Abstract
This review presents a synopsis of the current research in the field of peripheral and central neurostimulation for dysphagia and its relationship to advancing our knowledge in the field of human swallowing neurophysiology. Advances in the field of neurorehabilitation of motor systems in general have led to a wide range of approaches and are currently under rigorous investigations. Our field of dysphagia neurorehabilitation is sharing some of the formulated hypotheses and concepts for functional rehabilitation with neurostimulation. Importantly, results from studies looking into the cortical and subcortical control of human swallowing have been used as working hypotheses in the dysphagia neurorehabilitation field. For instance, based on our knowledge that peripheral and central inputs influence the swallowing network, experimental paradigms targeting swallowing neural reorganization have been trialled recently, prior to their translation into clinical practice for dysphagia rehabilitation. Here, we highlight the recent findings in the past year with the intention to stimulate potential research questions not yet investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The science of ‘dysphagia rehabilitation’ is continuously evolving, both in research and clinical practice, mainly due to two influential frameworks. Firstly, the framework of evidence-based practice, which ensures that we promote health and provide care by integrating the best available evidence. Secondly, the emerging role of neuroplasticity, which allows us to understand ‘how’ and ‘why’ positive long-lasting changes in neural pathways and synapses can be promoted by rehabilitation. Both concepts have evolved increasingly in recent decades. As a result of the convergence of these ideologies, neurostimulation approaches in dysphagia rehabilitation have now surfaced. Promising published evidence of the past year is reviewed in this context, together with some questions and future directions that remain to be answered and investigated. Rather than attempting to produce a comprehensive systematic review, here we provide information about the breadth of neurostimulation in rehabilitation, how the dysphagia field is currently incorporating these concepts into working hypotheses by exploring different forms of neurostimulation, followed by a review of the current evidence on neurostimulation in dysphagia rehabilitation.
‘Exposition’: Neurostimulation in Rehabilitation
Undoubtedly, the range of ‘neurostimulation approaches’ in rehabilitation sciences is increasing. Initially, the term neurostimulation referred to approaches in neurological rehabilitation such as deep brain stimulation for Parkinson’s disease and vagal stimulation for epilepsy. Nevertheless, studies on the neurophysiological properties of other systems [1] such as the limb function [2] or visual cortex [3] have pioneered the search for components and networks (neural and neuronal) of the peripheral and central nervous systems that could be modulated and harnessed for therapeutic purposes in the rehabilitation of neurological disease/impairment. For instance, brain stimulation with transcranial magnetic stimulation (TMS) has been utilised for diagnostic and therapeutic purposes related to neurological diseases [4]. Transcranial magnetic stimulation is a safe and non-invasive technique which uses strong electric currents delivered though a coil of wire to generate rapidly changing high-intensity magnetic field. Perpendicular currents of sufficient strength are generated to depolarize neuronal elements and evoke electromyographic responses on the targeted musculature [4].
Widely explored in the past two decades, brain stimulation techniques have proven their potential to modulate brain activity. These neurostimulation techniques include the repetitive TMS (rTMS), during which TMS pulses are delivered at specific frequencies to either excite or suppress neuronal processes (depolarizing or hyperpolarizing neurons). Research with neuroimaging following stroke showed a period of critical increase in activity within the intact limb primary motor cortex (MI) (unaffected hemisphere) [5] corresponding behavioural gains in limb function [6]. In stroke patients, following the observation of abnormal interhemispheric inhibition from the unaffected to the affected MI [5], inhibitory rTMS (low-frequency) has been used to suppress the cortical excitability of the unaffected MI in stroke patients with hemiparesis, in an attempt to restore excitatory interhemispheric balance [7–9], while excitatory rTMS has been used to potentiate the excitability of the affected MI [10].
Transcranial direct current stimulation (tDCS) is another neurorehabilitation technique in which a weak electric current (approximately 1–2 mA) is passed over the brain. The effects are dependent on a combination of parameters such as the current strength, duration of stimulation and electrode montage [11]. It appears to be both safe and well tolerated. Transcranial DCS can alter brain excitability with further behavioural effects depending on the site of stimulation in stroke patients [12]. As for the translational aspect of this neurostimulation technique, tDCS offers advantages if used in the clinical setting, since the equipment is small, relatively cheap and portable.
Since the mid 1960s, peripheral neurostimulation has shown encouraging effects within the rehabilitation field. It has been used in several forms and disciplines, from physiotherapy to management of refractory pain and migraine management. Peripheral neurostimulation with different stimuli (but mainly electric) provides a dynamic afferent input. Electrical stimulation can elicit an action potential in nerve axons through the delivery of an electric charge to an axon, inducing localised polarisation. When applied to motor neurons, this can be used to generate muscle contractions (musculo-cutaneous reflex), with specific components of this reflex being at a latency consistent with activity in a transcortical pathway. If electrical stimulation is applied to ascending axons of sensory neurons, studies have shown potential contribution to cortical motor reorganization. Peripheral electrical stimulation has been shown to have a direct effect on intracortical inhibition [13]. In neurorehabilitation, electrical stimulation from the periphery can be used with the end result of increasing dynamic synchronisation activity between cortical sensorimotor areas and muscle activity during voluntary movements [14–16].
