Abstract
This review describes the various applications of graphene derivative (GO/rGO) with the tungsten oxide nanocomposite such as supercapacitor, electrochromism, photocatalysis and energy sensing. This review article also presents the properties of tungsten oxide with the graphene derivatives and their classification on basis of transition metals, metal oxides, nonmetals, sulfide, and polymers. Graphene oxide is a wonder material that has the potential to impart extraordinary properties into several hybrid materials, resulting in distinctive application in enormous domains. The impressive application and properties of the graphene derivatives have been discussed in this review article. The transition metal oxides (TMOs) have gained considerable research attention due to their unique physicochemical characteristics. Among TMOs, tungsten oxide (WO3) is a versatile material with excellent properties, diverse applications, stability, and low fabrication cost. The enhanced property of tungsten oxide by incorporation of graphene derivatives is also discussed in this review. The main focus of this review article is to summarize the 5-year applications of GO/rGO-based tungsten oxide nanocomposite in energy storage (super capacitors and batteries), gas sensor devices, electrochromism, and photocatalyst. This review article will also provide the research gap and can excite new ideas for further improvement of GO/rGO-based tungsten oxide nanocomposite.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology deals with nano-size materials and plays a vital role in improving the performance of materials in different areas of research. The nanocomposite bought increase in the various applications such as in biotechnology, packaging, aerospace, electronics, photocatalysis and sensor [1]. In this regard, graphene-based nanomaterials are significant addition. Graphene is carbon-based material that gains more popularity due to its extraordinary physio-chemical properties, high surface area, and fewer health hazards effects [2, 3]. Graphene comprises of 2-D arranged carbon atoms and hexagonal crystalline structure. It possesses extraordinary adsorption rate and surface reactions due to its large specific surface area. Graphene derivates also possesses high thermal conductivity and insulating ability, high mechanical strength, and electron mobility [4, 5].
Many nanoadsorbents such modified zeolite, alumina, biochar and activated carbon can be used in air pollution, metal extraction, hydrogen storage, medicine and for wastewater remediation in removing uranium (VI) [6,7,8]. Activated carbon due to its high pore size, large surface area to volume ratio is being used as adsorbent [9]. The photocatalyst ZIF-8/g-C3N4 was fabricated to photo-reduce U(VI) from aqueous solutions [10]. Biochar material can be used to support the environmental friendly application of nanoscale materials and served as an ideal adsorbent for the removal of heavy metals [11] and Be(II) [12]. For example, the adsorbent showed excellent removal efficiency for the Cr(VI) [13]. However, these materials offer limited applications due to low removal rate, high cost, secondary pollution and complicated operating process [14]. To be economical feasible, the nanomaterial should possess high removal rate, simple synthesis process, environment friendly behavior, and low cost. Derivatives of graphene such as GO and rGO can be used as a dopant or an adsorbent for enhancing the properties of other materials with improved mobility of charge carriers [15, 16].
The advanced materials (such as Mxenes, metal organic framework, g-C3N4 [17], quantum dots and covalent-triazine frameworks) can be used in photocatalysis, sensing devices, and supercapacitor. Their effective modification (e.g., loading noble metal and doping) have indeed promoted the in-depth photocatalytic mechanism research. However, costly preparation, complex operation, and possible secondary pollution limit the development of industrial application of these advanced materials [18]. BiOCl is UV active photocatalyst which can degrade the organic contaminants and antibiotics. The BiOCL has a band gap of 3.2–3.5 eV which limits its photocatalytic performance under natural light [19].
To overcome the limitations caused by these nanomaterials/nanoadsorbents, researchers are struggling to develop engineered materials, i.e. the properties of pristine materials can be enhanced by the combination of two or more than two components to form composite materials. Graphene due to sp2 hybridization exhibited good electronic conduction which facilitates the electron delocalization. Metal oxide NPs have significant advantages such as electrochemical change due to quantum confinement effect, increases the band gap due to change in surface properties, and undergo structural changes which allow the cell parameter altering along with the change in lattice symmetry [20]. Several metal oxides such as zinc oxide, tin oxide, and titanium oxide gained a lot of attention due to their easy synthesis, low cost, and gas sensing properties. These oxides still have some limitations and were studied only for the bio-molecules electrochemical detection [21] [22]. The incorporation of metal oxide nanoparticles in graphene resulted in enhanced properties of metal oxide-graphene composites. Its surface area, chemical inertness, great thermal conductivities, and mechanical strength have been improved [23]. These composite materials like ZnO/rGO [24], Ta@GO@MgO [25], SnO2/Rgo [26], α-Fe2O3/rGO [27] rGO/In2O3 [28] rGO/Co3O4 [29] rGO/WO3 [30] serve potential applications in chemical and biological sensors, energy storage devices, energy conservation, electronics, and heterogeneous photocatalysis. The key advantage of graphene is its ability to integrate with the other materials to form new composites with the desired properties [31, 32]. The benefit of metal oxide with graphene derivative composite is that it can be used in different fields of study.
The metal oxide-decorated graphene derivative maintains good electro conductivity [33]. Their applications have been explored in supercapacitor, gas sensing, photocatalysis, and energy conversion devices [34, 35]. The composite of metal oxide (Co3O4, Mn3O4, ZnO, Fe2O3, and WO3) with graphene derivatives due to their unique and enhanced features are of great interest. Recently tungsten oxide has a good potential for catalytic activity, Electrochromic material, gas sensing [36]. These composite (rGO/GO/WO3) focus on two main parts; one is a combination of metal oxide pseudocapacitors and carbonaceous material while the second part comprises on new distinct structure and morphologies [37, 38]. The electrochemical properties of carbon material are individually low due to low rate capacity and poor ability of stored energy. The conductive materials like Ag, Au, Cu, Pt, rGO, and GO are most commonly used as electron mediators for the transportation of the electrons [39]. With the increasing demand for energy-based devices, the environment has suffered a lot. Some efforts have been made to develop better technology which depends on the property and structure of the material used and are also environment friendly. Among various oxides, tungsten oxide is considered an important metal oxide due to strong photo absorption properties in the NIR region for various applications [40, 41].
This review article aims in composing the literature survey on the binary and ternary composite of WO3 with graphene derivatives as supercapacitor, Electrochromic material, gas sensor, and photocatalyst. Among all of the electron mediators, the graphene-based (rGO and GO) materials are used widely due to their low cost, great biocompatibility, and high stability [42]. The combination of tungsten oxide with carbon materials (GO and rGO) shows good conductivity with enhanced electrical, Electrochromic properties, and surface area [43]. This review article highlights graphene oxide/reduced graphene oxide synthesis with tungsten trioxide, their composite, and their application in various fields. No review article on multidisciplinary application of graphene derivatives with tungsten oxide has been published before up to the best of our knowledge.
Tungsten oxide
Among the metal oxide, the tungsten oxide is abundantly present with less toxic effects as compared to others. Tungsten oxide also is known as tungsten anhydride or trioxide containing oxygen and tungsten metal and its composite has been known from the 1900s [44]. Tungsten oxide is naturally present in the form of hydrates such as meymacite, hydro tungstite, and tungstite. WO3 is an n-type oxide of the metal, having an energy bandgap in the range of 2.4–2.8 eV, and has been used in various applications such as a sensor for volatile organic compounds detection, electrochromism, and photocatalyst [45, 46].
In the late 1960s, the 3 important applications of tungsten oxide were emerged such as hydrogen detection using platinum-activated tungsten oxide by shaver in 1967 [47]. In 1973, the electrochromism properties of the tungsten oxide were discovered by Deb by depositing the tungsten oxide films on quartz substrates [48], other tungsten oxide Electrochromic devices in 1984 were also made for the energy-efficient windows. In 1976, tungsten oxide suitability in photo electrochemical cells was studied for the first time [49], and shown to be a very stable semiconducting anode for wastewater remediation due to its low energy band gap as compared to the TiO2 [50]. These three major applications lead to further study of tungsten oxide in different fields. Recently, due to an increase in energy requirement, the WO3 is used in many fields like CO2 reduction, air purification, antimicrobial agent, and dye-sensitized solar cells [51].
Structure of tungsten oxide
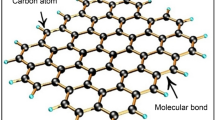
Tungsten oxide shows absorbance in NIR region; the strong photo absorption ability of WO3 is due to string local surface plasma resonance, and oxygen defect structure [52]. Tungsten oxide consists of a polymorphous structure with trioxide. The crystalline structure of WO3 is like ReO3 in two ways: one is WO6 octahedron tilting and the other is tungsten displacement from the octahedron center. The tungsten atom is located at the center of the octahedron each with an oxygen atom at the corner of the structure. Figure 1 elucidates the structure of tungsten oxide [53].
The structure of WO3 changes with the change in temperature. As a result, the electrical, optical, or magnetic behaviors of tungsten oxides are greatly influenced by the different crystalline phases of tungsten oxide. Pure WO3 crystal transforms according to the sequence given in Table 1 as the temperature is lowered from 900 to 189 °C [54].
Moreover, the various crystal structures of WO3 depend on the temperature. As the temperature changes, WO6 octahedral undergo displacement of the central W atom, which exhibit different polymorphic forms having different temperature ranges, including the tetragonal form (αWO3, > 740 °C),the orthorhombic (βWO3, 330–740 °C), monoclinic I form (γWO3, 17–330 °C), monoclinic II (ε/WO3, > − 43 °C)and triclinic (δ/WO3, − 43–17 °C) [55]. WO3 shows eight phases of conversion that occurred in the temperature range 0–1200 K. Among the different forms of tungsten oxide, the most stable form is monoclinic; in this form each unit cell contains eight WO6 octahedral, and a neighboring tungsten atom with 0.375 nm distance. The orthorhombic and tetragonal structures are formed when the monoclinic form is heated at high temperatures. Nevertheless, it cannot return to alternate phases once it returns to room temperature. The transition from the monoclinic to orthorhombic form is differentiated by a decrease in W–O bond displacement which shifts W atom by tilting WO6 octahedra [56, 57]. The Liu et al. studied the defects engineering for improving the metal oxide performance. The engineering defect affects the separation, electrical conductivity, optical absorption, migration of photogenerated carriers, and surface properties of materials [58]. However, the crystal defect can have negative and positive aspect on the photocatalysis. The surface lattice defect tends to increase the photocatalytic performance as compared to surface lattice defect of crystal [59]. Therefore, the appropriate defect on high crystalline surface of WO3 may be an effective way to enhance its photocatalytic activity in visible light. The Liu et al. studied the economical and simple vacuum de-oxidation method to create oxygen defect in WO3. This showed that surface oxygen vacancies are very advantageous to enhance the separation and absorption of photogenerated electron hole pair for tungsten oxide. The treated WO3 at temperature of 150–350 °C exhibited high photocatalytic activity and photocurrent density due to more surface oxygen vacancies. WO3–X at temperature of 450 and 550 °C showed lowered photocurrent density and activity due to bulk defects. This study showed the importance of surface oxygen vacancy defects for the application in photocatalysis [60]. Investigation of point defects is the base in studying the mechanism of macro-performance of materials under irradiations [61].
Application of tungsten oxide
Tungsten oxide (WO3) among other multifunctional TMOs, exhibits unique structure and properties [62, 63]. WO3, MxWO3, and their hybrids achieved much attention in many fields, e.g. photocatalysis, gas-sensor, heat generation, and energy-based applications [52]. In reports, WO3 and WO2.72 are described as sensor materials that can monitor gases that are toxic, and flammable such as H2, CH4, and COx [64]. WOx gas sensors still have some disadvantages like it has poor stability, selectivity is low, and operating temperature is high. The hybrid of tetrahedron-tungsten oxide palladium nanoplates (Pd/WO2.72) can improve electro catalytic activity [65]. For LIBs, WO3 composites are used as anodic material, such as the flexible tungsten–carbon nanofibers composite (WOx-C-NF) membrane used as an anode material in LIB with high reversible capacity of (481 mA hg−1) and good cycle stability [66]. WO3 is a promising candidate for supercapacitor due to stable and good conductivity [67, 68]. The CO2 photocatalytic reduction can be achieved by WO3 to obtain methane [54]. WO3 unique properties make it possible for commercial applications including sterilization, desalination, and steam generation [69]. WO3 could be promising material in field of visible light driven photocatalyst. It has many advantages such as low cost, good stability in oxidative and acidic medium, and narrow band gap [70].
A summary of the application of tungsten oxide in different fields is shown in Fig. 2.
Graphene derivatives (GO and rGO)
In 1960, intercalated graphite material with high conductivity was discovered, at the same time, Fernandez–Moran created the thin graphite crystal. During the next 30 years, huge research was done on graphitic material due to the discovery of fullerenes and nanotubes which explore the study of carbon material (graphene oxide, carbon sphere, carbon nanotubes, and porous carbon) [71]. Graphene is one-atom-thick sheet of carbon and is arranged in the form of a honeycomb lattice which acts as a building block for other graphitic materials [72]. It is lightest, thinnest material with zero band gap. Its main challenge is the synthesis of graphene on large scale, this lead to discovery of graphene derivatives such as graphene oxide (GO) and reduces graphene oxide (rGO). The production of these materials is relatively easier on large scales [73].
Graphene oxide is the oxidized form of graphene (sp3 hybridized) and 2.2 eV bandgap [74]. After the discovery of graphene, its derivative graphene oxide attracted huge attention as a feasible way for obtaining graphene. GO can be used in different applications discuss in sec 3.2. rGO is the reduced form of graphene oxide (sp3 hybridized) and considered as an attractive route in obtaining graphene-like behavior [75]. Different reduction methods such as thermal, chemical, or photo-thermal are used to obtain rGO. Depending on reductions, its band vary from 1.00 to 1.69 eV [76]. However, as result of reduction methods, the reduced graphene oxide is unable to reach the pristine structure of graphene. It still contains structural defects and residual oxygen even after on severe reduction. Some chemical reducing agents such as organics (phenyl hydrazine hydrate or hydroxylamine) or NaB can be used. The huge difference between the GO and rGO is-based on the number of oxygen atoms present in them. The GO is insulator and rGO is electrical conductor [77].
Properties and structure of the graphene oxide/reduced graphene oxide
The properties of graphene can be changed by the functionalization of graphene oxide. It can be used as an intermediary in single and few-layer graphene sheet production. The process of reduction and oxidation has been developed due to which the carbon layer can be isolated and separated without changing the structure. Graphene oxide has the property of electrical insulation due to the disturbance in the sp2 bonding network [78]. To obtain the graphene honeycomb hexagonal lattices for the electrical conductivity to be restored, it is important to reduce the graphene oxide. The process of reduction is the removal of the oxygen atom, when a large number of the oxygen atoms are removed the rGO does not disperse easily due to the formation of aggregates [79]. The graphene oxide sheet structure (Fig. 3) depends on the type and distribution of the oxygen-containing functional groups (hydroxyl, epoxy, and carbonyl). For a variety of surface modification, the reactive sides have been provided by functional groups, which is used in making functionalized graphene oxide and graphene-based materials [80] [81]. The rGO and GO can be used as integral material towards progression of improved and new products. The graphene derivatives could motivate the design and development of many applications such as in electronics, sensors, catalysis and photoluminescence. The researchers have explored the idea of increasing the metal oxide life time, charge separation, absorption by incorporating the graphene derivatives [82] [83].
Reduced graphene oxide exhibits high conductivity, optical behavior, stability, absorptive, and dispersibility. Graphene derivative also avoids the leaching and corrosion of many metal oxides due to their good electrical, mechanical, and thermal properties. The GO and rGO showed the ferromagnetism effect due to existence of vacancy, hydrogen chemisorption at their edges and topological defects. Many defects are formed when graphite treated with strong oxidizing agent. rGO sheets capped on nanorod of ZnO exhibits tunable ferromagnetic behavior[84]. In WO3, the presence of rGO shows the magnetization order significantly higher than that of pristine WO3. The magnetization of composites is found to increases with the incorporation of graphene sheets. The interaction between WO3 and rGO sheet leads to the formation of WO3 lattice on rGO sheets. These indicate that the extended bond of C–C conducts stronger coupling between the orbitals (5d and 6 s) of the tungsten and carbon atoms. The insertion of rGO sheets increases the rGO/WO3 composites magnetization. The studied showed that defect like oxygen vacancies at nanorods surface are responsible for the antiferro- and ferromagnetism at the room temperature [85].
Application of graphene oxide and reduced graphene oxide
The presence of the different oxygen-containing functional groups and the 2D structure of graphene oxide exhibit excellent properties in the field of thermal, electronic, optical, and electrochemical residence mechanical properties. Most importantly, these excellent properties of GO/rGO can be tuned and controlled to some extent to find promising and outstanding applications in many research [86]. The GO and rGO electronic properties can be tuned by adjustment of the coverage of functional groups, morphology and chemical compositions sample thickness, and the average flake size. When the parameters of the reduction and the deposition are tuned appropriately, the rGO and GO films can be turned into insulators, semiconductors, or semimetals. Because of this, the use of GO has been found in many electronic devices such as in the field-effect transistors (FETs) [87].
