Abstract
Over the last decades, press hardening or hot forming has become all pervasive for automotive body-in-white design. Press-hardened boron-microalloyed steels are widely used in modern body structure for high safety standard and lightweight achievement. Considering the welding of press-hardened components, some special considerations have to be taken into account compared to conventional deep-drawing steel grades. The welding process results in a significant hardness drop in the heat-affected zone (HAZ) which may lead to a change in failure mode or premature failure. Furthermore, the mechanical properties of the welded joints are influenced by the weld metal hydrogen content. Hydrogen pickup of press-hardened steel may result from the heat treatment or the welding process. Due to the martensitic microstructure and the high strength level, a critical hydrogen content may cause hydrogen embrittlement or hydrogen-assisted cold cracking (HACC). This paper contributes to the determination of the diffusible hydrogen content in resistance spot-welded and gas metal arc-welded joints of press-hardened boron-microalloyed steel. It also promotes the understanding of the effect of diffusible hydrogen on the mechanical properties of those joints. Hydrogen was deliberately introduced during welding by wetting of the sheet surface with hydrogenated fluids. The hydrogen content in the weld metal was quantified by thermal desorption mass spectrometry (TDMS) technique. Finally, the influence on the mechanical properties of the welded joints was determined based on a simple component test. A GMA-welded component was set under a constant static load and evaluated for delayed hydrogen-assisted cracking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The demand for improved fuel efficiency and rising CO2 targets has led to a lot of engineering effort on car body weight reduction since the 1990s [1]. Today, a broad variety of high-strength steel grades is used in modern body-in-white design in order to reduce the weight and enhance the safety of automobiles.
Press hardening (also called hot forming or hot stamping) involving the use of boron manganese-alloyed steel (22MnB5) is a preferred process for complex, high-strength components such as lateral impact protection, door sills, or A and B pillars. The 22MnB5 steel has a ferritic-pearlitic microstructure in as-received condition with a 500-MPa minimum tensile strength and a total elongation of approximately 20 % [2]. These blanks are heated in a roller hearth furnace to austenitization temperature of about 900–950 °C. Forming as well as quenching is then performed in the die tool in the fully austenitic condition. The use of cooled dies guarantees rapid cooling, and thus, a martensitic microstructure with an ultimate tensile strength of about 1,500 MPa is obtained by phase transformation. The critical cooling rate to transform 22MnB5 into martensite is around 30 K/s.
To avoid oxidation (scaling) and surface decarburization, heating of uncoated 22MnB5 steel blanks must be done in a protective atmosphere. Besides this, there are aluminum-silicon and zinc surface-coated 22MnB5 steels, which form a diffusion layer during heat treatment and thus offer scaling protection. The properties of the surface coating, which are affected by furnace temperature and dwell time, have a strong influence on the weldability of these steels [3].
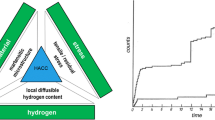
Despite the advantages of the press-hardening process for the production of crash-relevant sheet metal components, one significant point of concern involves the hydrogen embrittlement susceptibility of press-hardened steels. This is with respect to hydrogen pickup during heat treatment in the roller hearth furnace as well as during subsequent welding of press-hardened components. High-strength steel grades are well known to be particularly susceptible to hydrogen embrittlement as well as hydrogen-assisted cold cracking (HACC) due to welding processes. Hydrogen in welds exists in two forms: residual hydrogen, which is captured or trapped by microstructural features in the metal, and diffusible hydrogen, which is free to diffuse in the metal microstructure and is therefore primarily responsible for hydrogen degradation [4]. Diffusible hydrogen either effuses from the metal or migrates to flaws or microcracks in the weld and therefore accelerates cracking. The delayed cracking phenomenon in welded joints of high-strength steels is a combined effect of different parameters (Fig. 1):
-
Hydrogen absorption during welding or the amount of diffusible hydrogen in the weld metal, respectively;
-
A martensitic structure with high hardness and strength, but low ductility; and
-
A stress state of the welded joint caused by tensile residual stresses and/or externally applied loads [5].