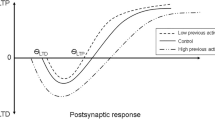
It is of interest to mention at this stage that the efficacy of the different neurorehabilitation approaches in the literature is subject to supportive evidence that any long-term beneficial effects are due to changes in neuronal activity. For instance, the cortical motor neuronal activity is subject to changes of the major excitatory neurotransmitter (glutamate) and major inhibitory neurotransmitter (gamma-aminobutyric acid, GABA). The balance between these neurotransmitters plays a vital role during the acquisition of new skills. Moreover, one of the most important concepts in rehabilitation is long-term potentiation (LTP) and its role in the induction of plastic changes [17]. LTP is a long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously. It is one of several phenomena underlying synaptic plasticity, the ability of chemical synapses to change their strength. The reduction in synaptic strength is called long-term depression (LTD). The neurostimulation approaches reported in the literature attempt to drive neuronal networks changes and to mimic these LTP/LTD effects, previously observed in animal studies.
Ideally, non-invasive brain stimulation and peripheral stimulation could serve as complementary or adjunct therapeutic modalities, boosting adaptive neurophysiological processes following lesions and suppressing or even preventing maladaptive neural damage [18]. Latest reviews on the effects of neurostimulation targeting the brain, the peripheral nervous system, or both the CNS and PNS in combination in relation to different functions, (i.e. speech, limb movement, language) conclude that neurostimulation has the capacity to promote positive rehabilitation outcomes, and that further evidence is needed [19].
‘Overture’: Neurostimulation in Dysphagia
Swallowing is the output of a very precise multidimensional interplay between different brain areas, translated into a well-tuned coordinated muscle activity. The working hypothesis for the use of neurostimulation in dysphagia rehabilitation derives in part from work in primates and subprimates [20–26] and others, which has provided evidence for the effects of descending cortical command signals on brainstem pathways in regulating the swallowing mechanism.
The swallowing neural network regulating the oropharyngeal midline structures is different from models of limb functions in several accounts but most importantly with regards to (a) the existence of a cortico-bulbar-cortical loop [20], (b) the bilateral non-competitive interhemispheric cortical processes, showing a form of dominance as opposed to strict ‘laterality’ and the less competitive hemispheric interplay compared to the limb model [27], and (c) the importance of the afferent inputs in swallowing. These important parameters warrant consideration in working hypotheses regarding the induction of changes not only with neurostimulation, but also with experience-dependent and behavioural rehabilitation in dysphagia. Recently, physiological studies with electrostimulation of superior laryngeal nerve (SLN) in animals have provided additional information about the relationship between laryngeal sensory input and the jaw opening reflex following swallowing [28] and the role of SLN sensory inputs in aspects of pharyngeal swallowing and esophageal reflexes [29].
In summary, networks of neurons in areas of interest for swallowing, i.e. brainstem central pattern generator and fiber tracts along the projection from cortical to brainstem levels, may be amendable to the use of peripheral stimuli from the oropharynx or central manipulation of cortical neuronal processes within the representations of swallow-related musculature in humans, as observed in animal studies. Peripheral stimuli used in neurostimulation techniques for swallowing rehabilitation attempt to affect or modulate the ‘threshold’ for the fine-tuned drives and processes with the ultimate result to increase synaptic output of these populations of neurons [30]. Alternatively, in the case of neurological damage, the end-result might well be the restoration or unravelling of plastic capacities of the brain that will allow behavioural gains. Transcranial magnetic stimulation has also been heavily used in dysphagia rehabilitation, in several forms (both excitatory and inhibitory). However, it is important to state here that TMS on cortical areas in a subthreshold modality does not elicit reflexive swallowing, but simpler responses are excited in swallowing musculature.
Working Hypotheses in Dysphagia Rehabilitation
As mentioned before, based on pioneering work in animal studies, there are now different neurostimulation approaches in dysphagia rehabilitation. It is also now realised that neurostimulation approaches should be of the optimal dose, intensity, frequency, repetition and duration for the swallowing network to adapt in a positive manner [31]. Swallowing network circuits are sensitive pools of neurons interconnected to coordinate the vital sensorimotor function of eating. Information about how neuronal processes for neuroplastic changes can be amended by neurorehabilitation is therefore important.
Neuroimaging and neurostimulation studies have provided insights into the activation patterns of the swallowing sequence and muscle activities (for reviews [32, 33]). The most consistent areas that are activated in these neuroimaging studies include the primary sensorimotor cortex, sensorimotor integration areas, the insula and frontal operculum, the anterior cingulate cortex, and supplementary motor areas (SMAs). We also have evidence for the patterns and processes of brain adaptation to brain lesions, in particular hemispheric acute and focal (stroke) [34], but less clearly defined for the diverse and sparse neurodegenerative models [35, 36], although recently we observed some evidence for patterns of cortical adaptation in Parkinson’s disease when ‘on’ medication compared to healthy aged-matched controls [37•]. For stroke in particular, studies with TMS showed that the cortical map representation of the pharyngeal musculature in the undamaged hemisphere markedly increased in size in dysphagic stroke patients who recovered swallowing, whilst there was no change in patients who had persistent dysphagia or in patients who were non-dysphagic throughout [34]. These observations imply that over a period of weeks or months, the recovery of swallowing after stroke may be reliant on compensatory strategies of cortical reorganisation, through neuroplastic changes that are mainly observed in the undamaged hemisphere, which has been observed in an fMRI study recently [38].
During the past year, research in dysphagia neurostimulation has been built upon the results or used the framework provided mainly from the following studies: (a) stimulation of the pericentral cortex or the frontal cortex can evoke swallowing in primates [24, 26, 27], (b) electrical stimulation of the pharyngeal branch of glossopharyngeal nerve can elicit the swallowing reflex [39], (c) bulbar-cortical-bulbar feedback loops participate in the pharyngeal phase of swallowing [20, 25], (d) swallow-related neurons in the medulla are influenced by spatial summation of afferent stimuli [40, 41], and lastly (e) the repetitive electrical stimulation of SLN can evoke swallowing reflex in a number of animal species [26].