GO is used to make thin-film transistors, and electronic sensors [88]. The GO and rGO can also be used in transparent conductors due to their transparent and conduction properties [89]. For making the flexible electronic materials, the mechanical properties of the GO films are very useful. The excellent optical properties of GO already found applications in optical detectors and optical sensors [88]. The GO is useful in several applications such as imaging of cells, therapy of the cancer cells, and drug delivery due to intrinsic fluorescence property [90]. Moreover, water purification using GO/rGO-based adsorbents or in filtration membranes is another exciting application in environment remediation [91, 92]. GO has a sheet resistance of about (1012 Ÿ sq−1) or even higher due to its insulating intrinsic nature that mainly correlates to the number of the sp3 bonding in C–O. When GO is reduced with various chemical or thermal treatments, its conductivity can be increased up to ~ 1000 S/m [93].
Along with oxygen functional groups and large surface area, the GO can be used as catalysts for the storage and production of hydrogen; also, in GO-based photo and electro-catalyst [88] and super capacitors [94]. Graphene oxide is used as an electrochromism biosensor which converts the biological response to an electrical response that determines the concentration of the analyte. GO-based electrodes are widely used due to their very fast response time, high sensitivity, and high signal-to-noise ratio [95]. Graphene oxide/reduced graphene oxide-based nanocomposite have also been widely studied/reviewed for their application in variety of research domains such as wastewater treatment [96,97,98,99,100,101,102,103], EMI shielding [104,105,106,107], antimicrobial applications [108,109,110], sensors [21, 88, 111, 112], energy storage [56, 113, 114], etc.
The electronic devices can be fabricated using graphene oxide as starting material [115]. The GO or rGO-based sensor are able to detect the avidin, hormonal catecholamine molecules, DNA, and also useful in electrochemical glucose sensing [116]. rGO nanocomposites can be used for high-capacity storage in Li ion batteries. High surface area of rGO is useful in energy storage material like in supercapacitor. GO can be a component in drug delivery systems. GO-based magnetite can be used for targeted delivery of the drug [117, 118]. The rGO and GO have been used as an essential component for detecting the biologically relevant molecules. GO has been used as quenching fluorescence materials in biosensors which utilizes the FRET effect (fluorescence resonance energy transfer) [119]. GO functionalized folic acid helps in the detection of human breast cancer and cervical cancer cells. Therefore, the graphene derivatives have electronic, energy storage, biomedical and biosensor practical applications [120]. As compared to graphene, the GO has the advantages of low cost, easy workability, polar functionalization, and water dispersibility. While rGO is less expensive, provides good control on functionalization and has high thermal and electrical conductivities [121,122,123,124].
Synthesis methods of WO3/GO and WO3/rGO composite
Many graphene-based composites with metal halides, metal oxide, and metal sulfides have been reported for the improvement in the supercapacitor, electrochromism, photocatalysis, and gas sensing applications [125]. However, among metal oxide, the WO3/graphene composites have paid very little attention. Different synthesis methods have been employed for WO3/graphene-based composite preparation such as the hydrothermal technique and a photo reduction process [126]. The morphology of WO3 is generally in form of nanoparticles or nanorods, and few studies have concentrated on the synthesis of WO3/rGO plate-shaped composites used in the field of photocatalysis. The orientation of the 2D sheet structure provides more active surface sites for catalysis. The hybrid nanocomposite between 2D rGO and WO3 is more conducive for the formation of intimate surface contacts, which optimize the transfer of interface charge and subsequently improve the photocatalytic activity, electrochromism, gas sensing, and supercapacitor applications [126]. To analyze the effect of the WO3/graphene-based composite in various applications, many researchers have been working on it. Moreover, nanoplates of WO3 on nanosheet graphene were synthesized by Nagaraju et al. through in situ microwave irradiation [127, 128].
In recent years, many methods such as electrospinning, ultrasonication, laser ablation, sol–gel synthesis and hydrothermal synthesis of GO/WO3 an rGO/WO3 have been reported. Each of these methods comprises of their own unique impact on the nanocomposite structures [129]. These properties include the conductivity, particle size, and stability. Hence, it is vital to study these methods for the uniformity in synthesis of derivatives of graphene-based nano-structured tungsten oxide for their wide range applications.
Sol gel spin coating: this method leads to formation of WO3/rGO nanocomposite film. This sol–gel spin-coating approach served to be an effective way to prepare rGO/WO3 nanocomposite and electrochromic film for energy saving smart window and electrochromic devices [130]. However, there are some demerits like high cost of raw material required to produced sol and have long processing time.
Hydrothermal technique considered to be an effective method in synthesis of metal oxide. Nano-material under high vapor pressures can be produced by this technique with less loss of final product [131]. Yadav prepared rGO/WO3 by a simple and cost-effective ultrasound-assisted hydrothermal method [132]. Electrochemical deposition is also an attractive method due to low cost and precise thickness control. It has demerit of not ensuring the uniform distribution of particles on film which is important to be needed in nanocomposites [133]. Table 2 presents an overview of the synthesis of various methods for WO3/GO and WO3/rGO composites.
Application of GO or rGO with WO3 binary composites
The graphene-based tungsten oxide has good reliability, stability, detecting power and splitting power. The supercapacitors as electrochemical energy storage device is becoming most popular power supplies in various applications. The supercapacitor also exhibits a superior life cycle and high-power density as compared to conventional batteries. The graphene-based supercapacitors are preferred due to their high chemical stability, high conductivity, low cost, and low environmental impact. Large work has been focused on electrode WO3 as positive electrode and little work has been reported for WO3 as negative electrode for supercapacitor. The tungsten oxide on graphene sheets act as a negative electrode and has good pseudo-capacitive performance which can be useful in energy storage and can be a promising negative electrode material for the asymmetry capacitors. The graphene-based tungsten oxide indicated a great potential application in energy storage devices, this could be served to be a good super capacitor in future [134, 135].
The graphene-based tungsten oxide composite shows the faster bleaching/coloring time, larger coloration efficiency and longer life cycle as a result it could be promising material for the potential application in energy saving smart windows [136] and electrochromic devices which have a huge marketing success. This composite is also useful for detecting the toxic gases, organic vapors and monitoring of the gases present in environment and useful in environmental remediation [12, 137]. This composite also have a good water pollutant splitting ability so it can be useful in removing the toxic dyes present in the water discharged from industries [129].
GO/rGO-WO 3 as gas sensors
The distinctive physicochemical properties, including the electrical conductivity, easy surface modification and strong light response, the 2-D materials such as graphene, black phosphorus, and transition metal dichalcogenides (TMDs, e.g., MoS2, WS2, TiS2) can be applied in various fields. The features such as easy operation, low cost, high sensitivity makes an electrochemical sensor as a suitable candidate to use as electrode. The 2D crystal class of the monoelement such as Xenes, undergo a beneficial development in both experimental and theoretical researchers. About 15 monoelemental 2D material predicted theoretically and synthesized experimentally including the phosphorene, graphene, and IIIA, IVA, VA, and VIA group elements. They have gathered an explosive attraction due to their unique physical properties and applications in energy, electronics, and optoelectronics. There is no standard method to synthesize or prepare the 2-D Xenes on a large scale which is a huge challenge. For the use in transparent and flexible electronic devices, many 1-D or quasi-1-D nanomaterials have been investigated. Many 3-D and 2-D nanomaterials have been introduced into the electrochemical sensor as a substrate layer [138]. Beside this, the carbon nanotubes-based films are prevalent. These films have excellent conductivity, chemical stability, optical transparency, intrinsic mobility, good flexibility, and high surface area. The sensor-based on these CNT films have a high electrical conductivity (a sheet resistance of around 100 Ω/at a transmittance of ca. 80%), which has been unsatisfactory so far. Although CNT films can recognized the analytes present in gaseous form but these have no transparency and low sensing performance [139]. Multifunctional CNT-based sensors possess excellent thermal and conductive properties [140]. The sensor-based on MoO3 materials can detect the gases but there sensing properties needed to be enhanced to detect the gas in fast response time [139].
Along with metal oxides (RuO2 and In2O3), the gold nanoparticles are employed vastly in energy storage devices due to their high specific capacitance and supercapactive performance. However, some drawbacks have been observed such as toxicity, high costs and scarce resources have been hindering their use, which result in extensive search for new materials with minimum limitations. Recently, tungsten oxide overcome these challenges especially for its great intercalation, good chemical stability, low cost, storage of electrolyte ion as like lithium and environmentally friendly. The graphene-based tungsten oxide nanocomposites have been reported as sensors, in water splitting devices and supercapacitors. The graphene-based tungsten oxide became an ideal material for the energy storage applications and gas sensing because the graphene act as electrical double capacitor layer which contributes to final capacitance [141]. The WO3 incorporation into 2-D structure provide a great reactive site for sensitive and selective detection of gases. Many other researchers reported the graphene-based hybrid material in supercapacitor [142].
Another emerging sensor, that is, black phosphorus has attracted the researchers due to its first application in biomedicine field and provides a limitless application in various medical and biochemical areas. Due to its finite bandgap and effective charge carrier mobility, BP exhibited a great sensing performance and can detect the biological molecules. Gold nanoparticles decorated in the BP showed wide linear range of 1 pg mL−1 to 10 μg mL−1 and a low detection limit of 0.20 pg mL−1 for carcinoembryonic antigen. The BP-based FET biosensor can detect the human immunoglobulin G (IgG) by a mechanical exfoliation method. Beside advantages of BP-based sensors, there are some drawbacks such as BP production rate is low, and its bulk preparation is still limited to the laboratory. Therefore, an effective and robust method is required which can control the size, layer number and surface medications of BP [143].
The 2-D materials can be used in cancer imaging, biosensing, and drug delivery platform. There are some limitations in 2-D materials which hinder its application in clinical field. Graphene although has high charge mobility but it has limited applications in bio-imaging and bio sensing due to no bandgap. But its derivatives have bandgap which make it a suitable candidate for sensing devices. While TMDs although has definite bandgap but its practical applications are not vital due the low carrier mobility. So, it is important to find a novel 2D material which will have well-balanced performance. The graphene-based tungsten oxide sensors have large potential for gas sensing applications as compared to clinical application. The sensitivity, detection range and stability of composite needed to be enhanced to make it effective sensor in clinical field.
The volatile organic compounds are released into the environment from different sources every day, so they should be tested to minimize their harmful effects [144, 145]. The metal oxide with graphene derivatives could increase the gas sensing properties of the bare metal oxides [146]. Many metal oxides such as TiO2, CuO, NiO, and ZnO, etc. are used for appropriate gas detection [147]. The addition of TiO2 NPs in transformer oil can boost the power frequency breakdown voltage of transformer oil [148]. Among various MOs as gas sensors, WO3 is an important n-type gas sensor. Pd- and Pt-doped WO3 have been reported for improved gas sensing performance [149, 150] but high-temperature operating restrains its application as a gas sensor [151]. This limitation can be overcome by the incorporation of graphene derivatives which are p-type materials [88, 112, 152,153,154]. To evaluate the gas sensing process of the WO3/rGO, a popular mechanism of gas sensing model was used. Regular variation in the signal produced by the resistance of the sensor is caused due to the adsorption reaction, and desorption of molecules of gas on the surface. On the surface of the sensing film, the oxygen molecules will chemisorb and dissociate when the sensor is exposed to air. The O2 molecule will capture the electron, transferring an electron to the chemisorbed oxygen from the conduction band, thus generating the species of oxygen (such as O2−, O− or O2−) film on the sensing layer surface. This causes carriers and electron depletion region (EDR) formation to decrease near sensors surface [155, 156].
The nanosheets of h/WO3/rGO sensor were prepared hydrothermally and then undergo post-calcination treatment for the detection of H2S [157]. The sensitivity of rGO/h/WO3 was 168.58 towards the 40 ppm concentration of H2S and exhibited a 3.7 times higher detection limit as compared to WO3 alone. For the H2S sensing mechanism, the reaction and surface adsorption model was used. In the case of WO3, the oxygen molecules from the air get adsorbed on the sensor surface, form O2−, O− or O2− by electron capturing from the WO3 conduction band, resulted in electron depletion layer with high-resistance state. When sensor WO3 was exposed to H2S gas, then gas reacted with chemisorbed oxygen (mainly O−), releasing electrons to sensor conduction band resulted in the low-resistance state. As the working temperature increased, a higher response was obtained due to the enhanced activity of chemisorbed oxygen; this process continued up to a certain limit beyond this, the gas adsorption decrease and started to desorb leading to a drop in the response of the sensor (Fig. 4a).
The sensing mechanism of H2S by (a) pure WO3 nanosheets and (b) rGO/h WO3 composite, reproduced with permission from Ref. [157]: copyright (2016), Elsevier
The 3D hybrid nanostructure combination of tungsten oxide with reduced graphene oxide provides channels for H2S gas diffusion into the sensor, resulting in good contact between H2S and inner WO3 grains in the response process as well as quick desorption in the recovery process. The rGO facilitated the charge carriers' transport. More molecules of oxygen on surface materials captured the electrons from CB of tungsten oxide and chemisorbed oxygen concentration O− increased and resulted in excessive release of electrons into the sensor (Fig. 4b). At the interface between rGO and WO3, a heterojunction formed. Holes and electrons formed in rGO and WO3, so electrons transferred to rGO from WO3, and holes moved to WO3 from rGO. After that, the hole and electrons having the opposite charge combine and results in vacant space due to a decrease in effective charge carrier and depletion layer is formed leading to increase in air resistance and good sensing of H2S.
Hao and coworkers performed a gas testing for NO2 using the WO3–rGO nanocomposite prepared hydrothermally. The WO3/rGO nanocomposite showed good sensitivity and kinetic response towards the NO2 and for other VOCs at low temperature (90 °C). The WO3/rGO presented p-type gas sensing behavior indicated by the sensing test. The comparison of gas sensing test between pure rGO and WO3-GO at the nanolevel and the experiment showed that the WO3/rGO has improved the sensing properties towards NO2 gas [158].
The GO/WO3 composite was prepared by the electro spinning method. This nanofiber sensors showed better sensing performances than that of the WO3 nanofiber [92]. First, due to the generation of the ohmic contact between WO3 nanograins and GO nanosheets resulting in improved sensing performances and enhanced resistance modulation. Figure 5a, c shows the WO3, and 1 ml WO3–GO composite SEM images. It can be observed that continuous and long 1D nanofibers are oriented randomly. From the SEM enlarge images as shown in Fig. 5b, d, the average diameter of WO3 and 1 ml of the GO–WO3 nanofibers were observed to be 150 and 100 nm, respectively. With an increase in the GO content in WO3, there was a clear decrease in the sensing performance because the WO3 surface covered with a high amount of GO, forming a continuous GO layer that acted as a conduction channel responsible for reducing the total sensor resistance. The reduced sensor resistance by GO–WO3 was very beneficial for practical application and served as the best candidate for acetone detection. Particularly, about 1 mL of the nanofibers of 1 ml GO–WO3 showed a high response of 35.9 to the 100 ppm acetone at the temperature of 375 °C and 4.3 times higher response than that of pure WO3 nanofibers.
(a) SEM image, (b) high-magnification SEM image, and (c) SEM image, (d) high-magnification SEM image. Reproduced with permission from Ref: [92], copyrights (2018) Elsevier
Jiang et al. prepared WO3–rGO nanocomposites by hydrothermal method with different wt% of GO (0.5 wt %, 1 wt %, 2 wt %, and 4 wt %) for investigating C2H2 gas. The Gas sensing results showed that 1 wt % of GO exhibited a very high response of (15.0–50 ppm), Low detection limit (1.3 ppm), fast response recovery (57 s, 27 s), excellent dynamic response (0.5–50 ppm), and long-term stability and outstanding working at the low optimal temperature (150 °C). Therefore, WO3/rGO nanocomposite sensor consider high-performance candidates for detection of C2H2 gas dissolved in oil-immersed at ppm-level [159].
Jeevitha et al. prepared porous rGO/WO3 conductive nanocomposite by simple ultrasonic method. The nanocomposites with different wt% (1%, 5%, 10% rGO/WO3) were prepared. The 5% rGO/WO3 nanocomposite had improved NH3 detection performance because of the improved pore size and surface area than that of pure WO3 nanospheres. As a result, the basic resistance is reduced, which results in a reduction in the width of the depleted region. WO3/rGO sensor showed p-type behavior for the detection of NH3. rGO has a high working function and provides numerous adsorption sites for the NH3. Therefore, the exposed surface of the sensor absorbed the NH3 molecules.