The major welding processes for press-hardened steels in automotive industry are resistance spot welding, gas metal arc welding, and laser beam welding [6]. Sources of hydrogen when welding press-hardened components may be hydrogenated surface layers at the weld joint due to condensate or torch leaking water as well as remains of anticorrosion oil or paint on the steel blanks. Hydrogen input in the weld pool can also be a result of moisture in the shielding gas or air turbulence. In addition, the welding consumable and the base metal can have significant hydrogen content due to fabrication (e.g., heat treatment) and thus may also be a source of hydrogen in the weld. However the latter is not discussed within the scope of this study.
The press-hardened 22MnB5 steel is characterized by a high-strength martensitic structure, but offers only low ductility and toughness with an elongation less than 6 %. It is therefore particularly susceptible to hydrogen embrittlement and hydrogen-assisted cold cracking when sufficient levels of diffusible hydrogen and tensile stresses are present at the same time. The stresses in welded joints of 22MnB5 steel are essentially determined by residual stresses due to manufacturing processes (e.g., welding) along with externally applied stresses caused by assembly or handling of the components.
Based on these considerations, this study contributes to the determination of the hydrogen input when welding press-hardened steel and the evaluation of the resulting hydrogen-assisted cracking potential of welded components.
2 Determination of diffusible weld metal hydrogen content
The determination of the hydrogen content in arc weld metal is specified in ISO 3690:2012 [7]. The document gives a standardized procedure for the production of a weld sample in the form of a rapidly quenched single bead-on-plate deposit using a specified welding jig. The hydrogen input during welding is referred to the deposited filler metal weight. Therefore, the weight of each test piece has to be determined before and after welding, and the balance is used as a reference weight.
The ISO standard distinguishes between the diffusible hydrogen HD, milliliters per 100 g of the deposited weld metal (refers to the deposited filler metal weight only), and the diffusible hydrogen HF, parts per million of the fused weld metal, which also includes fused base metal material. The diffusible hydrogen in the fused weld metal H F is thus calculated as by the following equation:
where AD and AF are the cross-sectional areas of the deposited metal and the fused metal, respectively (Fig. 2).
Analytical determination of diffusible hydrogen is required in terms of concentration in the carrying medium. This is why there is a necessary reference weight for the determination of diffusible hydrogen in weld metal, which is either the deposited filler metal weight or the fused metal weight (as seen above). However, this approach is specified in the ISO standard for arc welding processes with filler metal only. There is currently no standardized procedure for welding processes without filler metal like resistance spot welding or laser beam welding. Therefore, within the scope of this study, another approach had to be considered to determine diffusible hydrogen in spot-welded joints of 22MnB5 steel.
In [8], the diffusible hydrogen content in laser beam-welded joints of high-strength steels was determined by referring to the fused weld metal weight as derived from the ISO standard. For that purpose, the cross-sectional area of the weld and the weld length were used. A similar approach was applied in the current study for the determination of diffusible hydrogen in resistance spot welds. For resistance spot welding, the fused weld metal correlates to the weld nugget volume. The weld nugget has approximately the form of an ellipsoid of revolution with a pair of equal semi-axes (a) and a distinct third semi-axis (b) which is an axis of symmetry (Fig. 3). The semi-axes were measured in the cross section at the center of the weld nugget, and the nugget volume was approximated as
The weld nugget weight, which was used for H F determination, was then calculated as by the following equation:
where ρ St is the density of 22MnB5 steel with 7.85 g/cm3 [9].
There are several distinct analytical methods for the quantification of hydrogen in metallic materials. Within the scope of this study, the thermal desorption mass spectrometry (TDMS) technique was used, which is a widely established procedure and allows the quantitative determination of very small concentrations of hydrogen [10]. The diffusible hydrogen was released from the sample by hot solid extraction at a temperature of 400 °C as derived from the ISO standard. The hydrogen particles were then carried by an inert gas (pure nitrogen 5.0) into the quadrupole mass spectrometer, where they were ionized and accelerated through the mass analyzer into the detector. The integral of the resulting ion current signal correlates to the detected hydrogen content in the sample. The analyzer used in this study was the Bruker G8 GALILEO ON/H with a mass spectrometer IPI ESD 100.