In this review, we are only discussing literature with peripheral (electrical and non-electrical), central-brain neurostimulation and a combination of both peripheral and central in patients (Fig. 1), acknowledging the fact that behavioural exercise approaches are also being promoted through rigorous research in providing evidence for driving neurophysiological changes [42–45].
Peripheral Neurostimulation
There are several different neurostimulation approaches delivering peripheral neurostimulation in patients. Although the initial work in animal studies produced more ‘stereotypical’ responses [21, 39, 46], there is a marked variability in the elicitation of the swallowing reflex in humans. Some of the neurostimulation approaches, not utilising direct electrical stimulation are thermal, tactile, gustatory and air-puff stimulation.
Air-puff pulse stimulation is a promising technique, employing bilateral repeated air-puffs to the posterior peritonsillar regions, resulting in an urge to swallow, as already investigated in young [47] and older [48] healthy adults. This year, a case-series proof-of-principle study was published showing increased rates of saliva swallowing in dysphagic stroke patients when bilateral air-puff stimulation was applied [49•]. Neurophysiological studies using fMRI to examine the effects of air-puff stimulation with fMRI showed bilateral brain activation within primary somatosensory and motor cortices, thalamus, SMA and polymodal areas in the past [50, 51]. Further work with controlled trials to determine the clinical efficacy of this promising technique in larger number of dysphagic patients is anticipated over the coming years.
There is also recent evidence for potential changes in neurophysiological processes by gustatory afferent stimulation. As mentioned before, afferents in the oropharyngeal areas enable the elicitation of the swallowing reflex while transferring information via mechanoreceptors, taste receptors, chemical receptors etc. In several research studies afferent pathway stimulation of the swallowing network has been utilised as a means to aid swallowing performance [52]. A recent example of the effects of gustation on swallowing is the use of cannabioids in the animal literature to facilitate the swallowing reflex elicited by SLN electrical stimulation [53]. Another such example is a recent study in healthy participants with carbonated water swallowing, which showed that carbonated liquids had a direct effect on reaction latencies of the pharyngeal swallowing and increased the number of correctly performed challenged swallows (swallows within a pre-determined time-window) [54]. Moreover, there is evidence that orophangeal afferents express the polymodal transient receptor potential vanilloid 1 (TRPV1) [55], projecting to the supramedullar structures and to the nucleus tractus solitarius in the brainstem, allowing the involuntary onset of swallow response and modulating volitional swallowing. Recently, a large case-series study observed that stimulation of TRPV1 by capsaicinoids strongly improved safety and efficacy of swallow and shortened the swallow response in older patients with dysphagia (mixed stroke, neurodegenerative and aged patients with dysphagia) [56•]. These findings suggest the clinical potential of capaicinoids in dysphagia rehabilitation. We anticipate larger and randomised trials to validate the efficacy of the stimulation of TRPV1 as a pharmacologic strategy for oropharyngeal dysphagia management, as well as further neurophysiological outcome measures to assess the underlying mechanism in various dysphagic populations.
Interestingly, the majority of published clinical studies in the past year employed neuromuscular electrical stimulation (NMES), which uses an externally applied electrical current on the area of the anterior neck and/or in the suprahyoid area at motor or sensory threshold levels. The rationale behind this technique is that stimulation of the muscle fibres can ‘re-educate’ the functional swallow-related muscle contraction patterns [57, 58]. In the past year, the technique has been applied in stroke [59–61 and others], head and neck cancer [62], Parkinson’s disease [63–65], paediatrics [66] and mixed aetiologies patients populations [67–69]. The results of these studies, which employed various study designs (case series, cohorts, RCTs) are not conclusive since the stimulation parameters used across the studies were different. Moreover, researchers have used various combinations of stimulation and behavioural interventions in their protocols (i.e. effortful swallowing [70]). This heterogeneity is the direct outcome of the insufficient preliminary background work on the different parameters, such as the stimulation repetitions, optimal duration of therapeutic regimen, dosage and electrode positioning [71•, 72]. Nevertheless, recent evidence is surfacing (physiological [73–76] and neurophysiological [77, 78]). Meanwhile, a recent metanalysis [71] showed that NMES is not superior to traditional swallowing therapy in clinical functional outcomes in stroke population, but there may be some benefit when applied to dysphagic patients of varied disease aetiologies. Lastly, an example of rigorous investigation on the effects of combined volitional swallowing and NMES to the submental area has been already observed [75, 78]. We anticipate increasing our understanding of this technique in the future with neuroimaging studies, such as the one by Humbert and Joel [79], showing that electrical stimulation on the anterior neck bilaterally at a (low) sensory level administered during swallowing, activated fewer areas of the motor cortex and the insula.