Among the different ppm solutions of NH3 exposed to rGO/WO3 nanocomposite, an increase in the resistance value (Fig. 6a–c) was observed with p-type gas sensor. While the resistance of WO3-based sensor decreased when exposed to the NH3 indicating the behavior of the WO3 n-type material. The rGO/WO3 nanocomposite sensing response towards NH3 is shown in Fig. 6d. rGO/WO3 nanocomposite with 5wt% showed the maximum response as compared to WO3. For different NH3 concentrations like 10, 20, 40, 60, 80, and 100 ppm, the response values of the 5% rGO/WO3 nanocomposite were 4.50, 5.22, 7.53, 9.69, 12.88 and 15.83, respectively. Nanocomposite sensors at 5% of rGO/WO3 have been found very effective for NH3 detection in presence of the interfering gases, and exhibit significant stability features at room temperature [160]. The interaction between adsorbed O2 and NH3, free electrons released which neutralized the rGO holes reducing the small charges and resulting in an increase in width of electron layer depletion thereby increases the sensor resistance as shown in Fig. 6e.
(a, b, c) A dynamic response along with the recovery curve of (1%, 5%, 10%) rGO/WO3 nanocomposite on exposure to the 10–100 ppm NH3 (d) rGO/WO3 response curve nanocomposite with varying the concentration of NH3 (e) rGO/WO3 gas sensing mechanism for NH3 at room temperature [160]
Biofunctionalized (WO3/rGO) nanocomposite synthesized hydrothermally and used as electrochemical immune-sensor for the cardiac biomarker detection. The nano-composite WO3/rGO further functionalized with APTES which provide active amino group that can covalently bind to antibodies. The results presents that this immunosensor exhibited a good sensitivity of 58.24 mA/cm2 per decade with a wide detection range of 0.01–250 ng/mL. The WO3/rGO showed a superior performance due to (i) strong synergistic effect between the WO3 and rGO which enhanced the electron transfer kinetics, (ii) this nanocomposite showed a strong covalent coupling of antibody molecules through APTES which leads to the high stability of the device, and (iii) WO3 has large oxygen moieties present leading to increase in the loading capacity of antibody resulting in wider detection range. Thus, all these features are responsible for high sensing performance of the proposed electrochemical immunosensor [161].
The work showed that various metal oxides with graphene are used as gas sensors for the testing of organic volatile products to minimize the harmful effects among all, tungsten oxide (n-type) shows tremendous results with graphene derivatives (p-type). Tungsten oxide and graphene derivatives (i.e. h/WO3/rGO prepare hydrothermally) are used as a sensor for hydrogen sulfide, similarly hydrothermally synthesized WO3/rGO for nitrogen dioxide and acetylene, WO3/GO prepared by electrospinning for acetone, and WO3/rGO prepared by ultrasonic method for ammonia has been used as good sensors.
GO/rGO-WO3 as an electrochromic material
WO3 has emerged as promising electrochromic (EC) material due to its high Electrochromic features, and low cost. However, the WO3 exhibits poor EC stability. Zhao et al. prepared the WO3/rGO composite film which has better optical, electrochemical, and EC properties than the WO3 films alone. The WO3/rGO composite membrane has very fast kinetics, supported by EIS measurements. The rGO in the composite film can effectively increase the electron transfer rate, provide a very larger surface for charge transfer reactions, shorten the path of ion diffusion in bulk material and allow the electrolyte to penetrate easily through the membrane. It can be concluded that the composite membrane can achieve rapid ion diffusion and high conductivity during the staining and bleaching cycle. The experiment showed that rGO/WO3 not only has a very high response time (tc = 9.0 s, tb = 4.5 s), better cycle performance (1000 cycles) but also has higher ΔT (64.2% at 633 nm) [162].
Khan et al. experiment using rGO/WO3 composite as a supercapacitor and to know its EC features for different applications such as in displays, antiglare mirrors, and smart windows. The porous rGO/WO3 composite films were prepared using the hydrothermal method. The stress applied to the WO3 by rGO, reinforces the transformation of the WO3 structure. At 663 nm the coloration efficiency (CE) and Delta OD values of rGO/WO3 film were 181.5 cm2/C and 0.46 cm2/C and optical modulation of about 58.8% higher than that of bare WO3 films which is around 122.2 cm2/C (Fig. 7a, b) without the EC stability 2500c/b cycles. Compared with pure thin films of WO3, the rGO/WO3 film exhibited an improved EC performance due high electrical conducting property of rGO incorporated into tungsten oxide. The rGO/WO3 composite constituted a very large optical modulation of about 58.8% at the wavelength of 633 nm with a great increase in electrochromic stability and coloration efficiency [163].
Optical transmittance spectra of (a) bare WO3 and (b) thin-film WO3/rGO composite in its colored and bleached state at 400–800 nm, reproduced with permission from Ref. [163]: copyrights (2020) Elsevier
Tungsten oxide has popularity due to its low cost and electrochromic features, but it has less stability as electrochromic material. So, the WO3/rGO is used because rGO provides larger surface area for electron transfer and shorten the ion diffusion path to penetrate electrolyte easily through membrane. Also, it is used as supercapacitor in smart windows, antiglare mirrors, and displays. Coloration efficiency and electrochromic stability is increased by WO3/rGO composite as it exhibits large optical modulation.
GO/rGO-WO 3 for electrochemical energy storage
Tungsten oxide can be considered as an attractive candidate for fast charging and discharging [164,165,166], as it displays pseudo-capacitive properties where the main charge storage occurs via the surface-based faradaic reactions between W6+ and W5+[167]. However, due to relatively low pseudo-capacitive performance, nanostructured WO3 is desirable to improve physicochemical characteristics such as electrical conductivity. Very little research has been focused on tungsten oxide and carbon composites-based on a capacitive properties [168]. So, if the tungsten oxide is incorporated into the graphene sheet, its conductivity can be increased. The inclusion of graphene into the tungsten oxide improves the availability of charge on the surface of the electrode and provides an effective surface area [168].
Huang et al. reported Gr/WO3 hybrid exhibiting good specific capacitance [169]. Ma et al. [170] combined the tungsten oxide with graphene using hydrothermal conditions, and the graphene–tungsten oxide composite exhibit very high specific capacitance with stable cycling stability. However, the composite has an interfacial contact area limited between tungsten oxide and graphene. The composite of tungsten oxide with rGO was synthesized using simple electrostatic self-assembly. It has been mentioned that the non-aqueous electrolytes do not provide large capacitance because of their smaller ionic radius whereas, large capacitance is provided by the aqueous electrolyte. EIS technique has been used for the characterization of the charge transfer between electrolyte and electrode [171]. WO3 can be used as an energy storage material [168] and its storage capacity can be enhanced by the addition of GO [172].
Chu et al. prepared WO3/rGO composite by coating WO3 nano-flower with rGO through electrostatic attraction between WO3 positive charge and negatively charged GO. It was also observed that the WO3 retained 83.7% of initial capacity while the WO3/rGO electrode retained 87.5% of initial capacity. The insertion of rGO to the WO3 surface by electrostatic interaction improved the capacitance, cycling stability and makes it a promising supercapacitor electrode material [173]. Wong et al. proposed two electrodes cell system, consisted of WO3 and WO3/rGO nanocomposite electrodes in the 1 M Na2SO3 neutral aqueous electrolyte using nickel foam and cellulose membrane and to evaluate their electrochemical properties. The supercapacitor capacitance of the one electrode can be calculated using the following follow,
In the above formula, the C is specific capacitance (F g−1), dt = time of discharge (s), I is the current that applied to the cell (A), m is the active material weight (g), and dV sweep potential window (V). rGO and WO3·H2O coupling results improved electrochemical performance of 244.0F g−1 (at the 1A g−1) as compared to the pure WO3 [174].
Korkmaz et al. synthesized the WO3/GO supercapacitor. These thin films are very useful as supercapacitors in electric devices. The graphene oxide provides the active region in WO3 decreasing the ion diffusion distance. Different substrates were used such as glass, PMMA, ITO, FTO for GO/WO3 coating forming the GO/WO3/Glass, ITO/GO/WO3 PMMA/GO/WO3, and FTO/GO/WO3 films. The optical properties of the GO/WO3 composite change with the nature of substrate. This resulted change in a film thickness, and surface properties with high transmittance and not high reflectivity and refraction index. The WO3-GO thin films are suitable to be used in optical lenses. Among the different substrates, the glass has shown maximum specific capacitance having a value of 268.5 F/g, on GO/WO3/Glass [175]. Ibrahim et al. prepared (WO3/rGO) nanocomposite, through pulsed laser ablation in liquids (PLAL). The synthesized nanocomposite used in the supercapacitor and had shown the 577 F g−1 specific capacitance value measured by the galvano-static charge–discharge (GCD) method [176].
For energy storage application dual ion battery system is being developed [177,178,179,180]. For battery applications, ordered meso porous tungsten oxides and their nanocomposite with carbon were prepared via various template methods such as hard silica templating and block-copolymer templating [181]. These nanostructured electrodes were then characterized by XRD and implemented in lithium-ion batteries where the enhanced conductivity and ordered meso porous structure improved the specific capacity and rate capability. WO3 nanorods were also anchored onto the three-dimensional nitrogen doped graphene by hydrothermal method. The composite electrode WO3/N-graphene demonstrated high capacity retention and high capacity of 313 mAh g−1 at 1.6 A g−1 [182]. Template-assisted method has been also used for the synthesis of WO3 nanorods with crystalline 3D orientation which significantly improved in columbic efficiency up to 99% and a high capacity of 253 mAh g−1 for 200 cycles [183]. Dual ion batteries are also attractive candidates.
WO3 has pseudocapacitive properties so it can be used in fast charging and discharging; however, improvement is needed in physicochemical property like electrical conductivity. WO3 with graphene sheets can increase conductivity by increasing the surface area and hence availability of charge on electrode will be increased. Only WO3 and graphene combination improves the conductivity but has limited interfacial contact area, so tungsten oxide was synthesized with rGO with improved capacitance and cycling stability. WO3/rGO can be used as energy storage material but adding GO can improve the storage capacity.
GO/rGO/WO 3 composite as photocatalysts
Catalysis is an important industrial process for obtaining clean energy. Catalysis can be divided in two subgroups, (the homogeneous and heterogeneous). The difference lies in the phase distribution of the catalysts i.e., reactants, and products. Due to the ease of separation, heterogeneous catalysis is an attractive field for researchers. In this type of catalysis, photocatalysts are generally solid materials which can generate or produce the electron–hole pairs under light irradiation. Photocatalytic processes are widely regarded as viable solutions to environmental problems, sustainable clean energy, bacterial disinfection, self-cleaning and water treatment [184]. In 1972 Honda and Fujishima introduced the first photoelectrochemical cell (PEC), which uses ultraviolet (UV) irradiations for the splitting of water. Catalysts accelerates photoreaction. Although a variety of catalysts can be used, at that time the titanium dioxide was considered the most fascinating photocatalyst in both hetero and homo catalysis with distinctive properties. The heterogeneous photocatalysis is more efficient than homogeneous photocatalysis because it enhances the efficiency of different semiconductor which inhibit the electron–hole recombination, exhibits high efficiency against organic pollutants, and exhibits extraordinary reusability [185]. The heterogeneous photocatalysis process is widely studied, especially in water purification [186,187,188,189,190,191], energy issues, and climate sustainability etc. [192, 193]. Heterogeneous photocatalyst such as g-C3N4-loaded Z-scheme nanohybrids, for the photocatalytic wastewater treatment including bacterial inactivation and pollutant degradation. Lately, graphitic carbon nitride (g-C3N4) can be employed in degradation of organic pollutant. However, pure g-C3N4 have high recombination rate and low visible light absorption [194]. These disadvantages can be overcome by the combination of pristine g-C3N4 with other semiconductors as reported [186, 195,196,197,198]. The BiOBr/CoFe2O4/Graphene served to be good photocatalyst for RhB dye as compared to pristine BiOBr/CoFe2O4. The increased photocatalytic activity of composite was attributed to the formation of heterojunction where photoexcited electrons can stabilize effectively [199]. The agglomeration of photocatalysts may results less effective activity and difficult separation of the nanoparticles. The coupling of two or more semiconductors can overcome the above drawback [200]. The photoactivity comprises of two sources (1) production of hydroxyl radical by oxidation of OH− ions, (2) production of oxygen radicals by reduction of O2. Both anions and radicals can destroy the pollutants. The process of the photocatalysis in water is divided into 5 stages:
-
Transfer of the reagents into the water on the surface of the photocatalyst.
-
Reagent’s adsorption on the surface.
-
Activation of the photon on the photocatalyst surface and adsorption reactions.
-
Product desorption.
These charge carriers in CB and VB independently diminish and oxidize the adsorbed compounds on the surface of the photocatalyst.
The photocatalyst materials are divided into three generations. The first generation photocatalyst consists of single or only one component materials (such as TiO2, CdS, and ZnO) [203], while the second generation of photocatalyst consists of several suspended components (such as WO3/NiWO4, C3N4/Ag3VO4, and BiOI/ZnTiO3) [204]. Photocatalysts that are immobilized on the solid substrate can be considered as third generation materials (such as steel/TiO2/WO3, FTO/WO3-ZnO, and glass/P-TiO2) [205]. Among the variety of visible light responsive photocatalysts, the tungsten oxide (WOX, X ≤ 3) has attracted large attention [206] because of its variable stoichiometry, structure, its appropriate bandgap, chemical stability, and relative abundance. However, the efficiency of WOX alone is relatively low because of the rapid recombination of electrons and the photo-generated holes [207].
Numerous strategies had been developed for enhancing the photocatalytic efficiency of the WOx, like doping of the elements, loading of nanoparticles of precious metals, semiconductor heterojunction coupling, and hybridization with carbon materials. The derivative of graphene (GO and rGO) when combined with several metal oxides such as Bi2WO6, Cu2O, ZnO, and ZnFe2O4 results in improved charge separation which facilitates the photocatalysis process [102, 168]. The rGO/GO acts as an electron acceptor for WO3 [103].
Chai et al. prepared WO3/rGO through a hydrothermal method for the decomposition of the methylene blue and proposed a photocatalytic degradation mechanism of the rGO/WO3 composite (Fig. 8). In the case of WO3/rGO the PL intensity decrease which indicated the better separation of electron–hole pairs. The rGO acts as an electron acceptor for WO3 and excited MB dye (the excited MB has the ability of electron transfer to CB of rGO or WO3 surface). As a result, the electrons transfer away from WO3 for avoiding the electron and holes pair recombination on the tungsten oxide surface. The nanoplate composite WO3/rGO degraded 95% of MB under visible light [208].
The antibiotics present in the aquatic system cause serious environmental issues, sulfonamide groups used commonly in antibiotics [209], which are difficult to be removed by conventional methods [210]. Zhu et al. prepared WO3/rGO composites using the one-step hydrothermal method for photocatalytic removal of sulfamethoxazole. Three types of WO3/rGO composites were prepared (RW400, RW200, RW100). Among them, RW-100 and RW-200 degraded the SMX more than 98% within 3 h under visible light. Therefore, the rGO/WO3 composite was effective and exhibits good photocatalytic activity for degrading the SMX [211].
The graphene oxide enhances photocatalysis due to its significant absorption and photocatalytic effect [119, 212, 213]. The highest visible light photocatalysis was achieved due to WO3 deposition on the smooth surface of GO. In 2019 Sajjad et al. used WO3–GO photocatalyst for degradation of MO under visible light. Heterojunction WO3–GO was prepared by hydrothermal method with different wt ratios i.e., 1.0% WO3–GO, 5.0% WO3–GO, 10.0% WO3–GO, and 15.0% WO3–GO. The 10.0 wt% WO3–GO demonstrated the highest photocatalytic activity after 4 h irradiation under visible light while WO3-GO (1.0%, 5.0%, 15.0%) degraded 70, 75, and 77% MO, respectively. As the WO3 amount increases on the surface of GO, the active site of the catalyst in a solution of dye also increases but up to a certain limit. It was experimentally found that 10.0% WO3–GO concentration showed the optimum value, after that the when the concentration is increased further, this leads to the blocking of the active site resulting in the decrease in efficiency of GO/WO3 photocatalyst and less light penetration [214].