3 Experimental
A road map of the experimental approach of this study is given in Fig. 4 and will subsequently be described in detail. The material used was a boron manganese-alloyed 22MnB5 steel with an aluminum-silicon surface coating (22MnB5 + AS150) and a sheet thickness of 1.5 mm. The chemical composition is given in Table 1. Press hardening was done in a laboratory-scale facility. The as-received material with a ferritic-pearlitic structure was heat-treated at an austenitization temperature of 950 °C for 6 min and subsequently formed and quenched in a die tool reaching a cooling rate of more than 30 K/s. The aluminum-silicon coating, which was applied by hot dipping into a molten aluminum bath before press hardening, serves as a scaling protection during austenitization inside the furnace and is transformed into a Fe-Al-Si alloy diffusion layer during heating. After press hardening, an additional dehydrogenated heat treatment at 180 °C for 20 min was performed to release all diffusible hydrogen from the material, which may have resulted from previous manufacturing processes. The press-hardened material had a 0.2 % yield strength of 1,007 ± 30 MPa, an ultimate tensile strength of 1,466 ± 11 MPa, and a total elongation of 5.9 ± 0.3 %.
3.1 Determination of hydrogen input when welding
The dimensions of the hot solid extraction analyzer used imposed some limitations to the sample size and geometry. As a reasonable compromise between the size of the infrared furnace (inner diameter of 30 mm) and an easy handling when welding, the press-hardened steel sheets were mechanically cut to relative small samples for resistance spot and gas metal arc welding as shown in Fig. 5.
To represent possible sources of hydrogen when welding press-hardened steels, the surface of the samples was deliberately wetted with hydrogenated fluids. Welding of the 22MnB5 steel was done in three sheet surface conditions: acetone-cleaned surface, water-wetted surface, and oil-wetted surface. The water was hand-applied by an aerosol dispenser, while the hydrocarbon anticorrosion oil was deposited by a roll-on applicator. The average amount of fluid on the sample surface was determined by weight as approximately 17 and 10 mg/cm2, respectively. The chemical composition of the used filler metal is given in Table 2, while the welding parameters for gas metal arc welding and resistance spot welding are listed in Table 3. The gas metal arc (GMA) welding was done using the synergic cold metal transfer (CMT), which is an established modified dip arc welding process for thin metal sheets in automotive industry [11].
Based on the ISO standard [6], the welded samples were immediately transferred into liquid nitrogen after the welding process. Before hydrogen measurement, the samples were removed from the storage coolant, raised to room temperature, rinsed with acetone, and air jet dried.
Determining the fused weld metal weight of the welded joints, which served as reference weight for the quantification of diffusible hydrogen in the weld metal (HF), was done as described above (Section 2). The GMA-welded samples were weighted before and after welding. For the spot welds, the measured cross-sectional area in conjunction with Eqs. 2 and 3 was used.
3.2 Evaluation of hydrogen-assisted cold cracking
The influence of hydrogen input when welding press-hardened 22MnB5 steel on the mechanical properties of the welded joints was investigated by a simple component-based test. The test components consisted of a C-shaped lower part and a flat upper part, both of press-hardened 22MnB5 + AS150 steel (Fig. 6).
The test was performed for gas metal arc welding only. The components were successively joined with a continuous lap joint on each side (Fig. 7, left). As before, the welding was done with acetone-cleaned sheet surfaces as well as with deliberate hydrogen input by wetting the sheet surface with water or anticorrosion oil. There were three welded components for each sheet surface condition.
After welding and cooling down to a temperature of 200 °C, a constant static test load was imposed on the components for a test duration of 1 week (Fig. 7, center). Afterwards, the HACC susceptibility of the components was assessed by visual observation of the welded joints.
Since there was no crack occurrence after the whole period of time, an additional tensile test was performed (Fig. 7, right). The maximum tensile force developed in the test component before rupture was measured.
4 Results and discussion
4.1 Determination of fused weld metal weight
The results from the determination of the fused weld metal weight for the GMA and spot-welded joints are presented in Fig. 8 (left) along with optical micrographs of the weld cross sections (Fig. 8, right). The fused weld metal of the spot-welded samples was three times less than that of the GMA welds. For each welding process, the average value of more than ten welds was taken as the reference weight for the H F determination (see Eq. 1). The surface condition (acetone-cleaned or wetted with hydrogenated fluids) had no influence on the fused weld metal weight. No defects, particularly porosity, were found in any of the welds inspected.