In the past year, we also observed additional evidence for the effects of pharyngeal electrical stimulation, employing electrical stimulation to the afferents in the mid-pharyngeal area. Interestingly, this neurostimulation approach has been included in the latest Cochrane review [80]. This form of neurostimulation has been rigorously investigated over the course of recent decades on individuals with nornal swallowing and dysphagic patients. Parameters such as duration of the treatment, repetitions, dose, frequency, intensity have been investigated [81–83] for the application of this technique in acute dysphagic stroke patients. Moreover, the effects of this neurostimulation technique on swallowing neural mechanism have been investigated with neurophysiological outcome measurements (fMRI [82]), showing an overt increase in activation of sensorimotor cortex compared to no stimulation. Further neurophysiological evidence was gathered with TMS [81, 83], physiological [81, 83] and clinical functional measurements [83]. Evidence from a recent RCT favors the use of this technique once a day for 3 days only in acute stroke patients (up to 2 weeks post-stroke) as an adjunct to mainstream swallowing therapy [83]; indeed we anticipate the results from long-term follow-up studies in acute stroke patients and larger multi-center trials. Additionally, a pilot randomised controlled study with pharyngeal electrical stimulation applied for five consecutive days on patients with multiple sclerosis, showed significant improvement in physiological and functional measures (penetration-aspiration scores) [84]. However, the duration of the treatment chosen by the research team was arbitrary, while the inclusion criteria for the patients with multiple sclerosis (MS) with regards to the exact period of clinical manifestation of dysphagia (relapsing-remitting vs. progressive vs. secondary progressive MS) requires further investigation.
Overall, the above reviewed approaches utilise the results and evidence from the same spectrum of animal studies as a means to validate the use of enhanced input to the afferent pathways of swallowing. However each of these techniques employ different mediums, modalities and localisation of electrode placements (for those with electrical stimulation). One of the questions usually raised at this point is whether each of the aforementioned different techniques would produce different neurophysiological and functional gains. However, these neurostimulation techniques are different in nature and the research teams used different primary and secondary outcome measures, except for penetration-aspiration scores. Lastly, the level of evidence of these studies (cohort studies, RCT) certifying the efficacy of the approaches is not the similar to allow comparisons.
Central Neurostimulation
In recent years, there has been an increase in the number of studies using central neurostimulation experimentally for dysphagia rehabilitation. The motor cortex, which mediates motor execution, has been the focus of the published research on central neurostimulation approaches. The neurostimulation technique mostly used has been repetitive TMS, which can excite a number of descending volleys from the area stimulated. The ultimate goal in these recent studies in dysphagia has been to manipulate the cortical neuronal processes and assist in the reorganisation of cortical representations in dysphagia following stroke. What is clearly observed in the literature is that researchers have been utilising rTMS while following different working hypotheses. In addition, different cortical musculature representations, i.e. representations of upper oesophageal sphincter [85], mylohyoid [86, 87], pharyngeal [88•], have been targeted with varying parameters or intensities.
In the absence of a metanalysis, here we examine those trials with the highest level of evidence in the past year (randomised controlled trials, RCTs). Park et al. [88•] have used physiological and functional outcome measurements to study the effects of contralesional 5 Hz (excitatory) rTMS delivered for 2 weeks to the pharyngeal MI, based on observations made earlier for the role of this hemispheric projection in the recovery of swallowing in stroke patients [34] and the evidence for the effects of 5 Hz rTMS restoring the inhibitory effects of a ‘virtual lesion’ when applied contralaterally [89]. Park et al. [88•] concluded that this neurostimulation technique can produce beneficial changes in aspiration and pharyngeal residue 2 weeks post-treatment. In another RCT, Kim et al. [87], used functional measurements (functional scales and penetration-aspiration scale) to measure changes in acute and subacute dysphagic stroke patients following 2 weeks of 5 Hz rTMS to the ipsilesional mylohyoid cortical representation delivered at low and high frequencies and including a sham arm. They concluded that both outcome measurements improved significantly in the low frequency group.
It is important to mention that the above RCT studies with small sample sizes showed changes in dysphagia measurements, but used different parameters in their protocols. Some of those parameters are the following: (a) intensities, (b) location of neurostimulation (affected vs. non-affected), (c) muscle cortical projection/representation targeted, and (d) functional measurements of dysphagia rehabilitation (apart from a single one measurement, penetration-aspiration scores were used in both Kim et al. [87] and Park et al. [88•]).
Similarly, the effects of tDCS have been also investigated in dysphagic stroke population, but again the results are inconclusive when all studies are taken together. Although as previously, studies in healthy swallowing have been conducted in the past [90], researchers have used different neurostimulation parameters for their studies in patients, without clear rationale for the dosage of the neurostimulation approach. A single-blinded RCT with 20 stroke patients randomised to either anodal stimulation of the ipsilesional or sham stimulation showed beneficial functional outcomes, when used as an adjunct to traditional swallowing therapy [91]. The parameters in this trial were again different to the parameters used in earlier case-controlled studies in patients (i.e. affected vs. unaffected [92, 93]). Therefore, no direct conclusions can be reported for the utilisation of this technique; however, results look promising and we are looking forward for some additional results for the optimal dosage and parameters, alongside the correct electrode placement over the cortex.
Point-of-View
Fact One
All the above studies with central-brain neurostimulation showed beneficial changes in dysphagia. Dysphagia severity and stroke types varied across groups in both studies.
Fact Two
The additional differences between these studies are the different parameters and the different cortical representations targeted (pharyngeal, upper oesophageal, mylohyoid). In one research study, excitatory neurostimulation was used to the ipsilesional mylohyoid cortical representation [87], while in the other excitatory neurostimulation was used to the contralesional pharyngeal MI [88•].
One could easily arrive at the conclusion that the effects of brain neurostimulation can also be ‘cortical representation-target’ specific, i.e. excitatory stimulation to the unlesioned pharyngeal MI, excitatory stimulation to the lesioned mylohyoid representation.