The photocatalytic evolution of O2 using WO3/rGO was studied. The rGO incorporation into WO3 can enhance the tungsten oxide photocatalytic activity. The function of rGO is to improve the photogenerated charge transfer between graphene and metal oxide semiconductor and improving the photocatalytic performance. It has been found that the rate of O2 evolution is enhanced by the WO3/rGO hybrid at a higher rate than the alone WO3. The sunlight irradiated WO3/rGO composite generates electrons which are excited to CB of the WO3, and then move to rGO sheets, results in efficient separation of the photogenerated electrons-hole pairs. While the photo induced holes still stay at VB of WO3 and participate in water oxidation reaction as shown in Figure 9b, c. Different wt%, i.e., 1wt%, 3wt%, and 5wt% WO3/rGO solutions were prepared for the photocatalytic O2 evolution. Among different wt% solutions of WO3–rGO, the 3wt% of WO3–rGO exhibited maximum photocatalytic activity for O2 evolution (Fig. 9a) [215]. Table 3 presents the overview of the functions of different graphene derivatives. Metal-based nanosheets are effective electrocatalysts for water oxidation [216].
(a) Evolution curve of O2 reproduced with permission from Ref. [215]: copyrights (2019) Elsevier, (b) rGO/WO3 band energy alignment and photocatalytic pathway (c) for water oxidation in presence of solar light
This portion showed the photocatalytic activity of derivatives of graphene with tungsten oxide. The rGO/WO3 synthesized through hydrothermal method used for decomposing of the methylene blue and sulfamethoxazole. The GO/WO3 photocatalyst used for degradation of MO under visible light and for the photocatalytic evolution of O2.
Classification and application of WO3/rGO and GO-based ternary composites
Both GO and rGO materials offer the possible fabrication of tungsten oxide [217,218,219] ternary composites such as ZnO/WO3 [219, 220], TiO2/WO3 [221, 222], Fe2O3/WO3 [223, 224], MoO3/WO3 [225], PPy/WO3 [226, 227], Ir/WO3 [228], WS2/WO3 [229, 230] to improve the applications in gas sensors, energy storage, photocatalysis, and electrochromism etc.
Based on transition metals and metal oxides
Transition metal oxides are important materials and have applications in photocatalysts, gas sensing, energy storage, and chromium materials (electrochromic and photochromic materials). The Hematite (α-Fe2O3) is a thermodynamically most stable phase of iron oxide having forbidden band ~ i.e2.1 eV [231], visible-light photocatalyst [232, 233], and has suitable band positions matching with WO3 [234]. Tungsten oxide with graphene derivates has emerged potential material towards the electrochromic and photocatalytic behavior in visible light. TiO2/WO3 combination enhances the photocatalytic activity of TiO2, as titanium oxide has a relative band gap of 3.2 eV, which limits its use in the visible light region [235, 236].
ZnO oxide also possesses comparable band gap energy as that of titanium [237, 238]. Tungsten trioxide (WO3) and molybdenum trioxide (MoO3) are semiconductors transition metal oxides, which exhibit photochromic properties under ultraviolet radiation. The reason is the formation of bronze with partial hydrogen and the formation of tungsten or molybdenum ions from W6+ (transparent) to W5+ (blue) (W → W) or Mo6+ (Transparent) to Mo5+ (blue) (Mo → Mo). The coloring of MoO3 can be enhanced by the addition of WO3/rGO [130]. The combination of binary tungsten oxide composites with GO/rGO resulted in higher performance especially in the field of photocatalysis.
TiO 2 /WO 3 /GO nanocomposite for BPA degradation
The presence of epoxy, carboxyl, hydroxyl, and carbonyl groups in graphene oxide provides great thermal, mechanical, and chemical properties [239]. Graphene oxide can provide a better separation of charges [240] as in GO/TiO2 [241] thereby increases the photocatalysis efficiency. Hao et al. prepared the nanocomposite (GO/TiO2/WO3) photocatalyst for degrading the bisphenols. GO/TiO2/WO3 nanocomposites with different wt% were prepared hydrothermally. The photocatalytic mechanism was proposed since charge separation facilitated due to photo-generated electrons transfer from TiO2 to WO3 to GO and photogenerated holes transfer from WO3 to TiO2. The experiment was performed for 7 h under sunlight (Fig. 10). The 2 mg/L WO3/TiO2/GO showed 93.2% of BPA degradation. The WO3/TiO2/GO exhibited great reusability and stability by removing 88.2% BPA after a five-cycles of reusability [242].
BPA photodegradability under sunlight; pH 7, [BPA] = 20 mg/L, reproduced with permission from Ref. [242]: copyrights (2017) Elsevier
The reusability of heterogenous nanocomposite is of great importance in photocatalysis. To test the reusability of the photocatalyst the experiments are being carried out many times in same conditions. The reusability is then determined by observing the percentage of photocatalytic degradation after multiple use of photocatalyst. For reusability, the photocatalyst dissolve in solution after first usage, should be separated from the reaction medium either by centrifugation or filtration and then dried in oven. After that, the photocatalyst is treated again for degradation and its degradation percent is calculated again. The same process is repeated many times. This assure the reusability of the photocatalyst [243]. Hasannia et al. observed the reusability of photocatalyst WO3/rGO and showed good degradation after 5 catalytic cycles [244]. Govindaraj et al. examined the rGO/WO3 nanorods reusability and showed a good photocatalytic degradation for Ciprofloxacin (i.e., 90%) after three consecutive cycles [245].
The Z scheme photocatalyst Fe 2 O 3 /GO/WO 3 for enhancing the solar light photocatalytic reaction in water remediation
In the Z scheme photocatalytic system, the photogenerated electrons of the photosystem I (PSI) in the CB recombine with photogenerated holes of photosystem II (PSII) in VB via the interface. This Z-system allows the effective utilization of visible light to improve photocatalysis [246, 247]. Z- scheme photocatalyst-based on graphene oxide (GO) was prepared by Mohamed et al. The Z-scheme mechanism of α-Fe2O3/GO/WO3 is shown in Fig. 11a. Through GO interface, the photogenerated CB electrons of the WO3 recombined with the VB holes of Fe2O3. This improves the separation of the electron and hole pair thus decrease the recombination in both WO3 and Fe2O3 and increase the abilities of the reduction and oxidation of photogenerated electrons inFe2O3 and holes in WO3 electron.
(a) Z-scheme Fe2O3/GO/WO3 nanocomposite photocatalytic mechanism, (b) MB photocatalytic degradation efficiency as the C/C° variations with irradiation time, reproduced with permission from Ref: [248], copyrights (2019) Elsevier
The remaining photogenerated electrons of Fe2O3 produced a highly reactive species (O2−), while the remaining photogenerated holes form the •OH radical by reacting with –OH. These reactive radicals O2− and OH− are the responsible species for wastewater pollutant degradation. The α-Fe2O3/GO/WO3 was tested for phenol and the result showed that 95.4% of the phenol was degraded within 120 min. The ternary Fe2O3/GO/WO3 nanocomposite were prepared by ex-situ mixing using 5, 10, 30, and 50% of GO, and nanocomposite represented in form of FGW5, FGW10, FGW30, and FGW50. Figure 11b shows the FWO nanocomposite photocatalytic efficiency for MB and CV degradation. The activity increases with increasing the ratio of GO in the composite in the order FGW5 < FGW10 < FGW30 while with FGW50 the activity slightly decreased for the MB and CV degradation [248].
rGO/WO 3 /MoO 3 nanocomposite as photochromic material
Photochromism is the reversible change in the color of the material in presence of the sunlight. rGO/WO3/MoO3 ternary composite has photochromic properties. A photochromic test was carried out under ultraviolet irradiation for 50 min. For WO3/MoO3 films with rGO, the color changes from gray to dark blue, while for WO3/MoO3 films, the color changes from white to deep blue. The WM2 with 1.3 × 10–3 wt% of rGO-doped WO3/MoO3 showed good coloration when heated at 80 °C for 10 min and resulted in 94% reversibility, with rapid photochromic response and stability. Figure 12 shows the color photograph of films exposed to UV light for 50 min and thermally treated at 80 °C for 20 and 360 min. Under UV irradiation, the WO3/MoO3 film changed its colors from white to dark blue while the rGO-doped WO3/MoO3 films changed their colors from gray to dark blue. The WO3 and rGO-doped WO3 having a pure orthorhombic phase changed color from yellow to dark yellow. Afterward, the samples were kept in an oven at 80 °C, WO3/MoO3 and rGO-doped WO3/MoO3 films can return to the original gray color but WO3 and rGO-doped WO3 films cannot return to the original yellow color. The change in color of WO3/MoO3 and rGO-doped WO3/MoO3 films was due to disordered crystal structure containing high oxygen vacancies which resulted from their amorphous and hexagonal phase (mixed-phase) containing more molecules of water, leading to high insertions of photon and high electron mobility [249].
The photographic images of samples: (a) W, (b) W4, (c) WM, (d) WM2, and (e) WM4 irradiated under UV light for 50 min and then kept in an oven at 80 °C for 20 and 360 min [249]
ZnO/WO 3 /rGO for methylene blue degradation
Ternary nanocomposite (WO3-ZnO@rGO) was prepared by Chaudhary et al. through the simple ultrasound-assisted fabrication of WO3-ZnO over 2D rGO nanosheets. The ZnO/WO3/rGO nanocomposite was used to degrade the MB dye. The WO3-ZnO@rGO degraded MB dye upto 94% under visible light within 90 min because it possesses high quantum yield, good separation of electron–hole pairs, and retained the photocatalytic degradation efficiency even after 4 cycles of reusability. Therefore can be served as a potential candidate for wastewater treatment [250].
Zr-MOF@WO 3 /GO for tetracycline and malathion photodegradation
The poor semiconductor properties of pristine MOFs make them less favorable for photocatalytic applications [251]. A novel MOF-based nanocomposite with tungsten oxide and graphene oxide was studied for the first time by Fakhri and Bagheri for tetracycline and Malathion photodegradation. The nanocomposites were prepared through the solvothermal method. The UiO-66@ WO3/graphene oxide had served best removal efficiencies about 100% for MA and 97% for TC, respectively, under visible light within 70 min. It was found that tungsten oxide and UiO-66 had proper valence and conduction band matching which resulted in faster-photoinduced carriers and electron–hole pair separation on the graphene oxide layer. Therefore, the ternary composite had shown high performance in purifying polluted water [252].
Ni:FeOOH/WO 3 /rGO for water oxidation
In 2020, ternary composite WO3/rGO/Ni:FeOOH photo anode designed by Zhang et al. exhibited a 2.05 time larger photocurrent as compared to bare WO3 NFs. The tungsten oxide alone possesses low activity and instability in photo electrochemical (PEC) for water splitting. Therefore Ni:FeOOH is used as a bi-functional modifier that increases and promotes light absorption and charge transfer with WO3 NFs. The addition of rGO facilitates charge separation. The coupling of rGO, Ni:FeOOH, and WO3 nanoflakes (NFs) (WO3/rGO/Ni:FeOOH) enhanced the PEC activity. As a result, the WO3/rGO/Ni:FeOOH photo anode exhibited 1.32 mA cm−2 (1.23 V vs. RHE) in a neutral solution under irradiation [253].
Ag@WO 3 @rGO nanocomposites as photocatalyst for degradation
In 2020, the ternary nanocomposite of Ag@WO3@rGO was studied by Tran et al. synthesized through a two-step hydrothermal method for degradation of Rhodamine B. The addition of rGO and Ag reduced the optical band of WO3 which ultimately enhanced the photocatalytic activity of WO3-based composites under sunlight as compared to bare Ag-1@WO3 and WO3 [254, 255]. Therefore, the Ag@WO3@rGO degraded about 99.5% of RhB due to the absorptive effect of rGO, along with the synergistic effect of the electron reservoir of Ag with the visible light responsive band gap of WO3. Figure 13 shows the RhB adsorption results in darkness for 30 min. The bare WO3 adsorbed 3.8 wt. % of RhB, while for the Ag-doped WO3, the process of adsorption occurred more efficiently and reached 16.2, 18.7, and 22.0wt. % for the Ag1@WO3, Ag3@WO3, Ag5@WO3, respectively. The highest adsorption capacity of 29.1wt% was exhibited by the Ag3@WO3@rGO composite [256].
RhB adsorption amount by bare WO3, Ag1@WO3, Ag3@WO3, Ag5@WO3, and Ag-3@WO3@rGO within 30 min under dark conditions, Reproduced with permission from Ref [256]: copyrights (2020) Elsevier
POM-Ir/WOx/rGO nanocomposites for electrocatalytic water splitting
The Ir and WO3 combination has been rarely reported, while the Ir/WO3 catalysts have not been applied to electro catalysis for water splitting due to the irregular dispersion and uncontrollable preparation. The POM-derived Ir/WOx/rGO nanocomposites originated from Ir colloid solution, and a POM precursor was prepared for the first time. This composite showed enhanced performance of electrochemical for OER, HER, and water splitting. This work also proved that POM can served as a precursor for constructing the metal oxide to support the iridium catalysts thereby providing a new vision for designing multi-metal electrocatalysts [257].
The classification of graphene derivatives with tungsten oxide ternary nanocomposites showed applications such as photocatalysis, sensing, electrochromism and photochromism. The photocatalyst GO/TiO2/WO3 used for degrading the bisphenols, ZnO/WO3/rGO for Methylene blue degradation, Ag@WO3@rGO for degradation of Rhodamine B. Also Fe2O3/GO/WO3 and Ni:FeOOH/WO3/rGO used for water oxidation. rGO/WO3/MoO3 nanocomposite used as photochromic Material. Zr-MOF@WO3/GO used for tetracycline and malathion photodegradation and POM-derived Ir/WOx/rGO nano-composites for water splitting.
Based on transition metal sulfides
rGO/WO 3 /WS 2 as supercapacitor
The WS2 and rGO combination prevents the restacking of tungsten disulfide and graphene for minimizing the variation in volumes, provide a large electrode interface during the electrochemical charge and discharge cycle, which gives rise to efficient rate capability [257, 258]. On the other side, the tungsten oxide and rGO improve the electrode conductivity, leading to enhanced performance in the supercapacitor devices. Therefore, Yang et al. prepared a nanohybrid of rGO/WS2/WO3 (G/TS/TO). The synergistic effect that arises from the high electrical conductivity of tungsten oxide, the high surface area of rGO, and large electrode interfaces of tungsten disulfide, the nanohybrid had shown cyclic stability retention of 90% after 3000 charges/discharge cycles. Therefore, the G/TS/TO had shown enhanced supercapacitor performance [259].
1 T‐MoS 2 /h/WO 3 /GO supercapacitors
Ternary 1 T‐molybdenumdisulfide hexagonal tungsten trioxide reduced graphene oxide (M‐W‐rGO) composite were prepared by Naz et al. through a one‐pot hydrothermal process. The EIS techniques used to study the electrochemical performance of ternary M‐W‐rGO composite. The Specific capacitance comparison was made between M‐rGO, W‐rGO, and M‐W‐rGO composites; among them, the M‐W‐rGO had a high specific capacitance value of 836 F g−1 at 1 A g−1, with large 86.35% capacitance retention after 3000 cycles at a high current density of 5 A g−1. The Nyquist plot is shown in Fig. 14(a) concluded that the M‐W‐rGO composite had the lowest Rct of 2.42 Ω as compared to all the electrodes due to the synergic effect of MoS2 andWO3 and conductive nature of 1 T‐MoS2, rGO, which ultimately lead to increased electrical conductivity. The M‐W‐rGO has a high discharge time as compared with the other electrodes, and highest specific capacitance of 836 in the M–W–rGO electrode than that of M‐rGO, and W‐rGO composites 442, and 350 F g−1 at a current density of 1 A g−1, respectively (Fig. 14b) [260].
(a) Nyquist plots of composites MrGO, w‐rGO, and MWrGO. (b) Super capacitance performance comparison for MrGO, WrGO, and MWrGO composites [260]
Based on metal sulfide, the graphene derivatives with tungsten oxide showed the super capacitive application. The combination of metal sulfide with graphene derivative prevents the restacking of material and result in efficient rate capability. rGO/WO3/WS2 and 1 T‐MoS2/h/WO3/GO used as supercapacitor and their advantage discussed in above section. Fabrication of supercapacitor-based on ionic liquids is another effective strategy [261].
Based on non-metal
NCQDs with GO/WO 3 nano-sheets
The graphene quantum dot (GQD) has promising functionalities in catalysis, sensing applications, drug delivering, and bioimaging, because of its low toxicity, water solubility, biocompatibility, and tunability of photophysical properties. One advantage of GQD lies in the fact that it can be easily synthesized at mild chemical conditions, and its properties can be easily tuned by chemical modifications and doping techniques [262]. The quaternary PGCN/AgI/ZnO/CQDs nanocomposite has been successfully fabricated by an efficient hydrothermal method using naturally occurring bamboo leaves as precursors for CQDs production, while excluding the use of toxic reducing agents. The g-C3N4 integration to AgI/ZnO with CQDs and accelerated the photocatalytic activity which is attributed to electron sink behavior of CQDs. The fabricated Z-scheme P-doped g-C3N4/AgI/ZnO/CQDs photocatalyst exhibited remarkable stability and recyclability for ten consecutive catalytic cycles [263]. NCQDs had extended light absorption in the visible region, provide the electron–hole recombination hindrance by electron trap, and increase the number of reactive species. As a result of these properties, the NCQDs enhance the photocatalytic activity of the photocatalyst composite. The main role of the NCQD is in modifying the morphology, optical properties, increase the absorbance of visible light, and increase in the photocatalytic activity of WO3 [264].
