the W can be calculated based on these formulas.
According to Table 5, Al–Mn and Al–Cr systems have difference in the value of W, which leads to the different number of Fe atoms replaced by Mn or Cr atoms in β-Al5FeSi phase during solidification, further causes the different morphological Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy with the addition of minor Cr compared to that of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy with the addition of minor Mn. Besides, considering that high W means the lower solubility of solid solution of intermediate phase in alloys, the addition of trace Mn or Cr leads to the increase in the formation tendency of iron-rich phases with different morphology, which results in the decrease in the solid solubility of Fe in the AlSi9Cu3Mg0.19(Fe) alloy. Accordingly, the results are consistent with the above analysis of linear scanning curves of elements. Notably, Al–Fe system has a maximum value of W. Based on the above theory, we can understand like this, Mn or Cr additions cannot totally change the needle-like morphology of Fe-rich phases. These results are in reasonable agreement with previous studies [25, 26].
The combination of Mn and Cr could promote the formation of Chinese script (Fig. 1c), fish-bone (Fig. 1f) or pentagonal morphologies of Fe-rich phases (Fig. 1g). The morphology evolution mechanism of Fe-rich phases could be understood by using the electronegativity. As is known to all, the forming ability of intermetallic compounds between two elements can be decided by their electronegativity difference. Large electronegativity difference means strong bonding force between the two elements and easy formation of the intermetallic compound [48, 49]. The electronegativities of Fe, Mn, and Cr and their differences are listed in Table 4. Among Fe–Mn, Fe–Cr, and Mn–Cr systems, the electronegativity difference between Fe and Mn is the largest, so it is very difficult for Cr to replace the Fe or Mn in α-Al (Fe, Mn) Si. Therefore, few Fe-rich phases with hexagonal morphologies are observed in the AlSi9Cu3Mg0.19(Fe) alloys. Based on the above analysis, a schematic illustration of the morphology evolution mechanism of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy with and without minor Mn and Cr could be established as shown in Fig. 10. However, the combination of Mn and Cr promotes the different morphologies of Fe-rich phases in the low iron-bearing and high Fe level AlSi9Cu3Mg0.19(Fe) alloys, which requires further analysis.
4 Conclusions
The morphological evolution of Fe-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy has been investigated with various contents of Fe, Mn, and Cr. The following conclusions can be drawn from this study.
-
(1)
The trace Mn promotes the formation of Fe-rich phases with coarse Chinese script morphologies by suppressing the development of needle-like iron-rich phases, whereas trace Cr benefits the formation of blocky or hexagonal Fe-rich phases. Most notably, the combination of Mn and Cr could promote the formation of fine Chinese script, fish-bone or pentagonal morphologies of Fe-rich phases.
-
(2)
The morphology evolution mechanism of Fe-rich phases is related to the atom radius and electronegativity of Fe, Mn, and Cr. As adjacent transition elements, Fe, Mn, and Cr have similar atomic structure, which makes it possible to produce the change of iron-rich phase’s macroscopic morphology. Meanwhile, the atom radius and electronegativity differences among Fe, Mn and Cr cause the different morphological evolution of Fe-rich phases.
-
(3)
The additions of trace Mn and Cr could lead to the decrease of the solid solubility of Fe in the AlSi9Cu3Mg0.19(Fe) alloys. Accordingly, the chemical composition of iron-rich phases is altered in the AlSi9Cu3Mg0.19(Fe) alloy with the addition of minor Mn and Cr, which produces the change of macroscopic morphology and microhardness of iron-rich phases in the AlSi9Cu3Mg0.19(Fe) alloy.