However, it might be hard to understand that this might be the case by reviewing only these small-sample studies from different laboratories. Moreover, there is valuable information missing about the overall effects and any potential spread on surrounding musculature representations.
In addition, previously with TMS studies, we have observed that pharyngeal, mylohyoid and oesophageal cortical representations show some overlap but also topographic differences [94], which is also validated by fMRI studies [95]. We have also observed that in patients pharyngeal electrical stimulation post-stroke increased the map size of the pharyngeal musculature cortical representation but induced a reciprocal contraction for the oesophageal cortical representation [81]. Therefore, from a neuroscientific point-of-view, it would be of interest to understand whether by exciting or inhibiting one particular cortical representation (i.e. pharyngeal MI) we could provide adaptive or non-adaptive processes to other cortical representations important for swallowing (i.e. oesophageal).
Moreover, it would be interesting to have the following important information: (a) what are the effects of a single application of neurostimulation to dysphagic patients and which is the optimal dosage of a therapeutic regimen, and (b) the extent to which training and behavioural exercises, that can be the usual routine care plan for the dysphagic patient, affect the results of the application of brain stimulation approaches. To elaborate on this latter question, it is worth mentioning that tDCS was observed to produce results due to changes in GABAergic networks [96] and, recently, Zimerman et al. [97] reported that older age participants experienced substantial improvements in the acquisition of novel skills when training was applied concurrent with tDCS. From a clinical perspective, it is really interesting to observe that the effects of neurostimulation approaches can boost and assist conventional therapy for dysphagia, returning patients to clinically functional swallowing state.
Combining Peripheral and Central Neurostimulation
Pairing peripheral and central neurostimulation is another promising technique. This neurostimulation technique, called paired associative stimulation (PAS), is capable of provoking long-lasting heterosynaptic plasticity in neuronal pathways following combination of peripheral stimuli in the targeted muscle with cortical stimuli over the targeted muscle representational area on MI [98]. The concept of this technique derives from evidence that peripheral input plays an important role in plastic reorganization of motor areas and can lead to long-lasting changes in cortical excitability of the targeted area. Research in animal neocortical slices have shown that when a weak excitatory synaptic input repeatedly arrives at a neuron shortly before the neuron has fired an action potential, then the strength and efficacy of that connection is increased, whereas if the input arrives after the neural discharge, the strength of the connection is reduced [99, 100]. Bi-directional modulation of synaptic efficacy in this manner is termed Hebbian plasticity [101]. For the swallowing neural system, the neurostimulation parameters have been investigated in detail (dose, duration, intensity, frequency) in healthy participants [102, 103] and the application of the technique on chronic dysphagic stroke patients showed promising results on neurophysiological and functional measurements in a sham-controlled randomised study [103]. Additionally, using magnetic resonance spectroscopy, with which we can quantify the concentrations of neurotransmitters, it has been demonstrated that one of the underlying mechanisms accounting for the changes observed following PAS on the cortical motor pharyngeal representation, involves changes in intracortical glutamate levels within motor cortex [102] and recently, we have also observed changes in the major inhibitory neurotransmitter, GABA, when PAS is applied in health [104•]. This is perhaps one of the neurostimulation paradigms in swallowing neurorehabilitation that has been investigated extensively, with research studies investigating not only the parameters (repetition within a treatment session, frequency, duration, [102, 103] repeated application for responders vs. non-responders to a single application [105]), but also the underlying mechanisms to account for the changes observed in health and dysphagic stroke patients.
Conclusions
Overall, recent research studies investigating the effects of neurostimulation approaches for dysphagia rehabilitation have shown promising results. There is some paucity that neurostimulation techniques will be viewed as powerful tools in the hand of a rehabilitation clinician in the future. However, currently the field of neurorehabilitation science in dysphagia is diverse in nature and methodological differences across research studies are accentuating the need for further investigations.
It is important to continue research into neurostimulation techniques for swallowing rehabilitation for two reasons. Firstly, there is a potential avenue for clinical utility of neurostimulation in dysphagia rehabilitation clinics. Secondly, by studying how we can modulate the swallowing network, the optimal time-window for swallowing modulation and the exact neurophysiological and behavioural effects of neurostimulation, we will be able to accumulate much knowledge about the adaptive changes we can promote to our patients.
Performing studies with neurostimulation in healthy population and understanding the roles of the different stimuli on swallowing neural network may eliminate several factors that play a role during rehabilitation sessions in the clinics. Interestingly, factors such as individual differences, attention span, fatigue and pre-learned skills have been shown to affect the modulability of neuronal processes with neurostimulation [105–107]. Recently, we have also observed evidence about the contrasting effects of the common polymorphism of BDNF on neurophysiological outcomes in experimentally induced plasticity paradigms in the intact human pharyngeal motor cortex [108], which is indicative of the fact that a more detailed understanding of the genetic basis of cortical plasticity of human swallowing motor pathways is warranted. Lastly, physiological studies in health are a vital perquisite prior to applying these treatments to patient populations, but only a few neurostimulation techniques have been investigated for their effects on both physiological and neurophysiological outcome measures. In patient populations, studies from all the different levels of evidence: case series, cohorts, case-controls and RCTs are important, prior to the adaptation of the techniques by dysphagia specialists.