Jamila et al. prepared an efficient three-way photocatalyst by coupling the N-doped CQD with the WO3 nanosheets modified by the GO. The 15.0% of GO was added to the WO3 solution and then stirred to form the binary complex. WO3/GO/NCQD composites were prepared by adding 0.5, 1.0, 1.5, and 2.0 mL of NCQD. MO is an acidic dye and organic pollutant [265]. The photocatalytic activities of the samples were studied by preparing 10 ppm dye solution. The photocatalytic activity of (a) Pure WO3, (b) WO3/GO, (c) WO3/GO/0.5 mL-NCQDs, (d) WO3/1.5 mL-NCQDs, (e) WO3/GO/1.0 mL-NCQDs, (f) WO3/GO/2.0 mL NCQDs, (g) GO/1.5 mL-NCQDs, (h) WO3/GO/1.5 mL- NCQDs was tested. The ternary photocatalyst WO3/GO/N-CQDs having 0.5 mL, 1.0 mL, and 1.5 mL amount of NCQDs has shown 53%, 68%, and 86% of MO degradation, respectively (Fig. 15a, c, e, h). The NCQDs with a 1.5 mL amount in WO3-GO had shown the maximum (86%) degradation of the MO under visible light. Above 1.5 mL of NCQS amount, the photocatalytic efficiency started to decrease due to the light scattering effect by NCQs. The MO degradation followed the first-order reaction kinetics as shown in Fig. 15b [266].
(a) Time-dependent degradation profile of MO under visible light, (b) lnCO/C for MO degradation, reproduced with permission from Ref. [266]: Copyrights (2020) Elsevier
Based on polymers
WO 3 /polyaniline-g-graphene oxide nanocomposite for chromium detection
Polyaniline is a conductive polymer containing NH2 functional group [267]. Khan and co/WOrkers prepared the PANI-g/rGO@WO3 nanocomposite thin film by a simple coating method with a conductive adhesive deposited on AgE for the ultra-sensitive identification of Cr3+ with a very short response time. Cr3+ions having a lower concentration in the aqueous solution, there was small surface coverage of Cr3+ ion on the film of WO3/polyaniline-g-graphene oxide PANI/AgE and the surface reaction proceed steadily. Therefore, by increasing in Cr3+ concentration, the surface reaction significantly increases. The current response is stable when the Cr3+concentration is 0.01 M, while there is a very fast increase in current response due to further increase in the concentration of the Cr3+ion on the surface of PANI-g/rGO@WO3/AgE in the unsaturation area which is the lower region of the Cr3+ concentration [268].
Tetraphenyl porphyrin/WO 3 /rGO for acid blue 25 photocatalytic degradation
The ternary composite TPP/WO3/rGO was studied and prepared by Malefane et al. through in situ method and the product was calcined at different temperatures for the degradation of Acid blue 25 under visible light. The nano-composite exhibited a bandgap of 2.14 eV, spherical shapes table upto 3 cycles, and degraded 85%of AB25. This composite can be efficiently used for the photocatalytic treatment of textile waste, energy conversion, hydrogen generation [269].
g-C 3 N 4 /rGO/WO 3 for ciprofloxacin photocatalytic degradation
Consequently, Ag/AgBr/VO/PCN photocatalyst has been reported. This has been synthesized effectively using a direct Z-scheme photocatalytic system with suitable band association. In this case the doping of phosphorous increases specific surface area of GCN and make it effective for adsorption of pollutants to perform the photodegradation activity. The Z-system can work in both gas and liquid phase environments, which are widely used in water splitting, photoreduction of the CO2, and decompose variety of the organic contaminations [270].
Z-scheme photocatalyst g-C3N4/rGO/WO3 was studied by Lu et al. prepared through the facile photo reduction method. The g-C3N4/rGO/WO3 prevented the reunion of the charges and enhanced electron mobility due to the electron transfer property of reduced graphene oxide as compared to the g-C3N4, g-C3N4/WO3, and WO3 alone. The photocatalytic degradation rate by g-C3N4/rGO/WO3 was twice as much as that of g-C3N4/WO3 for ciprofloxacin. So, as a result of Lu’s experiment, the Z scheme rGO/g-C3N4/WO3 system enhanced the efficiency of charge separation [271]. Table 4 presents a detailed overview of tungsten oxide with graphene derivatives composites.
The graphene derivatives with tungsten oxide ternary composite have applications in field of supercapacitor and photocatalysis. PANI-g/rGO@WO3 nanocomposite used for ultra-sensitive identification of Cr3+. TPP/WO3/rGO and g-C3N4/WO3 used for degradation of Acid blue 25 and ciprofloxacin.
Future challenges
Various metal and metal oxide-based sensors have drawn much attention in past decades due to their stability against dissolution, low-cost fabrication, and independence from cationic interference. However, they still face limitations, such as fabrication cost, high-energy consumption and design complexity. In recent years, tungsten oxide has been studied. It is possible to fabricate the tungsten oxide on large scale using wet-chemistry coating technologies, such sol–gel method which is applicable due to its low-cost production and large area of coating capability. The quality of the coatings and crystallinity can be controlled by changing the sputtering parameters such as oxygen content, power density, substrate temperature and pressure.
Conclusion
The tungsten oxide with the GO and rGO presents an important role in several fields such as supercapacitor, electrochromism, energy storage, electrocatalysis, and photocatalysis. The incorporation of the graphene derivatives enhances the tungsten oxide properties and makes it a suitable candidate to be used in various fields. The advancement of WO3-GO/rGO-based electrodes shows excellent energy storage features with environment friendly compatibility. Also, the WO3/rGO nanocomposite displays improved EC properties as compared to alone WO3. The WO3/GO and WO3/rGO served as a good and efficient gas sensor for the detection of the acetone and NH3 in a very quick response time. Overall, this review article provides the recent work in field of supercapacitor, photocatalyst, EC material and gas sensor by graphene-based tungsten oxide. The essential information of graphene-based tungsten oxide nanocomposite in above fields (their advantages and disadvantages) will fueled up further research on graphene-based tungsten oxide materials. The growing global demand of energy is one of the future challenges due to extinction of energy resources. The graphene-based WO3 are stable enough to be used as effective electrode in supercapacitor due to its low cost and high performance. Despite of several advantages of graphene-based WO3 nanocomposite as sensor and supercapacitor, there are still some challenges and difficulties associated with it. The main challenge is the lack of work-based on graphene/WO3 as sensor in bio-medical field. Therefore, new novel work should be needed to understand the mechanism, factor affecting the interaction between the nanocomposite and microorganism. Also, it is important to investigate the antibacterial properties of GO/rGO/WO3 nanocomposite, their antibiotic resistance so that these composites can have clinical applications. Although the GO/rGO/WO3-based composites show excellent properties, their photocatalysis applications are limited due to the fast reunion of the electron–hole pair of tungsten oxide. Some improvements have been made by introducing a ternary heterojunction Z-scheme system which has the advantage of better charge separation. So, researchers are developing new routes to overcoming the limitation of the composite of tungsten oxide with graphene derivative especially in the field of photocatalysis. Therefore, there is need for the urgent development of new and improved work on graphene-based tungsten oxide nanocomposite as sensor, supercapacitor and photocatalyst. We prospect that the data provided in this review about the recent 5-year application of advances on the graphene-based tungsten oxide nanocomposite will help the researchers to understand the ongoing development. This will help in improving the ideas and working to conquer the associated challenges.
Abbreviations
- TMOs:
-
Transition metal oxides
- MxWO3 :
-
M = metal
- NIR:
-
Near-infrared
- LIBs:
-
Lithium-ion batteries
- CV:
-
Crystal violet
- BPA:
-
Bisphenol A
- NRs:
-
Nanorods
- MOs:
-
Metal oxides
- AgE:
-
Silver electrode
- PANI-g/rGO@WO3:
-
Polyaniline-grafted-reduced graphene–tungsten oxide
- FGW:
-
Fe2O3/GO/WO3
- FGW 5:
-
5% GO
- FGW 10:
-
10% GO
- FGW 30:
-
30% GO
- FGW 50:
-
50% GO
- RhB:
-
Rhodamine blue
- RHE:
-
Reversible hydrogen electrode
- POM-Ir/WOx/rGO:
-
Polyoxometalate-derived iriduim/tungsten oxide/reduced graphene oxide
- EIS:
-
Electrochemical impedance spectroscopy
- M‐W‐rGO:
-
1 T-molybdenum disulphide-hexagonal tungsten trioxide-reduced graphene oxide
- PL intensity:
-
Photoluminescence intensity
- Rct:
-
Charge transfer resistance
- PEC:
-
Pulsed Eddy current
- NCQDs:
-
Nitrogen-doped carbon quantum dots
- RW400, RW200, RW100:
-
RGO/WO3 with different Na2WO4·2H2O dosages, the three samples were named RW-100, RW-200, and RW-400
- SMX:
-
Sulfamethoxazole
- WM:
-
WO3/MoO3 with 50% rGO
- WM1:
-
WO3/MoO3 with 0.65 × 10–3% rGO
- WM2:
-
WO3/MoO3 with 1.30 × 10–3% rGO
- WM3:
-
WO3/MoO3 with 2.0 × 10–3% rGO
- WM4:
-
WO3/MoO3 with 2.6 × 10–3% rGO
- OER:
-
Oxygen evolution reaction
- HER:
-
Hydrogen evolution reaction
- APTES:
-
3-Aminopropyltriethoxysilane
- MTV:
-
Multivariate metal
References
Bursten, J.R., Roco, M.C., Yang, W., et al.: Nano on reflection A number of experts from different areas of nanotechnology describe how the field has evolved in the last ten years. Nat. Nanotechnol. 11, 828–834 (2016)
Dao, T.D., Jeong, H.M.: Graphene prepared by thermal reduction–exfoliation of graphite oxide: effect of raw graphite particle size on the properties of graphite oxide and graphene. Mater. Res. Bull. 70, 651–657 (2015)
Hu, M., Yao, Z., Wang, X.: Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 56, 3477–3502 (2017)
Wang, J., Jin, X., Li, C., et al.: Graphene and graphene derivatives toughening polymers: toward high toughness and strength. Chem. Eng. Sci. 370, 831–854 (2019)
Dutta, V., Singh, P., Shandilya, P., et al.: Review on advances in photocatalytic water disinfection utilizing graphene and graphene derivatives-based nanocomposites. J. Environ. Chem. Eng. 7, 103132 (2019)
Matijašević, S., Zildžović, S., Stojanović, J., et al.: Removal of uranium (VI) from aqueous solution by acid modified zeolites. Zaštita materijala. 57, 551–558 (2016)
Dai, L., Li, L., Zhu, W., et al.: Post-engineering of biochar via thermal air treatment for highly efficient promotion of uranium (VI) adsorption. Bioresour. Technol. 298, 122576 (2020)
Duan, J., Ji, H., Xu, T., et al.: Simultaneous adsorption of uranium (VI) and 2-chlorophenol by activated carbon fiber supported/modified titanate nanotubes (TNTs/ACF): Effectiveness and synergistic effects. Chem. Eng. Sci. 406, 126752 (2021)
Rout, S., Muduli, B., Kumar, A., et al.: Removal of uranium (VI) from water using hydroxyapatite coated activated carbon powder nanocomposite. J. Environ. Sci. Health A. 55, 596–605 (2020)
Qiu, M., Liu, Z., Wang, S., et al.: The photocatalytic reduction of U (VI) into U (IV) by ZIF-8/g-C3N4 composites at visible light. Environ. Res. 196, 110349 (2021)
Liu, R., Wang, H., Han, L., et al.: Reductive and adsorptive elimination of U (VI) ions in aqueous solution by SFeS@ biochar composites. Environ. Sci. Pollut. Res. 28, 1–10 (2021)
Chen, R., Cheng, Y., Wang, P., et al.: Facile synthesis of a sandwiched Ti3C2Tx MXene/nZVI/fungal hypha nanofiber hybrid membrane for enhanced removal of Be (II) from Be (NH2) 2 complexing solutions. Chem. Eng. Sci. 421, 129682 (2021)
Liu, F., Hua, S., Wang, C., et al.: Adsorption and reduction of Cr (VI) from aqueous solution using cost-effective caffeic acid functionalized corn starch. Chemosphere 279, 130539 (2021)
Sabzehmeidani, M.M., Mahnaee, S., Ghaedi, M., et al.: Carbon-based materials: a review of adsorbents for inorganic and organic compounds. Adv. Mater. 2, 598–627 (2021)
Ciceroni, C., Agresti, A., Di Carlo, A., et al.: Graphene oxide for DSSC, OPV and perovskite stability. In: Korotcenkov, G. (ed.) The Future of Semiconductor Oxides in Next-Generation Solar Cells, pp. 503–531. Elsevier (2018)
Luo, Q., Zhang, Y., Liu, C., et al.: Iodide-reduced graphene oxide with dopant-free spiro-OMeTAD for ambient stable and high-efficiency perovskite solar cells. J. Mater. Chem. 3, 15996–16004 (2015)
Ashiq, H., Nadeem, N., Mansha, A., et al.: G-C3N4/Ag@ CoWO4: a novel sunlight active ternary nanocomposite for potential photocatalytic degradation of rhodamine B dye. J. Phys. Chem. Solids 161, 110437 (2021)
Zou, Y., Hu, Y., Shen, Z., et al.: Application of aluminosilicate clay mineral-based composites in photocatalysis. Res. J. Environ. Sci. 115, 190–214 (2022)
Yao, L., Yang, H., Chen, Z., et al.: Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere 273, 128576 (2020)
Rastogi, A., Zivcak, M., Sytar, O., et al.: Impact of metal and metal oxide nanoparticles on plant: a critical review. Front. Chem. 5, 78 (2017)
George, J.M., Antony, A., Mathew, B.: Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchim. Acta 185, 358 (2018)
Xu, H., Zhu, S., Xia, M., et al.: Three-dimension hierarchical composite via in-situ growth of Zn/Al layered double hydroxide plates onto polyaniline-wrapped carbon sphere for efficient naproxen removal. J. Hazard. Mater. 423, 127192 (2021)
Parnianchi, F., Nazari, M., Maleki, J., et al.: Combination of graphene and graphene oxide with metal and metal oxide nanoparticles in fabrication of electrochemical enzymatic biosensors. Int. Nano Lett. 8, 229–239 (2018)
Galstyan, V., Comini, E., Kholmanov, I., et al.: Reduced graphene oxide/ZnO nanocomposite for application in chemical gas sensors. RSC adv. 6, 34225–34232 (2016)
Sekhar, D.C., Diwakar, B.S., Babu, B.R., et al.: Development of graphene oxide-based hybrid metal oxide nanocomposites of GO-SnO2/ZnO/Fe3O4, GO-SiO2/ZnO/Fe3O4 for energy applications. Phys. B 603, 412749 (2021)
Kim, H.W., Na, H.G., Kwon, Y.J., et al.: Microwave-assisted synthesis of graphene–SnO2 nanocomposites and their applications in gas sensors. ACS Appl. Mater. Interfaces. 9, 31667–31682 (2017)
Achary, L.S.K., Kumar, A., Barik, B., et al.: Reduced graphene oxide-CuFe2O4 nanocomposite: a highly sensitive room temperature NH3 gas sensor. Sens. Actuators B Chem. 272, 100–109 (2018)
Borges, L.G.A., Savi, A., Teixeira, C., et al.: Mechanical ventilation weaning protocol improves medical adherence and results. J. Crit. Care. 41, 296–302 (2017)
Feng, Q., Li, X., Wang, J., et al.: Reduced graphene oxide (rGO) encapsulated Co3O4 composite nanofibers for highly selective ammonia sensors. Sens. Actuators B Chem. 222, 864–870 (2016)