4.2 Determination of hydrogen in weld metal by TDMS
The detected diffusible hydrogen H F of the GMA-welded samples is displayed in Fig. 9 (left). The results illustrate that the hydrogenated fluids applied on the sheet surface before welding had a significant influence on the diffusible hydrogen in the fused weld metal. Wetting the sheet surface with water or anticorrosion oil led to a considerably higher diffusible hydrogen content in the welded joints.
GMA welding with previous cleaning of the sheet surfaces with acetone, i.e., without any additional hydrogen source when welding, resulted in a diffusible hydrogen content of approximately 3.5 ppm. The detected diffusible hydrogen probably results from greasy contamination of the filler metal, e.g., due to drawing grease on the welding wire. A considerably higher hydrogen content was induced by wetting the sheet surface with water or anticorrosion oil. These samples exhibited diffusible hydrogen in the weld metal of 7.2 and 7.5 ppm respectively, which is more than twice as much as in the cleaned samples.
While the GMA-welded samples generated a clear peak in the ion current signal of the mass spectrometer, as illustrated in Fig. 9 (right), there was no such peak analyzing the spot-welded samples. The signal was, irrespective of the applied hydrogen source and the size of the weld nugget, so marginal that a quantitative determination of diffusible hydrogen was not possible. As displayed in Fig. 10, only very low diffusible hydrogen may be assumed by means of the signal received, but it is hardly more than the background noise. A Savitzky-Golay smoothing was performed to get a qualitative comparison between the spot-welded samples. There possibly is a slight peak in the signal when wetting the sheet surface with water or anticorrosion oil as compared to the cleaned samples (Fig. 10).
The results of the determination of diffusible hydrogen H F in the fused weld metal reveal a significant difference between the GMA- and spot-welded samples. With equal hydrogen sources, the hydrogen input in the weld is much higher for GMA welding than that for resistance spot welding. During arc welding, the hydrogen dissociates in the high-temperature regions of the welding arc and is highly absorbed by the weld pool as well as the large surface of the liquid drop [12]. On the contrary, resistance spot welding is not an open-arc process and the weld pool surface is much smaller, but with considerably higher heating and cooling rates. Therefore, much less hydrogen is absorbed during resistance spot welding. Overall, there is a variety of parameters which influences the hydrogen input of the different welding processes, such as the highly distinct energy input, the different welding and cooling times, and particularly the time-dependent size of the weld pool surface in GMA and resistance spot welding.
4.3 Determination of HACC susceptibility by a component-based test
The visual observation of all welded test components (see Figs. 6 and 7) after holding the applied load for 1 week showed no cold cracking in the GMA-welded lap joints. The results of the subsequent destructive testing in the form of a tensile test conducted on all test components are presented in Fig. 11.
The highest maximum tensile force of approximately 16.9 kN was achieved by the components which where welded with a cleaned sheet surface. Wetting the sheet surface with hydrogenated fluids before welding resulted in a significantly lower maximum tensile force of 11.1 kN for water and 9.8 kN for anticorrosion oil.
The components with the acetone-cleaned sheet surface ruptured in the high-temperature tempering zone of the HAZ which usually occurs when welding 22MnB5 steel and is characterized by considerably lower hardness values [13]. Whereas, the failure of the wetted components occurred directly along the fusion line of the GMA-welded joints.
The results of the tensile test clearly illustrate that there is a correlation between the application of hydrogenated fluids on the sheet surface before welding and the achieved maximum tensile force of the welded joints. Although there was no external hydrogen-assisted cold cracking after welding and applying a constant load for a test period of 1 week, the results of the tensile test suggest that hydrogen diffused into the weld metal microstructure and to a certain extent resulted in embrittlement of the welded joints.
Additional scanning electron microscopy (SEM) was performed on the fractured surfaces of the tensile tested components. The topography of the acetone-cleaned components, which achieved the highest maximum tensile force and failed in the tempered zone of the HAZ, shows a ductile fracture mode with typical shear dimples (Fig. 12a). In contrast, the wetted components with a considerably lower tensile force exhibit a brittle intergranular fracture surface. With maximum amplification, gaping grain boundaries are visible as a typical feature for hydrogen embrittlement (Fig. 12b, c).
5 Summary
The high-strength 22MnB5 + AS150 steel was welded with deliberate hydrogen input in the form of hydrogenated fluids on the sheet surface before GMA and resistance spot welding. The diffusible hydrogen in the fused weld metal was quantified by TDMS. The resulting hydrogen-assisted cold cracking (HACC) potential of the GMA-welded joints was determined by a simple component-based test.