Even though we already commented above on each neurostimulation approach applied in patients, it would be of interest to add that we still have unanswered questions that, if addressed, will assist not only the application of neurostimulation but also the dysphagia rehabilitation field in general. For instance, most of the neurostimulation approaches have been vastly researched in stroke patients. Applying neurostimulation approaches to different disease aetiologies and accounting for several factors while measuring neurophysiological and functional outcome measures, will provide us further information about the endogenous plastic changes in humans with regards to swallowing function. In the literature, the differences in parameters of each of the neurorehabilitation technique have proven to be more confusing than comforting. Formulation of a hypothesis needs always to precede the interventional studies. Notably, central, peripheral and combined neurostimulation need further investigations on that account. Therewith, our field will benefit from multicenter trials with larger number of patients testing in a controlled manner the effects of neurostimulation approaches that have previously shown promising results when tested in small sized RCTs.
We, as clinicians working in accordance with evidence-based practice framework, should provide safe, comforting and beneficial neurorehabilitation to our patients for speedy recovery of swallowing function, arresting the maladaptive behaviours and increasing the potential of adaptive processing following disease. Hopefully, in the near future, we may be able to achieve this with a combination of tools, including neurostimulation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Cracco RQ, Cracco JB, Maccabee PJ, Amassian VE. Cerebral function revealed by transcranial magnetic stimulation. J Neurosci Methods. 1999;86(2):209–19.
Jacobs M, Premji A, Nelson AJ. Plasticity-inducing TMS protocols to investigate somatosensory control of hand function. Neural Plast. 2012;2012:350574. doi:10.1155/2012/350574.
Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–49.
Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–99.
Hummel FC, Cohen LG. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5(8):708–12.
Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126(Pt 6):1430–48.
Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64(10):1802–4.
Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–22.
Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681–6.
Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121(1):30–7.
Adeyemo BO, Simis M, Macea DD, Fregni F. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front Psychiatry. 2012;3:88.
Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50.
Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999;126(4):536–44.
Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241(1):5–8.
Feige B, Aertsen A, Kristeva-Feige R. Dynamic synchronization between multiple cortical motor areas and muscle activity in phasic voluntary movements. J Neurophysiol. 2000;84(5):2622–9.
Nielsen J, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. J Physiol. 1997;501(Pt 2):473–84.
Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129(Pt 7):1659–73.
Schulz R, Gerloff C, Hummel FC. Non-invasive brain stimulation in neurological diseases. Neuropharmacology. 2013;64:579–87.
Barwood CH, Murdoch BE. rTMS as a treatment for neurogenic communication and swallowing disorders. Acta Neurol Scand. 2013;127(2):77–91.
Sumi T. Reticular ascending activation of frontal cortical neurons in rabbits, with special reference to the regulation of deglutition. Brain Res. 1972;13(46):43–54.
Sumi T. Some properties of cortically-evoked swallowing and chewing in rabbits. Brain Res. 1969;15(1):107–20.
Miller AJ. Significance of sensory inflow to the swallowing reflex. Brain Res. 1972;43(1):147–59.
Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis). J Neurophysiol. 1999;82(3):1529–41.
Lamkadem M, Zoungrana OR, Amri M, Car A, Roman C. Stimulation of the chewing area of the cerebral cortex induces inhibitory effects upon swallowing in sheep. Brain Res. 1999;832(1–2):97–111.
Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res. 1999;824(1):140–5.
Doty RW. Influence of stimulus pattern on reflex deglutition. Am J Physiol. 1951;166(1):142–58.
Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510(Pt 1):249–59.
Fukuhara T, Tsujimura T, Kajii Y, Yamamura K, Inoue M. Effects of electrical stimulation of the superior laryngeal nerve on the jaw-opening reflex. Brain Res. 2011;19(1391):44–53.
Lang IM, Medda BK, Jadcherla S, Shaker R. The role of the superior laryngeal nerve in esophageal reflexes. Am J Physiol Gastrointest Liver Physiol. 2012;302(12):G1445–57.
Dobkin BH. Do electrically stimulated sensory inputs and movements lead to long-term plasticity and rehabilitation gains? Curr Opin Neurol. 2003;16(6):685–91.
Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–39.
Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):166–71.
Martin RE. Neuroplasticity and swallowing. Dysphagia. 2009;24(2):218–29.
Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–12.
Humbert IA, McLaren DG, Kosmatka K, Fitzgerald M, Johnson S, Porcaro E, Kays S, Umoh EO. Robbins J Early deficits in cortical control of swallowing in Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1185–97.
Michou E, Hamdy S. Dysphagia in Parkinson’s disease: A therapeutic challenge? Expert Rev Neurother. 2010;10(6):875–8.
• Suntrup S, Teismann I, Bejer J, et al. Evidence for adaptive cortical changes in swallowing in Parkinson’s disease. Brain. 2013;136(Pt 3):726–38. In this research study, the researchers showed evidence for potential adaptive cortical mechanisms for dysphagia in Parkinson’s disease.
Li S, Luo C, Yu B, et al. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J Neurol Neurosurg Psychiatry. 2009;80(12):1320–9.
Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1342–7.
Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull. 1995;37(4):397–404.
Sessle BJ. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Res. 1973;53(2):319–31.
Peck KK, Branski RC, Lazarus C, et al. Cortical activation during swallowing rehabilitation maneuvers: a functional MRI study of healthy controls. Laryngoscope. 2010;120(11):2153–9.
Kothari M, Svensson P, Jensen J, et al. Training-induced cortical plasticity compared between three tongue-training paradigms. Neuroscience. 2013;29(246):1–12. doi:10.1016/j.neuroscience.2013.04.040.
Robbins J, Kays SA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–8.
Crary MA, Carnaby GD, LaGorio LA, Carvajal PJ. Functional and physiological outcomes from an exercise-based dysphagia therapy: a pilot investigation of the McNeill dysphagia therapy program. Arch Phys Med Rehabil. 2012;93(7):1173–8.