Choi, S.-J., Choi, C., Kim, S.-J., et al.: Facile synthesis of hierarchical porous WO3 nanofibers having 1D nanoneedles and their functionalization with non-oxidized graphene flakes for selective detection of acetone molecules. RSC Adv. 5, 7584–7588 (2015)
Anand, T., Sahoo, S.K.: Cost-effective approach to detect Cu (II) and Hg (II) by integrating a smartphone with the colorimetric response from a NBD-benzimidazole-based dyad. Phys. Chem. Chem. Phys. 21, 11839–11845 (2019)
Allahbakhsh, A., Arjmand, M.: Graphene-based phase change composites for energy harvesting and storage: state of the art and future prospects. Carbon 148, 441–480 (2019)
Solomon, G., Kohan, M., Landström, A., et al.: Semiconducting metal oxides empowered by graphene and its derivatives: progresses and critical perspective on selected functional applications. Int. J. Appl. Phys. 128, 180905 (2020)
Zhao, C., Chou, S.-L., Wang, Y., et al.: A facile route to synthesize transition metal oxide/reduced graphene oxide composites and their lithium storage performance. RSC Adv. 3, 16597–16603 (2013)
Khan, M., Tahir, M.N., Adil, S.F., et al.: Graphene-based metal and metal oxide nanocomposites: synthesis, properties and their applications. J. Mater. Chem. A. 3, 18753–18808 (2015)
Korotcenkov, G., Cho, B.: Metal oxide composites in conductometric gas sensors: achievements and challenges. Sens. Actuators B Chem. 244, 182–210 (2017)
Chidembo, A., Aboutalebi, S.H., Konstantinov, K., et al.: Globular reduced graphene oxide-metal oxide structures for energy storage applications. Energy Environ. Sci. 5, 5236–5240 (2012)
Jeevitha, G., Abhinayaa, R., Mangalaraj, D., et al.: Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J Phys Chem Solids. 116, 137–147 (2018)
Martins, G., Salvador, A.F., Pereira, L., et al.: Methane production and conductive materials: a critical review. Environ. Sci. Technol. 52, 10241–10253 (2018)
Wang, Z., Gong, W., Wang, X., et al.: Remarkable near-infrared electrochromism in tungsten oxide driven by interlayer water-induced battery-to-pseudocapacitor transition. ACS Appl. Mater. Interfaces. 12, 33917–33925 (2020)
Godbole, R., Godbole, V., Bhagwat, S.: Surface morphology dependent tungsten oxide thin films as toxic gas sensor. Mater Sci Semicond Process. 63, 212–219 (2017)
Shehzad, N., Tahir, M., Johari, K., et al.: Fabrication of highly efficient and stable indirect Z-scheme assembly of AgBr/TiO2 via graphene as a solid-state electron mediator for visible light induced enhanced photocatalytic H2 production. Appl. Surf. Sci. 463, 445–455 (2019)
Cronin, J.P., Agrawal, A., Adams, L.: Electrochromic device structures with conductive nanoparticles. U.S. Patent Application No. 16/259195 (2019)
Pourbaix, M.: Atlas of electrochemical equilibria in aqueous solution, p. 307. National Association of Corrosion Engineers, Houston (1974)
Cavalcante, L., Sczancoski, J., Espinosa, J., et al.: Photoluminescent behavior of BaWO4 powders processed in microwave-hydrothermal. J. Alloys Compd. 474, 195–200 (2009)
Song, J., Huang, Z.-F., Pan, L., et al.: Oxygen-deficient tungsten oxide as versatile and efficient hydrogenation catalyst. ACS Catal. 5, 6594–6599 (2015)
Shaver, P.: Activated tungsten oxide gas detectors. Appl. Phys. Lett. 11, 255–257 (1967)
Deb, S.: Optical and photoelectric properties and colour centres in thin films of tungsten oxide. Philos. Mag. Lett. 27, 801–822 (1973)
Hodes, G., Cahen, D., Manassen, J.: Tungsten trioxide as a photoanode for a photoelectrochemical cell (PEC). Nature 260, 312–313 (1976)
Butler, M., Nasby, R., Quinn, R.K.: Tungsten trioxide as an electrode for photoelectrolysis of water. Solid State Commun. 19, 1011–1014 (1976)
Huang, Z.-F., Song, J., Li, K., et al.: Hollow cobalt-based bimetallic sulfide polyhedra for efficient all-pH-value electrochemical and photocatalytic hydrogen evolution. J. Am. Chem. Soc. 138, 1359–1365 (2016)
Wu, C.-M., Naseem, S., Chou, M.-H., et al.: Recent advances in tungsten-oxide-based materials and their applications. Front. Mater. Sci. 6, 49 (2019)
Huang, Z.F., Song, J., Pan, L., et al.: Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 27, 5309–5327 (2015)
Zheng, H., Ou, J.Z., Strano, M.S., et al.: Nanostructured tungsten oxide–properties, synthesis, and applications. Adv. Funct. Mater. 21, 2175–2196 (2011)
Chacón, C., Rodríguez-Pérez, M., Oskam, G., et al.: Synthesis and characterization of WO3 polymorphs: monoclinic, orthorhombic and hexagonal structures. J. Mater. Sci. Mater. Electron. 26, 5526–5531 (2015)
Shinde, P.A., Jun, S.C.: Review on recent progress in the development of tungsten oxide-based electrodes for electrochemical energy storage. Chem. Sus. Chem. 13, 11–38 (2020)
Wang, J., Ji, Y., Yin, R., et al.: Transition metal-doped ultrathin RuO2 networked nanowires for efficient overall water splitting across a broad pH range. J. Mater. Chem. 7, 6411–6416 (2019)
Kumar, A., Raizada, P., Hosseini-Bandegharaei, A., et al.: C–, N–vacancy defect engineered polymeric carbon nitride towards photocatalysis: viewpoints and challenges. J. Mater. Chemis. A 9, 111–153 (2021)
Yu, Y., Zhao, Y., Qiao, Y.-L., et al.: Defect engineering of rutile TiO2 ceramics: route to high voltage stability of colossal permittivity. J. Mater. Sci. Technol. 84, 10–15 (2021)
Liu, Q., Wang, F., Lin, H., et al.: Surface oxygen vacancy and defect engineering of WO3 for improved visible light photocatalytic performance. Catal. Sci. Technol. 8, 4399–4406 (2018)
Guan, H., Huang, S., Ding, J., et al.: Chemical environment and magnetic moment effects on point defect formations in CoCrNi-based concentrated solid-solution alloys. Acta Mater. 187, 122–134 (2020)
Davazoglou, D., Dritsas, T.: Fabrication and calibration of a gas sensor-based on chemically vapor deposited WO3 films on silicon substrates: application to H2 sensing. Sens. Actuators B Chem. 77, 359–362 (2001)
Ionescu, R., Llobet, E., Al-Khalifa, S., et al.: Response model for thermally modulated tin oxide-based microhotplate gas sensors. Sens. Actuators B Chem. 95, 203–211 (2003)
Stankova, M., Vilanova, X., Llobet, E., et al.: Influence of the annealing and operating temperatures on the gas-sensing properties of rf sputtered WO3 thin-film sensors. Sens. Actuators B Chem. 105, 271–277 (2005)
Lu, Y., Jiang, Y., Gao, X., et al.: Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 136, 11687–11697 (2014)
Lee, J., Jo, C., Park, B., et al.: Simple fabrication of flexible electrodes with high metal-oxide content: electrospun reduced tungsten oxide/carbon nanofibers for lithium ion battery applications. Nanoscale 6, 10147–10155 (2014)
Huang, X., Zhang, Z., Li, H., et al.: In-situ growth of nanowire WO2.72 on carbon cloth as a binder-free electrode for flexible asymmetric supercapacitors with high performance. J. Energy Chem. 29, 58–64 (2019)
Yan, J., Wang, T., Wu, G., et al.: Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Adv. Mater. 27, 1580–1586 (2015)
Chala, T.F., Wu, C.-M., Chou, M.-H., et al.: Melt electrospun reduced tungsten oxide/polylactic acid fiber membranes as a photothermal material for light-driven interfacial water evaporation. ACS Appl. Mater. Interfaces. 10, 28955–28962 (2018)
Dong, P., Hou, G., Xi, X., et al.: WO3-based photocatalysts: morphology control, activity enhancement and multifunctional applications. Environ. Sci 4, 539–557 (2017)
Van Bommel, A., Crombeen, J.V., Tooren, A.: LEED and auger electron observations of the SiC (0001) surface. Surf. Sci. 48, 463–472 (1975)
Kano, E., Kvashnin, D.G., Sakai, S., et al.: One-atom-thick 2D copper oxide clusters on graphene. Nanoscale 9, 3980–3985 (2017)
Nguyen, B.H., Nguyen, V.H.: Promising applications of graphene and graphene-based nanostructures. Adv. Nat. Sci. 7, 023002 (2016)
Singh, M., Yadav, A., Kumar, S., et al.: Annealing induced electrical conduction and band gap variation in thermally reduced graphene oxide films with different sp2/sp3 fraction. Appl Surf Sci. 326, 236–242 (2015)
Wu, S., Jia, Q., Dai, W.: Synthesis of RGO/TiO2 hybrid as a high performance photocatalyst. Ceram. Inter. 43, 1530–1535 (2017)
Sehrawat, P., Islam, S., Mishra, P., et al.: Reduced graphene oxide (rGO)-based wideband optical sensor and the role of temperature, defect states and quantum efficiency. Sci. Rep. 8, 1–13 (2018)
Malas, A., Bharati, A., Verkinderen, O., et al.: Effect of the GO reduction method on the dielectric properties, electrical conductivity and crystalline behavior of PEO/rGO nanocomposites. Polymers 9, 613 (2017)
Huang, X., Liu, F., Jiang, P., et al.: Is graphene oxide an insulating material? In 2013 IEEE International Conference on Solid Dielectrics (ICSD). (2013)
Zhu, Y., Murali, S., Cai, W., et al.: Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924 (2010)
Mohan, V.B., Liu, D., Jayaraman, K., et al.: Improvements in electronic structure and properties of graphene derivatives. Adv. Mater. Lett. 7, 421–429 (2016)
Rabchinskii, M.K., Ryzhkov, S.A., Kirilenko, D.A., et al.: From graphene oxide towards aminated graphene: facile synthesis, its structure and electronic properties. Sci. Rep. 10, 1–12 (2020)
Hu, Y., Shen, Z., Li, B., et al.: Solvent effects on photocatalytic anaerobic oxidation of benzyl alcohol over Pt-loaded defective SrTiO3 nanoparticles. ACS Appl. Nano Mater. 4, 9254–9264 (2021)
Mondal, A., Prabhakaran, A., Gupta, S., et al.: Boosting photocatalytic activity using reduced graphene oxide (RGO)/semiconductor nanocomposites: issues and future scope. ACS Omega 6, 8734–8743 (2021)
Geng, X., Lu, P., Zhang, C., et al.: Room-temperature NO2 gas sensors-based on rGO@ZnO1-x composites: experiments and molecular dynamics simulation. Sens. Actuators B Chem. 282, 690–702 (2019)
Thiyagarajan, K., Muralidharan, M., Sivakumar, K.: Defects induced magnetism in WO3 and reduced graphene oxide/WO3 nanocomposites. J. Supercond. Nov. Magn. 31, 117–125 (2018)
Zhang, X.-J., Wang, G.-S., Cao, W.-Q., et al.: Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces. 6, 7471–7478 (2014)
Yu, C., Chang, X., Liu, J., et al.: Creation of reduced graphene oxide-based field effect transistors and their utilization in the detection and discrimination of nucleoside triphosphates. ACS Appl. Mater. Interfaces. 7, 10718–10726 (2015)
Toda, K., Furue, R., Hayami, S.: Recent progress in applications of graphene oxide for gas sensing: a review. Anal. Chim. Acta. 878, 43–53 (2015)
Zhang, X., Hu, S., Wang, M., et al.: Continuous graphene and carbon nanotube-based high flexible and transparent pressure sensor arrays. Nanotechnology 26, 115501 (2015)
Kumar, S., Kumar, S., Srivastava, S., et al.: Reduced graphene oxide modified smart conducting paper for cancer biosensor. Biosens. Bioelectron. 73, 114–122 (2015)
Xu, Y., Bai, H., Lu, G., et al.: Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 130, 5856–5857 (2008)
Wei, Y., Zhang, Y., Gao, X., et al.: Multilayered graphene oxide membranes for water treatment: a review. Carbon 139, 964–981 (2018)
Li, D., Müller, M.B., Gilje, S., et al.: Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 3, 101 (2008)
Wang, X., Yan, C., Sumboja, A., et al.: Nickel cobalt oxide nanowire-reduced graphite oxide composite material and its application for high performance supercapacitor electrode material. J. Nanosci. Nanotechnol. 14, 7104–7110 (2014)
Bakker, E., Telting-Diaz, M.: Electrochemical sensors. Anal. Chem. 74, 2781–2800 (2002)
Lee, E., Hong, J.-Y., Kang, H., et al.: Synthesis of TiO2 nanorod-decorated graphene sheets and their highly efficient photocatalytic activities under visible-light irradiation. J. Hazard. Mater. 219, 13–18 (2012)
Tabasum, A., Alghuthaymi, M., Qazi, U.Y., et al.: UV-accelerated photocatalytic degradation of pesticide over magnetite and cobalt ferrite decorated graphene oxide composite. Plants 10, 6 (2021)
Tabasum, A., Bhatti, I.A., Nadeem, N., et al.: Degradation of acetamiprid using graphene-oxide-based metal (Mn and Ni) ferrites as Fenton-like photocatalysts. Water Sci. Technol. 81, 178–189 (2020)
Nadeem, N., Zahid, M., Tabasum, A., et al.: Degradation of reactive dye using heterogeneous photo-Fenton catalysts: ZnFe2O4 and GO-ZnFe2O4 composite. Mater. Res. Express. 7, 015519 (2020)
Qureshi, K., Ahmad, M.Z., Bhatti, I.A., et al.: Graphene oxide decorated ZnWO4 architecture synthesis, characterization and photocatalytic activity evaluation. J. Mol. Liq. 285, 778–789 (2019)
Asma, T., Muhammad, Z., Haq Nawaz, B., et al.: Fe3O4 -GO composite as efficient heterogeneous photo-Fenton’s catalyst to degrade pesticides. Mater. Res. Express. 6, 015608 (2019)
Upadhyay, R.K., Soin, N., Roy, S.S.: Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: a review. RSC Adv. 4, 3823–3851 (2014)
Xiang, Q., Yu, J., Jaroniec, M.: Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 41, 782–796 (2012)
Shakoor, F., Aldaghfag, S.A., Yaseen, M., et al.: Physical characteristics of barium-based cubic perovskites. Chem. Phys. Lett. 779, 138835 (2021)
Zahid, M., Nawab, Y., Gulzar, N., et al.: Fabrication of reduced graphene oxide (RGO) and nanocomposite with thermoplastic polyurethane (TPU) for EMI shielding application. J. Mater. Sci. Mater. Electron. 31, 967–974 (2020)
Singh, A.P., Mishra, M., Chandra, A., et al.: Graphene oxide/ferrofluid/cement composites for electromagnetic interference shielding application. Nanotechnology 22, 465701 (2011)
Jia, Z., Zhang, M., Liu, B., et al.: Graphene foams for electromagnetic interference shielding: a review. ACS Appl. Nano Mater. 3, 6140–6155 (2020)
Anand, A., Unnikrishnan, B., Wei, S.-C., et al.: Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—a minireview. Nanoscale Horiz. 4, 117–137 (2019)
Pandit, S., Gaska, K., Kádár, R., et al.: Graphene-based antimicrobial biomedical surfaces. Chem. Phys. Chem. 22, 250 (2021)
Fatima, N., Qazi, U.Y., Mansha, A., et al.: Recent developments for antimicrobial applications of graphene-based polymeric composites: a review. J Ind Eng Chem. 100, 40–58 (2021)
Vasseghian, Y., Dragoi, E.-N., Moradi, M., et al.: A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 148, 111931 (2021)
Chatterjee, S.G., Chatterjee, S., Ray, A.K., et al.: Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sens. Actuators B Chem. 221, 1170–1181 (2015)
Olabi, A., Abdelkareem, M.A., Wilberforce, T., et al.: Application of graphene in energy storage device—a review. Renew. Sust. Energ. Rev. 135, 110026 (2021)
Askari, M.B., Salarizadeh, P., Seifi, M., et al.: ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 860, 158497 (2021)
Sharma, N., Sharma, V., Jain, Y., et al.: Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 376, 1700006 (2017)