References
S.K. Shaha, F. Czerwinski, W. Kasprzak, J. Friedman, D.L. Chen, Mater. Sci. Eng. A 657, 441 (2016)
J.Q. Li, B. Gao, Y.X. Zhuang, K.H. Liu, G.F. Tu, Acta Metall. Sin. (Engl. Lett.) 23, 327 (2010)
L.J. Baruch, R. Raju, V. Balasubramanian, A.G. Rao, I. Dinaharan, Acta Metall. Sin. (Engl. Lett.) 29, 431 (2016)
R. Li, L. Liu, L. Zhang, J. Sun, Y. Shi, B. Yu, J. Mater. Sci. Technol. 33, 404 (2017)
P. Zhang, Z. Li, B. Liu, W. Ding, J. Mater. Sci. Technol. 33, 367 (2017)
C.B. Basak, N.H. Babu, Sci. Rep. 7, 1 (2017)
Y. Zhang, W. Liu, X. Liu, J. Mater. Sci. Technol. 32, 48 (2016)
S. Ferraro, A. Bjurenstedt, S. Seifeddine, Metall. Mater. Trans. A 46, 3713 (2015)
L. Liu, A.M.A. Mohamed, A.M. Samuel, F.H. Samuel, H.W. Doty, S. Valtierra, Metall. Mater. Trans. A 40, 2457 (2009)
S. Terzi, J.A. Taylor, Y.H. Cho, L. Salvo, M. Suéry, E. Boller, A.K. Dahle, Acta Mater. 58, 5370 (2010)
L.A. Narayanan, F.H. Samuel, J.E. Gruzleski, Metall. Mater. Trans. A 25, 1761 (1994)
S.G. Shabestari, Mater. Sci. Eng. A 383, 289 (2004)
E.R. Wang, X.D. Hui, S.S. Wang, Y.F. Zhao, G.L. Chen, Mater. Sci. Eng. A 527, 7878 (2010)
E. Zanini, S. Barison, L. Capra, G. Timelli, F. Voltazza, La Metall. Ital. 104, 3 (2012)
S. Ferraro, A. Fabrizi, G. Timelli, Mater. Chem. Phys. 153, 168 (2015)
L. Sweet, S.M. Zhu, S.X. Gao, J.A. Taylor, M.A. Easton, Metall. Mater. Trans. A 42, 1737 (2011)
Y. Yang, S.Y. Zhong, Z. Chen, M. Wang, N. Ma, H. Wang, J. Alloys Compd. 647, 63 (2015)
H.Y. Kim, S.W. Han, H.M. Lee, Mater. Lett. 60, 1880 (2006)
O. Elsebaie, A.M.A. Mohamed, A.M. Samuel, F.H. Samuel, A.M.A. Al-Ahmari, Mater. Des. 32, 3205 (2011)
L. Ceschini, I. Boromei, A. Morri, S. Seifeddine, I.L. Svensson, J. Mater. Process. Technol. 209, 5669 (2009)
H.Y. Kim, T.Y. Park, S.W. Han, H.M. Lee, J. Cryst. Growth 291, 207 (2006)
E.R. Wang, X.D. Hui, G.L. Chen, Mater. Des. 32, 4333 (2011)
L. Zhang, J. Gao, L.N.W. Damoah, D.G. Robertson, Miner. Process. Extr. Metall. Rev. 33, 99 (2012)
L. Lu, A.K. Dahle, Metall. Mater. Trans. A 36, 819 (2005)
S. Seifeddine, I.L. Svensson, Metall. Sci. Technol. 27, 11 (2009)
S. Seifeddine, S. Johansson, I.L. Svensson, Mater. Sci. Eng. A 490, 385 (2008)
A.M.A. Mohamed, A.M. Samuel, F.H. Samuel, H.W. Doty, Mater. Des. 30, 3943 (2009)
E. Taghaddos, M.M. Hejazi, R. Taghiabadi, S.G. Shabestari, J. Alloys Compd. 468, 539 (2009)
G. Timelli, S. Ferraro, A. Fabrizi, Int. J. Cast Met. Res. 26, 239 (2013)
G. Gustafsson, T. Thorvaldsson, G.L. Dunlop, Metall. Mater. Trans. A 17, 45 (1986)
A. Fabrizi, S. Ferraro, G. Timelli, The influence of Fe, Mn and Cr additions on the formation of iron-rich intermetallic phases in an Al–Si die-casting alloy. Paper presented at the Shape Casting: 5th International Symposium 2014, San Diego Convention Center, San Diego, 16–20 Feb 2014