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–69.
Theurer JA, Bihari F, Barr AM, Martin RE. Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia. 2005;20(4):254–60.
Theurer JA, Czachorowski KA, Martin LP, Martin RE. Effects of oropharyngeal air-pulse stimulation on swallowing in healthy older adults. Dysphagia. 2009;24(3):302–13.
• Theurer JA, Johnston JL, Fisher J, et al. Proof-of-principle pilot study of oropharyngeal air-pulse application in individuals with dysphagia after hemispheric stroke. Arch Phys Med Rehabil. 2013;94(6):1088–94. The proof-of-principle study for the efficacy of air-puff stimulation in dysphagic stroke patients is published in this paper. Interesting results for the effects of bilateral air-puff stimulation.
Soros P, Lalone E, Smith R, et al. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience. 2008;153(4):1300–8.
Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42(1):285–95.
Ebihara S, Kohzuki M, Sumi Y, Ebihara T. Sensory stimulation to improve swallowing reflex and prevent aspiration pneumonia in elderly dysphagic people. J Pharmacol Sci. 2011;115(2):99–104.
Mostafeezur RM, Zakir HM, Takatsuji H, Yamada Y, Yamamura K, Kitagawa J. Cannabinoids facilitate the swallowing reflex elicited by the superior laryngeal nerve stimulation in rats. PLoS ONE. 2012;7(11):e50703. doi:10.1371/journal.pone.0050703.
Michou E, Mastan A, Ahmed S, Mistry S, Hamdy S. Examining the role of carbonation and temperature on water swallowing performance: a swallowing reaction-time study. Chem Senses. 2012;37(9):799–807.
Hamamoto T, Takumida M, Hirakawa K, Tatsukawa T, Ishibashi T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol. 2009;129(5):560–8.
• Rofes L, Arreola V, Martin A, Clavé P. Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut. 2013;62(9):1280–7. The role of capsaicinoids in improving swallowing in dysphagic patients with mixed aetiologies is investigated by the group here. Adequate number of participants and physiological measurements.
Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46(5):466–74.
Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23(3):302–9.
Sun SF, Hsu CW, Lin HS, Sun HP, Chang PH, Hsieh WL, Wang JL. Combined neuromuscular electrical stimulation (NMES) with fiberoptic endoscopic evaluation of swallowing (FEES) and traditional swallowing rehabilitation in the treatment of stroke-related dysphagia. Dysphagia. 2013. doi:10.1007/s00455-013-9466-9.
Kushner DS, Peters K, Eroglu ST, Perless-Carroll M, Johnson-Greene D. Neuromuscular electrical stimulation efficacy in acute stroke feeding tube-dependent dysphagia during inpatient rehabilitation. Am J Phys Med Rehabil. 2013;92(6):486–95.
Beom J, Kim SJ, Han TR. Electrical stimulation of the suprahyoid muscles in brain-injured patients with dysphagia: a pilot study. Ann Rehabil Med. 2011;35(3):322–7.
Lin PH, Hsiao TY, Chang YC, et al. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19(1):91–9.
Baijens LW, Speyer R, Passos VL, Pilz W, Roodenburg N, Clavé P. The effect of surface electrical stimulation on swallowing in dysphagic Parkinson patients. Dysphagia. 2012;27(4):528–37.
Baijens LW, Speyer R, Passos VL, et al. Surface electrical stimulation in dysphagic Parkinson patients: a randomized clinical trial. Laryngoscope. 2013. doi:10.1002/lary.24119.
Heijnen BJ, Speyer R, Baijens LW, Bogaardt HC. Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson’s disease and oropharyngeal dysphagia: effects on quality of life. Dysphagia. 2012;27(3):336–45.
Christiaanse ME, Mabe B, Russell G, Simeone TL, Fortunato J, Rubin B. Neuromuscular electrical stimulation is no more effective than usual care for the treatment of primary dysphagia in children. Pediatr Pulmonol. 2011;46(6):559–65.
Verin E, Maltete D, Ouahchi Y, et al. Submental sensitive transcutaneous electrical stimulation (SSTES) at home in neurogenic oropharyngeal dysphagia: a pilot study. Ann Phys Rehabil Med. 2011;54(6):366–75.
Lee SY, Yang HE, Yang HS, Lee SH, Jeung HW, Park YO. Neuromuscular electrical stimulation therapy for dysphagia caused by Wilson’s disease. Ann Rehabil Med. 2012;36(3):409–13.
Barikroo A, Lam PM. Comparing the effects of rehabilitation swallowing therapy vs. functional neuromuscular electrical stimulation therapy in an encephalitis patient: a case study. Dysphagia. 2011;26(4):418–23.
Park JW, Kim Y, Oh JC, Lee HJ. Effortful swallowing training combined with electrical stimulation in post-stroke dysphagia: a randomized controlled study. Dysphagia. 2012;27(4):521–7.
• Tan C, Liu Y, Li W, Liu J, Chen L. Transcutaneous neuromuscular electrical stimulation can improve swallowing function in patients with dysphagia caused by non-stroke diseases: a meta-analysis. J Oral Rehabil. 2013;40(6):472–80. A recent metaanalysis for the effects of neuromusculare electrical stimulation for dysphagia in stroke patients.
Carnaby-Mann GD, Crary MA. Examining the evidence on neuromuscular electrical stimulation for swallowing: a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133(6):564–71.
Johnson AM, Connor NP. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve. 2011;43(2):203–11.