Ul Haque, S., Nasar, A., Rahman, M.M.: Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 10, 1–12 (2020)
Choi, Y.-J., Kim, E., Han, J., et al.: A novel biomolecule-mediated reduction of graphene oxide: a multifunctional anti-cancer agent. Molecules 21, 375 (2016)
Jose, P.P.A., Kala, M., Joseph, A.V., et al.: Reduced graphene oxide/silver nanohybrid as a multifunctional material for antibacterial, anticancer, and SERS applications. Appl. Phys. A. 126, 1–16 (2020)
Tian, F., Lyu, J., Shi, J., et al.: Graphene and graphene-like two-denominational materials-based fluorescence resonance energy transfer (FRET) assays for biological applications. Biosens. Bioelectron. 89, 123–135 (2017)
Zhong, X., Lu, Z., Liang, W., et al.: The fabrication of 3D hierarchical flower-like δ-MnO2@COF nanocomposites for the efficient and ultra-fast removal of UO22+ ions from aqueous solution. Environ. Sci. 7, 3303–3317 (2020)
Pu, F., Bai, Y., Lv, J., et al.: Yolk–shell Cu2O@CuO‐decorated RGO for high‐performance lithium‐ion battery anode. Energy Environ. Mater. 1–8 (2021)
Alkhouzaam, A., Qiblawey, H.: Functional GO-based membranes for water treatment and desalination: fabrication methods, performance and advantages. A review. Chemosphere 274, 129853 (2021)
Panahi-Sarmad, M., Goodarzi, V., Amirkiai, A., et al.: Programing polyurethane with systematic presence of graphene-oxide (GO) and reduced graphene-oxide (rGO) platelets for adjusting of heat-actuated shape memory properties. Eur. Polym. J. 118, 619–632 (2019)
Hao, P., Lin, Z., Song, P., et al.: rGO-wrapped porous LaFeO3 microspheres for high-performance triethylamine gas sensors. Ceram. Int. 46, 9363–9369 (2020)
Eda, G., Chhowalla, M.: Graphene-based composite thin films for electronics. Nano lette. 9, 814–818 (2009)
Zhou, M., Yan, J., Cui, P.: Synthesis and enhanced photocatalytic performance of WO3 nanorods@ graphene nanocomposites. Mater. Lett. 89, 258–261 (2012)
Nagaraju, P., Alsalme, A., Alkathiri, A.M., et al.: Rapid synthesis of WO3/graphene nanocomposite via in-situ microwave method with improved electrochemical properties. J Phys Chem Solids. 120, 250–260 (2018)
Guo, J., Li, Y., Zhu, S., et al.: Synthesis of WO3@graphene composite for enhanced photocatalytic oxygen evolution from water. Rsc Adv. 2, 1356–1363 (2012)
Hu, X., Xu, P., Gong, H., et al.: Synthesis and characterization of WO3/graphene nanocomposites for enhanced photocatalytic activities by one-step in-situ hydrothermal reaction. Materials 11, 147 (2018)
Zhi, M., Huang, W., Shi, Q., et al.: Sol–gel fabrication of WO3/RGO nanocomposite film with enhanced electrochromic performance. RSC Adv. 6, 67488–67494 (2016)
Firat, Y.: Pseudocapacitive energy storage properties of rGO/WO3 electrode synthesized by electrodeposition. Mater. Sci. Semicond. Process. 133, 105938 (2021)
Yadav, A., Hunge, Y., Kang, S.-W.: Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interfaces 24, 101075 (2021)
Kalaiarasi, S., Kavitha, M., Karpagavinayagam, P., et al.: Tungsten oxide decorated graphene oxide nanocomposite: chemical synthesis, characterization and application in super capacitors. Mater. Today Proc. 1–8 (2020)
Liu, B., Wang, Y., Jiang, H.-W., et al.: WO3 nanowires on graphene sheets as negative electrode for supercapacitors. J. Nanomater. 2017, 2494109 (2017)
Huang, S.-Y., Le, P.-A., Yen, P.-J., et al.: Cathodic plasma–induced syntheses of graphene nanosheet/MnO2/WO3 architectures and their use in supercapacitors. Electrochim. Acta. 342, 136043 (2020)
Shchegolkov, A.V., Jang, S.-H., Shchegolkov, A.V., et al.: A brief overview of electrochromic materials and related devices: a nanostructured materials perspective. Nanomaterials 11, 2376 (2021)
Demon, S.Z.N., Kamisan, A.I., Abdullah, N., et al.: Graphene-based materials in gas sensor applications: a review. Sens. Mater. 32, 759–777 (2020)
Su, L., Fan, X., Wang, C., et al.: Advances in photonics of recently developed xenes. Nanophotonics. 9, 1621–1649 (2020)
Wan, P., Wen, X., Sun, C., et al.: Flexible transparent films-based on nanocomposite networks of polyaniline and carbon nanotubes for high-performance gas sensing. Small 11, 5409–5415 (2015)
Li, X., Sheng, X., Guo, Y., et al.: Multifunctional HDPE/CNTs/PW composite phase change materials with excellent thermal and electrical conductivities. J Mater Sci Technol. 86, 171–179 (2021)
Tao, W., Ji, X., Zhu, X., et al.: Two-dimensional antimonene-based photonic nanomedicine for cancer theranostics. Adv. Mater. 30, 1802061 (2018)
Shi, M., Zhu, H., Yang, C., et al.: Chemical reduction-induced fabrication of graphene hybrid fibers for energy-dense wire-shaped supercapacitors. Chin. J. Chem. Eng. In press and available online (2021)
Luo, M., Fan, T., Zhou, Y., et al.: 2D black phosphorus–based biomedical applications. Adv. Funct. Mater. 29, 1808306 (2019)
Wang, L., Huang, H., Xiao, S., et al.: Enhanced sensitivity and stability of room-temperature NH3 sensors using core–shell CeO2 nanoparticles@cross-linked PANI with p–n heterojunctions. ACS Appl. Mater. Interfaces. 6, 14131–14140 (2014)
Meng, F., Hou, N., Ge, S., et al.: Flower-like hierarchical structures consisting of porous single-crystalline ZnO nanosheets and their gas sensing properties to volatile organic compounds (VOCs). J. Alloys Compd. 626, 124–130 (2015)
Abideen, Z.U., Katoch, A., Kim, J.-H., et al.: Excellent gas detection of ZnO nanofibers by loading with reduced graphene oxide nanosheets. Sens. Actuators B Chem. 221, 1499–1507 (2015)
Patil, S.J., Patil, A.V., Dighavkar, C.G., et al.: Semiconductor metal oxide compounds-based gas sensors: a literature review. Front. Mater. Sci. 9, 14–37 (2015)
Zhang, X., Sun, X., Lv, T., et al.: Preparation of PI porous fiber membrane for recovering oil-paper insulation structure. J. Mater. Sci. Mater. Electron. 31, 13344–13351 (2020)
Fardindoost, S., Rahimi, F., Ghasempour, R.: Pd doped WO3 films prepared by sol–gel process for hydrogen sensing. Int. J. Hydrog. Energy. 35, 854–860 (2010)
Xie, Z., Xu, H., Rong, F., et al.: Hydrogen activity tuning of Pt-doped WO3 photonic crystal. Thin Solid Films 520, 4063–4067 (2012)
Choi, S.-J., Fuchs, F., Demadrille, R., et al.: Fast responding exhaled-breath sensors using WO3 hemitubes functionalized by graphene-based electronic sensitizers for diagnosis of diseases. ACS Appl. Mater. Interfaces. 6, 9061–9070 (2014)
Tu, N.D.K., Choi, J., Park, C.R., et al.: Remarkable conversion between n-and p-type reduced graphene oxide on varying the thermal annealing temperature. Chem. Mater. 27, 7362–7369 (2015)
Phan, D.-T., Chung, G.-S.: p–n junction characteristics of graphene oxide and reduced graphene oxide on n-type Si(111). J. Phys. Chem. Solids. 74, 1509–1514 (2013)
Qin, N., Wang, X., Xiang, Q., et al.: A biomimetic nest-like ZnO: controllable synthesis and enhanced ethanol response. Sens. Actuators B Chem. 191, 770–778 (2014)
Shankar, P., Rayappan, J.B.B.: Gas sensing mechanism of metal oxides: the role of ambient atmosphere, type of semiconductor and gases—a review. Sci. Lett. J. 4, 126 (2015)
Yin, M., Yao, Y., Fan, H., et al.: WO3-SnO2 nanosheet composites: hydrothermal synthesis and gas sensing mechanism. J. Alloys Compd. 736, 322–331 (2018)
Shi, J., Cheng, Z., Gao, L., et al.: Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 230, 736–745 (2016)
Hao, Q., Liu, T., Liu, J., et al.: Controllable synthesis and enhanced gas sensing properties of a single-crystalline WO3–rGO porous nanocomposite. RSC Adv. 7, 14192–14199 (2017)
Jiang, Z., Chen, W., Jin, L., et al.: High performance acetylene sensor with heterostructure-based on WO3 nanolamellae/reduced graphene oxide (rGO) nanosheets operating at low temperature. Nanomaterials 8, 909 (2018)
Jeevitha, G., Abhinayaa, R., Mangalaraj, D., et al.: Porous reduced graphene oxide (rGO)/WO3 nanocomposites for the enhanced detection of NH3 at room temperature. Nanoscale Adv. 1, 1799–1811 (2019)
Sandil, D., Srivastava, S., Malhotra, B., et al.: Biofunctionalized tungsten trioxide-reduced graphene oxide nanocomposites for sensitive electrochemical immunosensing of cardiac biomarker. J. Alloys Compd. 763, 102–110 (2018)
Zhao, B., Lu, S., Zhang, X., et al.: Porous WO3/reduced graphene oxide composite film with enhanced electrochromic properties. Ionics 22, 261–267 (2016)
Khan, A., Bhosale, N., Mali, S., et al.: Reduced graphene oxide layered WO3 thin film with enhanced electrochromic properties. J. Colloid Interface Sci. 571, 185–193 (2020)
Jo, C., Hwang, I., Lee, J., et al.: Investigation of pseudocapacitive charge-storage behavior in highly conductive ordered mesoporous tungsten oxide electrodes. J. Phys. Chem. C. 115, 11880–11886 (2011)
Machida, K.I., Enyo, M.: Structural and electrochromic properties of tungsten and molybdenum trioxide electrodes in acidic media. J. Electrochem. Soc. 137, 1169–1175 (1990)
Conway, B.E., Birss, V., Wojtowicz, J.: The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sour. 66, 1–14 (1997)
Zhu, M., Meng, W., Huang, Y., et al.: Proton-insertion-enhanced pseudocapacitance-based on the assembly structure of tungsten oxide. ACS Appl. Mater. Interfaces. 6, 18901–18910 (2014)
Cai, Y., Wang, Y., Deng, S., et al.: Graphene nanosheets-tungsten oxides composite for supercapacitor electrode. Ceram. Int. 40, 4109–4116 (2014)
Xing, L.-L., Huang, K.-J., Fang, L.-X.: Preparation of layered graphene and tungsten oxide hybrids for enhanced performance supercapacitors. Dalton Trans. 45, 17439–17446 (2016)
Ma, L., Zhou, X., Xu, L., et al.: Hydrothermal preparation and supercapacitive performance of flower-like WO3·H2O/reduced graphene oxide composite. Colloids Surf. A Physicochem. Eng. 481, 609–615 (2015)
Ribeiro, D., Abrantes, J.: Application of electrochemical impedance spectroscopy (EIS) to monitor the corrosion of reinforced concrete: a new approach. Constr. Build. Mater. 111, 98–104 (2016)
Park, S.K., Lee, H.J., Lee, M.H., et al.: Hierarchically structured reduced graphene oxide/WO3 frameworks for an application into lithium ion battery anodes. Chem. Eng. Sci. 281, 724–729 (2015)
Chu, J., Lu, D., Wang, X., et al.: WO3 nanoflower coated with graphene nanosheet: synergetic energy storage composite electrode for supercapacitor application. J. Alloys Compd. 702, 568–572 (2017)
Wong, C.P.P., Lee, K.M., Lai, C.W.: Hydrothermal preparation of reduced graphene oxide/tungsten trioxide nanocomposites with enhanced electrochemical performance. J. Mater. Sci. Mater. Electron. 28, 14554–14567 (2017)
Korkmaz, S., Tezel, F.M., Kariper, İ: Facile synthesis and characterization of graphene oxide/tungsten oxide thin film supercapacitor for electrochemical energy storage. Physica E Low Dimens. Syst. Nanostruct. 116, 113718 (2020)
Ibrahim, Y.O., Gondal, M., Alaswad, A., et al.: Laser-induced anchoring of WO3 nanoparticles on reduced graphene oxide sheets for photocatalytic water decontamination and energy storage. Ceram. Int. 46, 444–451 (2020)
Ji, B., Zhang, F., Song, X., et al.: A novel potassium-ion-based dual-ion battery. Adv. Mater. 29, 1700519 (2017)
Tong, X., Zhang, F., Ji, B., et al.: Carbon-coated porous aluminum foil anode for high-rate, long-term cycling stability, and high energy density dual-ion batteries. Adv. Mater. 28, 9979–9985 (2016)
Wang, M., Jiang, C., Zhang, S., et al.: Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 10, 667–672 (2018)
Zhang, X., Tang, Y., Zhang, F., et al.: A novel aluminum–graphite dual-ion battery. Adv. Energy Mater. 6, 1502588 (2016)
Yoon, S., Pyo, S.G., Son, H., et al.: Investigation of novel nanostructured tungsten oxides as high volumetric and safe anode materials in lithium ion batteries. 224th ECS Meeting Abstracts. MA2013, 2, 1089 (2013)
Gu, X., Wu, F., Lei, B., et al.: Three-dimensional nitrogen-doped graphene frameworks anchored with bamboo-like tungsten oxide nanorods as high performance anode materials for lithium ion batteries. J. Power Sources. 320, 231–238 (2016)
Herdt, T., Deckenbach, D., Bruns, M., et al.: Tungsten oxide nanorod architectures as 3D anodes in binder-free lithium-ion batteries. Nanoscale 11, 598–610 (2019)
Kumar, A., Raizada, P., Singh, P., et al.: Perspective and status of polymeric graphitic carbon nitride-based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. Sci. 391, 123496 (2020)
Wang, Z., Li, C., Domen, K.: Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 48, 2109–2125 (2019)
Zahid, M., Nadeem, N., Tahir, N., et al.: Hybrid nanomaterials for water purification. In: Abd-Elsalam, K.A. (ed.) Multifunctional hybrid nanomaterials for sustainable agri-food and ecosystems, pp. 155–188. Elsevier, Amsterdam (2020)
Nadeem, N., Zahid, M., Rehan, Z A., et al.: Improved photocatalytic degradation of dye using coal fly ash-based zinc ferrite (CFA/ZnFe2O4) composite. Int J Environ Sci Technol. (2021) (In press and available online)
Nadeem, N., Abbas, Q., Yaseen, M., et al.: Coal fly ash-based copper ferrite nanocomposites as potential heterogeneous photocatalysts for wastewater remediation. Appl. Surf. Sci. 565, 150542 (2021)
Nadeem, N., Zahid, M., Bhatti, H.N., et al.: Wastewater remediation using coal fly ash nanocomposites, in aquananotechnology, pp. 149–174. Elsevier, Amsterdam (2021)
Nadeem, N., Zahid, M., Hanif, M.A., et al.: Silver-doped metal ferrites for wastewater treatment, in silver nanomaterials for agri-food applications, pp. 599–622. Elsevier, Amsterdam (2021)
Nadeem, N., Yaseen, M., Rehan, Z.A., et al.: Coal fly ash supported CoFe2O4 nanocomposites: Synergetic Fenton-like and photocatalytic degradation of methylene blue. Environ. Res. 112280 (2021)
Singh, P., Shandilya, P., Raizada, P., et al.: Review on various strategies for enhancing photocatalytic activity of graphene-based nanocomposites for water purification. Arab. J. Chem. 13, 3498–3520 (2020)
Sonu., Dutta, V., Sharma, S., et al.: Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc.s. 23, 1119–1136 (2019)
Raizada, P., Sudhaik, A., Singh, P., et al.: Ag3PO4 modified phosphorus and sulphur co-doped graphitic carbon nitride as a direct Z-scheme photocatalyst for 2, 4-dimethyl phenol degradation. J. Photochem. Photobiol. A. 374, 22–35 (2019)
Zahid, M., Nadeem, N., Hanif, M.A., et al.: Metal ferrites and their graphene-based nanocomposites: synthesis, characterization, and applications in wastewater treatment. In: Magnetic nanostructures. Springer, Cham, 181-212 (2019)
Rahman, M.U., Qazi, U.Y., Hussain, T., et al.: Solar driven photocatalytic degradation potential of novel graphitic carbon nitride-based nanozero-valent iron doped bismuth ferrite ternary composite. Opt. Mater. 120, 111408 (2021)
Saher, R., Hanif, M.A., Mansha, A., et al.: Sunlight-driven photocatalytic degradation of rhodamine B dye by Ag/FeWO4/g-C3N4 composites. Int J Environ Sci Technol. 18, 927–938 (2021)