The conclusions of this study may be summarized as follows:
-
1.
The fused weld metal weight of the spot-welded joints was determined by a geometrical approximation in the form of an ellipsoid of revolution and used as the reference weight for the determination of the diffusible hydrogen H F in the weld metal as derived from the ISO 3690 standard.
-
2.
For GMA welding, the detected diffusible hydrogen in the fused weld metal was considerably higher when wetting the sheet surface with hydrogenated fluids before welding. The diffusible hydrogen when welding with deliberate hydrogen input was twice as much as when acetone cleaning of the sheet surface before welding.
-
3.
No diffusible hydrogen content could be detected in the spot-welded joints whether the sheet surface was wetted with hydrogenated fluids or not. The detected ion current signal was for all samples hardly more than the background noise of the mass spectrometer.
-
4.
The performed simple component-based test showed no external hydrogen-assisted cold cracking in the GMA-welded joints. However, the components welded with previous wetting of the sheet surface with hydrogenated fluids achieved significantly lower maximum tensile forces in the subsequent tensile testing.
-
5.
SEM observation of the fractured surfaces of the tensile tested components showed evidence of hydrogen embrittlement for the samples, which were wetted with hydrogenated fluids before welding.
References
Prange W, Schneider C (2001) Automobile lightweight initiatives of the international steel industry. Stahl Eisen 121(7):23–29 (in German)
Hoff C (2007) Investigation of the influence of process variables during press hardening of high-strength steel 22MnB5. Dissertation, University Erlangen-Nürnberg (in German)
Fan DW, De Cooman B (2012) State-of-the-knowledge on coating systems for hot stamped parts. Steel Res Int 83(5):412–433
Wendler-Kalsch E (1986) Basis and mechanisms of H-induced corrosion of metallic materials. Kuron D (Ed.). Wasserstoff und Korrosion, Irene Kuron, Bonn pp. 8–47 (in German)
Schulze G (2010) Welding metallurgy—ferrous–nonferrous metals. 4th Edition, Springer, Berlin (in German)
Jüttner S (2011) Materials and joining technology developments in body-in-white design. Proceedings of Joining Technology Congress, Magdeburg, Germany, 12 May 2011 (in German)
ISO 3690 (2012) Welding and allied processes—determination of hydrogen content in arc weld metal
Laser beam welding of innovative, high-strength steels with respect to hydrogen and cracking susceptibility (LaHRissa), Final Report, BMBF-FH3-Joint research project, 2007 (in German)
Timm H (2008) Efficiency of materials in lightweight body design. Proceedings of 10th VDA technical congress, Ludwigsburg, Germany, 2–3 April 2008 (in German)
Bergers K, deSouza Camisao E, Thomas I et al (2010) Determination of hydrogen in steel by thermal desorption mass spectrometry. Steel Res Int 81(7):499–507
Schlott S (2011) 2nd international congress—new concepts and joining technologies in automotive industry. ATZ Prod 4(2):8–9 (in German)
Mikula J (1994) The role of hydrogen in the initiation of cold cracking (Part I). Weld Int 8(10):761–765
Schwedler O, Zinke M, Jüttner S (2012) Properties of welded joints of press hardened automotive components with respect to hydrogen embrittlement. Proceedings of DVS Congress, Saarbrücken, Germany, 17–18 September 2012 (in German)
Acknowledgments
These tests were funded by the Federal Ministry of Economics and Technology (BMWi) assisted by the Arbeitsgemeinschaft industrieller Forschungsvereinigungen (AiF—industrial research consortium) “Otto von Guericke” e. V. (AiF-no. 17.016 BR/EFB-no. 08/210) and supported by the Europäische Forschungsgesellschaft für Blechverarbeitung e. V. (EFB—European Research Association for Sheet Metal Working). The authors gratefully acknowledge the support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Doc. IIW-2434, recommended for publication by Commission II “Arc Welding and Filler Metals.”
Rights and permissions
About this article
Cite this article
Schwedler, O., Zinke, M. & Jüttner, S. Determination of hydrogen input in welded joints of press-hardened 22MnB5 steel. Weld World 58, 339–346 (2014). https://doi.org/10.1007/s40194-014-0119-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-014-0119-x