Nam HS, Beom J, Oh BM, Han TR. Kinematic effects of hyolaryngeal electrical stimulation therapy on hyoid excursion and laryngeal elevation. Dysphagia. 2013. doi:10.1007/s00455-013-9465-x.
Heck FM, Doeltgen SH, Huckabee ML. Effects of submental neuromuscular electrical stimulation on pharyngeal pressure generation. Arch Phys Med Rehabil. 2012;93(11):2000–7.
Kagaya H, Baba M, Saitoh E, Okada S, Yokoyama M, Muraoka Y. Hyoid bone and larynx movements during electrical stimulation of motor points in laryngeal elevation muscles: a preliminary study. Neuromodulation. 2011;14(3):278–83.
Humbert IA, Michou E, MacRae PR, Crujido L. Electrical stimulation and swallowing: How much do we know? Semin Speech Lang. 2012;33(3):203–16.
Doeltgen SH, Huckabee ML. Swallowing neurorehabilitation: from the research laboratory to routine clinical application. Arch Phys Med Rehabil. 2012;93(2):207–13.
Humbert IA, Joel S. Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. Neuroimage. 2012;59(2):1485–90.
Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;10:CD000323.
Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1(1):64–8.
Fraser C, Power M, Hamdy S, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34(5):831–40.
Jayasekeran V, Singh S, Tyrrell P, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138(5):1737–46.
Restivo DA, Casabona A, Centonze D, Marchese-Ragona R, Maimone D, Pavone A. Pharyngeal electrical stimulation for dysphagia associated with multiple sclerosis: a pilot study. Brain Stimul. 2013;6(3):418–23.
Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand. 2009;119(3):155–61.
Verin E, Leroi AM. Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: a noncontrolled pilot study. Dysphagia. 2009;24(2):204–10.
Kim L, Chun MH, Kim BR, Lee SJ. Effect of repetitive transcranial magnetic stimulation on patients with brain injury and dysphagia. Ann Rehabil Med. 2011;35(6):765–71.
• Park JW, Oh JC, Lee JW, Yeo JS, Ryu KH. The effect of 5 Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol Motil. 2013;25(4):324–e250. RCT examining the effects of excitatory rTMS on the unaffected pharyngeal motor cortex. Interesting results in physiological and functional outcome measures.
Jefferson S, Mistry S, Michou E, Singh S, Rothwell JC, Hamdy S. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contralesional brain stimulation. Gastroenterology. 2009;137(3):841–9.
Jefferson S, Mistry S, Singh S, Rothwell JC, Hamdy S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1035–40.
Shigematsu T, Fujishima I, Ohno K. Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair. 2013;27(4):363–9.
Kumar S, Wagner CW, Frayne C, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42(4):1035–40.
Yang EJ, Baek SR, Shin J, et al. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci. 2012;30(4):303–11.
Hamdy S, Aziz Q, Rothwell JC, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2(11):1217–24.
Martin RE, MacIntosh BJ, Smith RC, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92(4):2428–43.
Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–6.
Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol. 2013;73(1):10–5. doi:10.1002/ana.23761.
Classen J, Wolters A, Stefan K, et al. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–9.
Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86(3):1033–48.
Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14(3 Pt 2):1634–45.
Donoghue JP. Plasticity of adult sensorimotor representations. Curr Opin Neurobiol. 1995;5(6):749–54.
Singh S, Mistry S, Jefferson S, et al. A magnetic resonance spectroscopy study of brain glutamate in a model of plasticity in human pharyngeal motor cortex. Gastroenterology. 2009;136(2):417–24.
Michou E, Mistry S, Jefferson S, Singh S, Rothwell J, Hamdy S. Targeting unlesioned pharyngeal motor cortex improves swallowing in healthy individuals and after dysphagic stroke. Gastroenterology. 2012;142(1):29–38.
• Michou E, Mistry S, Vidyasagar R, Downey D, Williams S, Hamdy S. Bilateral modulation of functional cortical swallowing activity and neurotransmitters concentrations after paired associative brain stimulation: a FMRI and magnetic resonance spectroscopy (MRS) study. Presented at European Society for Swallowing Disorders, Barcelona, 25–27 Oct 2012. Interesting results showing a drop in inhibitory neurotransmitter (GABA) with MRS in MI following the paired associative stimulation, targeting the pharyngeal cortical representation in health and concurrent increase in bilateral MI in activity as measured with Blood Oxygenation Level-Dependent signal with functional MRI.
Michou E, Mistry S, Rothwell J, Hamdy S. Priming pharyngeal motor cortex by repeated paired associative stimulation: implications for dysphagia neurorehabilitation. Neurorehabil Neural Repair. 2013;27(4):355–62.
Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181:615–26.
Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72.
Jayasekeran V, Pendleton N, Holland G, Payton A, Jefferson S, Michou E, Vasant D, Ollier B, Horan M, Rothwell J, Hamdy S. Val66Met in brain-derived neurotrophic factor affects stimulus-induced plasticity in the human pharyngeal motor cortex. Gastroenterology. 2011;141(3):827–36.
Compliance with Ethics Guidelines
Conflict of Interest
E. Michou declares no conflicts of interest. S. Hamdy’s institution has received research grants from Wellcome Trust and MRC and Dr. Hamdy is partial owner of Phagenesis Ltd.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Michou, E., Hamdy, S. Neurostimulation as an Approach to Dysphagia Rehabilitation: Current Evidence. Curr Phys Med Rehabil Rep 1, 257–266 (2013). https://doi.org/10.1007/s40141-013-0034-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-013-0034-x