S. Murali, K.S. Raman, K.S.S. Murthy, Mater. Charact. 33, 99 (1994)
G. Timelli, F. Bonollo, Mater. Sci. Eng. A 528, 273 (2010)
G. Timelli, A. Fabrizi, S. Capuzzi, F. Bonollo, S. Ferraro, Mater. Sci. Eng. A 603, 58 (2014)
S.G. Shabestari, M. Mahmudi, M. Emamy, J. Campbell, Int. J. Cast Met. Res. 15, 17 (2002)
J.Y. Hwang, H.W. Doty, M.J. Kaufman, Mater. Sci. Eng. A 488, 496 (2008)
Y. Liu, S. Shao, K. Liu, X. Yang, D. Lu, Mater. Sci. Eng. A 531, 141 (2012)
L. Balogh, T. Ungár, Y. Zhao, Y.T. Zhu, Z. Horita, C. Xu, T.G. Langdon, Acta Mater. 56, 809 (2008)
D.A. Porter, K.E. Easterling, Phase Transformations in Metals and Alloys, 2nd edn. (Chapman & Hall, London, 1992), pp. 1–29
J.W. Mullin, Crystallization, 4th edn. (Butterworth-Heinemann, Oxford, 2001), pp. 216–218
G. Zhong, S.S. Wu, C. Lin, H. Nagaumi, Mater. Sci. Forum 879, 2286 (2016)
N.A. Belov, D.G. Eskin, A.A. Aksenov, Multicomponent Phase Diagrams (Elsevier Science, Amsterdam, 2005), pp. 287–340
A.M. Samuel, F.H. Samuel, H.W. Doty, Proc. Mater. Sci. 1, 19 (2016)
K.A. Gschaeidner, J. Mater. Sci. 31, 5529 (1996)
M.A. Moustafa, J. Mater. Process. Technol. 209, 605 (2009)
K.A. Gschaeidner, Theory of Alloy Phase Formation (The Metallurgical Society of AIME, Englewood, 1980), pp. 1–39
J.T. Wang, J.Z. Cui, L.X. Ma, J. Xi’an Univ. Arch. Technol. 25, 445 (1993). (in Chinese)
H. Liu, Y. Chen, Y. Tang, D. Huang, G. Niu, Mater. Sci. Eng. A 437, 348 (2006)
W. Hume-Rothery, R.E. Smellman, C.W. Hawworth, The Structure of Metals and Alloys, 4th edn. (Bungay, Institute of Metals, 1962), pp. 101–105
Acknowledgements
This work was supported by the National Key Research and Development Plan (Nos. 2017YFB1103701, 2016YFB0701201, 2016YFB0701203), the National Natural Science Foundation of China (Nos. 51671101, 51464034, 51761037), the Natural Science Foundation of Jiangxi Province (Nos. 20161ACB21003, 20162BCB23013, and 20172BCB22002), the Scientific Research Foundation of the Education Department of Jiangxi Province (No. GJJ150010), the Innovative Funding for Graduate Students in Nanchang University (No. cx2016089), and the Funding of Key Laboratory of Superlight Materials & Surface Technology (Harbin Engineering University), Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195
Rights and permissions
About this article
Cite this article
Qiu, ZQ., Meng, XC., Yuan, QH. et al. Morphological Evolution of Fe-Rich Phases in the AlSi9Cu3Mg0.19(Fe) Alloy with the Addition of Minor Mn and Cr. Acta Metall. Sin. (Engl. Lett.) 31, 629–640 (2018). https://doi.org/10.1007/s40195-017-0688-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-017-0688-y