Saeed, H., Nadeem, N., Zahid, M., et al.: Mixed metal ferrite (Mn0.6Zn0.4Fe2O4) intercalated g-C3N4 nanocomposite: efficient sunlight driven photocatalyst for methylene blue degradation. Nanotechnology 32, 505714 (2021)
Dutta, V., Sharma, S., Raizada, P., et al.: Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 23, 1119–1136 (2019)
Raizada, P., Kumari, J., Shandilya, P., et al.: Kinetics of photocatalytic mineralization of oxytetracycline and ampicillin using activated carbon supported ZnO/ZnWO4. Desalination 79, 204–213 (2017)
Philippopoulos, C., Nikolaki, M.: Photocatalytic processes on the oxidation of organic compounds in water. In: Šramová, B. (ed.) New Trends in Technologies. IntechOpen (2010)
Saravanan, R., Gracia, F., Stephen, A.: Basic principles, mechanism, and challenges of photocatalysis. In: Khan, M.M., Pradhan, D., Sohn, Y. (eds.) Nanocomposites for Visible Light-Induced Photocatalysis, pp. 19–40. Springer, Cham (2017)
Serpone, N.: Emeline, A, semiconductor photocatalysis—past, present, and future outlook. J. Phys. Chem. Lett. 3, 673–677 (2012)
Riboni, F., Bettini, L.G., Bahnemann, D.W., et al.: WO3–TiO2 vs. TiO2 photocatalysts: effect of the W precursor and amount on the photocatalytic activity of mixed oxides. Cat. Today 209, 28–34 (2013)
Anwer, H., Mahmood, A., Lee, J., et al.: Photocatalysts for degradation of dyes in industrial effluents: opportunities and challenges. Nano Res. 12, 955–972 (2019)
Cong, S., Geng, F., Zhao, Z.: Tungsten oxide materials for optoelectronic applications. Adv. Mater. 28, 10518–10528 (2016)
Thangavel, S., Elayaperumal, M., Venugopal, G.: Synthesis and properties of tungsten oxide and reduced graphene oxide nanocomposites. Mater. Express. 2, 327–334 (2012)
Chai, B., Li, J., Xu, Q., et al.: Facile synthesis of reduced graphene oxide/WO3 nanoplates composites with enhanced photocatalytic activity. Mater. Lett. 120, 177–181 (2014)
Peiris, C., Gunatilake, S.R., Mlsna, T.E., et al.: Biochar-based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresour. Technol. 246, 150–159 (2017)
Ahmed, M.B., Zhou, J.L., Ngo, H.H., et al.: Adsorptive removal of antibiotics from water and wastewater: progress and challenges. Sci. Total Environ. 532, 112–126 (2015)
Zhu, W., Sun, F., Goei, R., et al.: Facile fabrication of RGO/WO3 composites for effective visible light photocatalytic degradation of sulfamethoxazole. Appl. Catal. B. 207, 93–102 (2017)
Jiang, G., Lin, Z., Chen, C., et al.: TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 49, 2693–2701 (2011)
Kasap, H., Godin, R., Jeay-Bizot, C., et al.: Interfacial engineering of a carbon nitride-graphene oxide–molecular Ni catalyst hybrid for enhanced photocatalytic activity. ACS Catal. 8, 6914–6926 (2018)
Sajjad, S., Khan, M., Leghari, S.A.K., et al.: Potential visible WO3/GO composite photocatalyst. Int. J. Appl. Ceram. Technol. 16, 1218–1227 (2019)
Ke, J., Zhou, H., Liu, J., et al.: Enhanced light-driven water splitting by fast electron transfer in 2D/2D reduced graphene oxide/tungsten trioxide heterojunction with preferential facets. J. Colloid Interface Sci. 555, 413–422 (2019)
Wang, Z., Lei, Q., Wang, Z., et al.: In-situ synthesis of free-standing FeNi-oxyhydroxide nanosheets as a highly efficient electrocatalyst for water oxidation. Chem. Eng. Sci. 395, 125180 (2020)
Qin, J., Cao, M., Li, N., et al.: Graphene-wrapped WO3 nanoparticles with improved performances in electrical conductivity and gas sensing properties. J. Mater. Chem. 21, 17167–17174 (2011)
Chen, D., Zhang, H., Liu, Y., et al.: Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy Environ. Sci. 6, 1362–1387 (2013)
Park, S., Hong, T., Jung, J., et al.: Room temperature hydrogen sensing of multiple networked ZnO/WO3 core–shell nanowire sensors under UV illumination. Curr Appl Phys. 14, 1171–1175 (2014)
Sajjad, A.K.L., Sajjad, S., Iqbal, A.: ZnO/WO3 nanostructure as an efficient visible light catalyst. Ceram. Int. 44, 9364–9371 (2018)
Luo, X., Deng, F., Min, L., et al.: Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nanocomposite and its molecular recognitive photocatalytic degradation of target contaminant. Environ. Sci. Technol. 47, 7404–7412 (2013)
Song, X.C., Wang, X., Zheng, Y.F., et al.: Electrochromic Properties of WO3-MoO3 nanocomposite films prepared by electrodeposition method. Curr. Nanosci. 9, 330–334 (2013)
Vallejos, S., Gracia, I., Figueras, E., et al.: Nanoscale heterostructures-based on Fe2O3@WO3-x nanoneedles and their direct integration into flexible transducing platforms for toluene sensing. ACS Appl. Mater. Interfaces. 7, 18638–18649 (2015)
Bai, S., Zhang, K., Sun, J., et al.: Surface decoration of WO3 architectures with Fe2O3 nanoparticles for visible-light-driven photocatalysis. Cryst. Eng. Comm. 16, 3289–3295 (2014)
Gesheva, K., Arvizu, M.A., Bodurov, G., et al.: Optical, structural and electrochromic properties of sputter-deposited W–Mo oxide thin films. J. Phys. 764, 012010 (2016)
Su, P.-G., Peng, Y.-T.: Fabrication of a room-temperature H2S gas sensor-based on PPy/WO3 nanocomposite films by in-situ photopolymerization. Sens. Actuators B Chem. 193, 637–643 (2014)
Mane, A., Navale, S., Patil, V.: Room temperature NO2 gas sensing properties of DBSA doped PPy–WO3 hybrid nanocomposite sensor. Org. Electron. 19, 15–25 (2015)
Inomata, H., Shimokawabe, M., Kuwana, A., et al.: Selective reduction of NO with CO in the presence of O2 with Ir/WO3 catalysts: influence of preparation variables on the catalytic performance. Appl. Catal. B. 84, 783–789 (2008)
Zhou, P., Xu, Q., Li, H., et al.: Fabrication of two-dimensional lateral heterostructures of WS2/WO3·H2O through selective oxidation of monolayer WS2. Angew. Chem. 127, 15441–15445 (2015)
Shang, X., Rao, Y., Lu, S.-S., et al.: Novel WS2/WO3 heterostructured nanosheets as efficient electrocatalyst for hydrogen evolution reaction. Mater. Chem. Phys. 197, 123–128 (2017)
Negreiros, F.R., Pedroza, L.S., Souza, F.L., et al.: Surface Fe vacancy defects on haematite and their role in light-induced water splitting in artificial photosynthesis. Phys. Chem. Chem. Phys. 19, 31410–31417 (2017)
Zhang, X., Li, H., Wang, S., et al.: Improvement of hematite as photocatalyst by doping with tantalum. J. Phys. Chem. C. 118, 16842–16850 (2014)
Sugrañez, R., Balbuena, J., Cruz-Yusta, M., et al.: Efficient behaviour of hematite towards the photocatalytic degradation of NOx gases. Appl. Catal. B. 165, 529–536 (2015)
Xie, J., Zhou, Z., Lian, Y., et al.: Synthesis of α-Fe2O3/ZnO composites for photocatalytic degradation of pentachlorophenol under UV–vis light irradiation. Ceram. Int. 41, 2622–2625 (2015)
Dozzi, M.V., Marzorati, S., Longhi, M., et al.: Photocatalytic activity of TiO2–WO3 mixed oxides in relation to electron transfer efficiency. Appl. Catal. B. 186, 157–165 (2016)
Patrocinio, A.O.T., Paula, L.F., Paniago, R.M., et al.: Layer-by-layer TiO2/WO3 thin films as efficient photocatalytic self-cleaning surfaces. ACS Appl. Mater. Interfaces. 6, 16859–16866 (2014)
Pérez-González, M., Tomás, S., Morales-Luna, M., et al.: Optical, structural, and morphological properties of photocatalytic TiO2–ZnO thin films synthesized by the sol–gel process. Thin Solid Films 594, 304–309 (2015)
Hernández, S., Hidalgo, D., Sacco, A., et al.: Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. 17, 7775–7786 (2015)
Park, S., Lee, K.-S., Bozoklu, G., et al.: Graphene oxide papers modified by divalent ions—enhancing mechanical properties via chemical cross-linking. ACS Nano 2, 572–578 (2008)
An, D., Yang, L., Wang, T.-J., et al.: Separation performance of graphene oxide membrane in aqueous solution. Ind. Eng. Chem. Res. 55, 4803–4810 (2016)
Kusiak-Nejman, E., Wanag, A., Kowalczyk, Ł, et al.: Graphene oxide-TiO2 and reduced graphene oxide-TiO2 nanocomposites: insight in charge-carrier lifetime measurements. Catal. Today. 287, 189–195 (2017)
Hao, X., Li, M., Zhang, L., et al.: Photocatalyst TiO2/WO3/GO nano-composite with high efficient photocatalytic performance for BPA degradation under visible light and solar light illumination. J Ind Eng Chem. 55, 140–148 (2017)
Yashni, G., Al-Gheethi, A., Mohamed, R., et al.: Reusability performance of green zinc oxide nanoparticles for photocatalysis of bathroom greywater. Water Pract. Technol. 16, 364–376 (2021)
Hasannia, S., Kazemeini, M., Seif, A., et al.: Oxidative desulfurization of a model liquid fuel over an rGO-supported transition metal modified WO3 catalyst: experimental and theoretical studies. Sep. Purif. Technol. 269, 118729 (2021)
Govindaraj, T., Mahendran, C., Manikandan, V., et al.: Fabrication of WO3 nanorods/RGO hybrid nanostructures for enhanced visible-light-driven photocatalytic degradation of Ciprofloxacin and Rhodamine B in an ecosystem. J. Alloys Compd. 868, 159091 (2021)
Dong, Z., Wu, Y., Thirugnanam, N., et al.: Double Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 430, 293–300 (2018)
Patial, S., Raizada, P., Hasija, V., et al.: Recent advances in photocatalytic multivariate metal organic frameworks-based nanostructures toward renewable energy and the removal of environmental pollutants. Mater. Today Energy. 19, 100589 (2021)
Mohamed, H.H.: Rationally designed Fe2O3/GO/WO3 Z-scheme photocatalyst for enhanced solar light photocatalytic water remediation. J. Photochem. Photobiol. A. 378, 74–84 (2019)
Sikong, L., Choopoo, P., Kooptarnond, K.: The photochromic properties of reduced graphene oxide doped tungsten/molybdenum trioxide nano-composites. Dig. J. Nanomater. Biostruct. 11, 821–831 (2016)
Chaudhary, K., Shaheen, N., Zulfiqar, S., et al.: Binary WO3–ZnO nanostructures supported rGO ternary nanocomposite for visible light driven photocatalytic degradation of methylene blue. Synth. Met. 269, 116526 (2020)
Patial, S., Raizada, P., Hasija, V., et al.: Recent advances in photocatalytic multivariate metal organic framework (MOFs)-based nanostructures toward renewable energy and the removal of environmental pollutants. Mater. Today Energy. 19, 100589 (2020)
Fakhri, H., Bagheri, H.: Highly efficient Zr-MOF@WO3/graphene oxide photocatalyst: synthesis, characterization and photodegradation of tetracycline and malathion. Mater. Sci. Semicond. Process. 107, 104815 (2020)
Zhang, X., Bian, X., Xu, H., et al.: Fabrication of WO3/RGO/Ni: FeOOH heterostructure for synergistically enhancing photoelectrochemical water oxidation. Appl. Surf. Sci. 542, 148579 (2021)
Tang, X.-Z., Cao, Z., Zhang, H.-B., et al.: Growth of silver nanocrystals on graphene by simultaneous reduction of graphene oxide and silver ions with a rapid and efficient one-step approach. Chem. Comm. 47, 3084–3086 (2011)
Kamali, K.Z., Pandikumar, A., Jayabal, S., et al.: Amalgamation-based optical and colorimetric sensing of mercury (II) ions with silver@graphene oxide nanocomposite materials. Microchim. Acta. 183, 369–377 (2016)
Tran, V.A., Nguyen, T.P., Kim, I.-T., et al.: Excellent photocatalytic activity of ternary Ag@WO3@rGO nanocomposites under solar simulation irradiation. J. Sci: Adv Mater Dev. 6, 108–117 (2021)
Huang, B., Ma, Y., Xiong, Z., et al.: Polyoxometalate-derived Ir/WOx/rGO nanocomposites for enhanced electrocatalytic water splitting. Energy Environ. Mater. 4, 681–686 (2021)
Gao, J., Ma, Y., Li, J., et al.: Free-standing WS 2-MWCNTs hybrid paper integrated with polyaniline for high-performance flexible supercapacitor. J Nanopart Res. 20, 298 (2018)
Yang, Z., Zhang, H., Ma, B., et al.: Facile synthesis of reduced graphene oxide/tungsten disulfide/tungsten oxide nanohybrids for high performance supercapacitor with excellent rate capability. Appl. Surf. Sci. 463, 150–158 (2019)
Naz, R., Liu, Q., Abbas, W., et al.: One-pot hydrothermal synthesis of ternary 1T-MoS2/hexa/WO3/graphene composites for high-performance supercapacitors. Chem. Eur. J. 25, 16054–16062 (2019)
Zhu, H., An, Y., Shi, M., et al.: Porous N-doped carbon/MnO2 nanoneedles for high performance ionic liquid-based supercapacitors. Mater. Lett. 296, 129837 (2021)
Jabed, M.A., Zhao, J., Kilin, D., et al.: Understanding of light absorption properties of the N-doped graphene oxide quantum dot with TD-DFT. J. Phys. Chem. C. 125, 14979–14990 (2021)
Hasija, V., Sudhaik, A., Raizada, P., et al.: Carbon quantum dots supported AgI/ZnO/phosphorus doped graphitic carbon nitride as Z-scheme photocatalyst for efficient photodegradation of 2, 4-dinitrophenol. J. Environ. Chem. Eng. 7, 103272 (2019)
Wei, J., Wang, H., Zhang, Q., et al.: One-pot hydrothermal synthesis of N-doped carbon quantum dots using the waste of shrimp for hydrogen evolution from formic acid. Chem. Lett. 44, 241–243 (2015)
Arabatzis, I., Stergiopoulos, T., Bernard, M., et al.: Silver-modified titanium dioxide thin films for efficient photodegradation of methyl orange. Appl. Catal. B. 42, 187–201 (2003)
Jamila, G.S., Sajjad, S., Leghari, S.A.K., et al.: Nitrogen doped carbon quantum dots and GO modified WO3 nanosheets combination as an effective visible photo catalyst. J. Hazard. Mater. 382, 121087 (2020)
Gupta, V., Miura, N.: Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta. 52, 1721–1726 (2006)
Khan, A., Khan, A.A.P., Rahman, M.M., et al.: Preparation of polyaniline grafted graphene oxide–WO3 nanocomposite and its application as a chromium(III) chemi-sensor. RSC Adv. 5, 105169–105178 (2015)
Malefane, M.E., Ntsendwana, B., Mafa, P.J., et al.: In-situ synthesis of tetraphenylporphyrin/tungsten(VI) oxide/reduced graphene oxide (TPP/WO3/RGO) nanocomposite for visible light photocatalytic degradation of acid blue 25. ChemistrySelect 4, 8379–8389 (2019)
Raizada, P., Sudhaik, A., Singh, P., et al.: Converting type II AgBr/VO into ternary Z scheme photocatalyst via coupling with phosphorus doped g-C3N4 for enhanced photocatalytic activity. Sep. Purif. Technol. 227, 115692 (2019)
Lu, N., Wang, P., Su, Y., et al.: Construction of Z-scheme g-C3N4/RGO/WO3 with in situ photoreduced graphene oxide as electron mediator for efficient photocatalytic degradation of ciprofloxacin. Chemosphere 215, 444–453 (2019)
Choobtashani, M., Akhavan, O.: Visible light-induced photocatalytic reduction of graphene oxide by tungsten oxide thin films. Appl Surf Sci. 276, 628–634 (2013)
Khan, M.E., Khan, M.M., Cho, M.H.: Fabrication of WO3 nanorods on graphene nanosheets for improved visible light-induced photocapacitive and photocatalytic performance. RSC Adv. 6, 20824–20833 (2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nisa, MU., Nadeem, N., Yaseen, M. et al. Applications of graphene-based tungsten oxide nanocomposites: a review. J Nanostruct Chem 13, 167–196 (2023). https://doi.org/10.1007/s40097-021-00464-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-021-00464-z