Abstract
Green synthesis of nanoparticles has attracted significant attention as an alternative to chemical synthesis procedure. The bulk availability of plants, microbial biomass and the use of eco-friendly solvents has significantly reduced the cost in addition to the hazards associated with the chemical synthesis of the nanoparticle. In this study, we demonstrated the biosynthesis of titanium nanoparticles (TiO2NPs) with the extract of Trichoderma citrinoviridae as a reducing agent. The physicochemical properties of biogenic TiO2NPs were studied using FESEM, Zeta sizer, FTIR and XRD. The size (10–400 nm), morphology, crystallinity, zeta potential (29.5 mV), and polydispersity index (0.327) suggested that the biogenic TiO2NPs were polymorphic, crystalline and stable. FESEM revealed that the synthesized TiO2NPs were majorly irregular, and some interesting TiO2NPs structures, i.e., triangular, pentagonal, spherical and rod were also observed. The biogenic TiO2NPs showed excellent antibacterial activity (100 µg/mL) against planktonic cells of extremely drug-resistant (XDR) Pseudomonas aeruginosa clinical isolates. The TiO2NPs also had better antioxidant potential as compared to standard gallic acid. This study indicates the use of T. citrinoviridae for synthesizing biogenic TiO2NPs and their potential use against XDR bacteria.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvements in green nanotechnology have found prospects in areas of medicine, health-care, agriculture and other commercial sectors [1]. Biogenic nanoparticles (NPs) are now looked upon as a promising and attractive alternative to costly and chemically synthesized NPs that involve the use of hazardous chemicals. Green synthesis procedure with plant and microbial extracts are more safe, reliable, sustainable and eco-friendly. The molecules from plant and microbial extracts act as capping/reducing agents for NPs and, thus, increase their bioactivity, biocompatibility, and environmental sustainability [2].
Among microbes, extensive reports are available on the fungal extract mediated green synthesis of NPs. Fungi-mediated synthesis of metal/metal oxide NPs (Ag, Au, ZnO, CeO, TiO, CuO) is well studied [3,4,5,6,7,8]. Trichoderma is one such genus of fungi in the family Hypoceraceae, that are prevalent in all soils and are counted amongst the most laboratory culturable fungi. Various species belonging to the genus Trichoderma are studied for biogenic NPs synthesis. For instance, T. koningiopsis, T. harzianum, T. viride, T. asperellum, T. longibrachiatum, T. pseudokoningii, and T. virens are used for synthesizing NPs of Ag, Au, Cu and CdS [9,10,11,12,13]. Therefore, due to the laboratory tractable nature, Trichoderma sp. may attract significant attention for preparing fungus mediated biogenic metal and metal oxide NPs.
To the best of the authors' knowledge, Trichoderma genus is yet to be explored for biogenic synthesis of titanium oxide NPs (TiO2NPs). TiO2NPs are important semiconducting transition metal oxides used in paints owing to their excellent optical, magnetic, and electrical properties. They are extensively used in photocatalytic applications to remove toxins or contaminants from wastewater [14]. For many years, TiO2NPs are looked as an attractive alternative to antimicrobial compounds due to their photocatalytic properties, low cost, biocompatibility, eco-friendly nature, generally recognized as safe (GRAS) status, chemical and thermodynamic stability [15, 16]. Therefore, the TiO2NPs hold great potential to be used in the areas of food, health, and medicine.
Thus, in the present study, TiO2NPs were biosynthesized using the extract of an endophytic fungus Trichoderma citrinoviride and characterized for size, morphology, surface charge, crystallinity with FESEM, FTIR, UV, zeta sizer, and XRD. Therefore, this investigation could be a source of producing biogenic nano-TiO2 from T. citrinoviride and use them as antioxidants source. Additionally, they displayed antimicrobial property against XDR Pseudomonas aeruginosa clinical isolates.
Materials and methods
Isolation of Trichoderma
The isolation of endophytic fungus was carried out by the procedure described earlier [17]. The roots of Sorghum bicolor (Dehugaon, Pune, India) were washed under running tap water for 10 min followed by surface sterilization with 4% (v/v) sodium hypochlorite. The roots were cut transversely resulting in 2-mm-thick root discs, which were then transferred on Potato Dextrose Agar (PDA) plates supplemented with chloramphenicol 150 mg/L. The PDA plates were incubated at 28 °C for 1 week for the growth of endophytic fungal colonies.
Collection and characterization of Pseudomonas aeruginosa clinical isolates
The two clinical isolates of Pseudomonas aeruginosa (PA01 and PA02) were collected from the Department of Microbiology B. J. Govt. Medical College, Pune, India. The isolates were characterized by 16S rRNA gene sequencing and the sequences were submitted to GenBank with the accession numbers MK072805 and MK072806. The cultures were further characterized as extremely drug resistant based on their resistance profile evaluated as per CLSI 2017 guidelines (Fig. 1) [18].
Biological synthesis of TiO2 nanoparticles
For the biosynthesis of TiO2NPs, T. citrinoviride was inoculated in PDB (Potato Dextrose Broth) (HiMedia, Mumbai, India). The flask with T. citrinoviride was then kept on a rotatory shaker (150 rpm) at 25 °C for 7 days. The mycelial suspension was then passed through Whatman’s filter paper no. 42 and collected separately. The mycelia were extensively washed with sterile distilled water. Five grams of mycelial biomass was dried and powdered followed by suspending it in 100-mL sterile distilled water before overnight incubation. On the next day, the mycelial solution was centrifuged at 10,000 rpm for 15 min at 4 °C, and the cell lysate was used for biosynthesis of TiO2NPs. Here, 0.2-ml HNO3 (Himedia, Mumbai, India) was added to 10-mL titanium isopropoxide (Sigma Aldrich, India), 90-mL ethanol (Himedia, Mumbai, India) and 10-mL mycelial cell lysate. The resultant reaction mixture was stirred properly and incubated for 30 min. This was followed by drying and calcination of the incubated reaction mixture in the furnace at 450 °C for 2 h to synthesize TiO2NPs powder.
Characterization of Trichoderma TiO2NPs
The ultraviolet–visible (UV–Vis) spectrum (Shimadzu Corp., Tokyo, Japan) of TiO2NPs were recorded at regular intervals and the absorption maxima were recorded between 400 and 700 nm with a resolution of 1 nm (Data not shown).
Morphological details of TiO2NPs were analyzed using field emission scanning electron microscope (FESEM) (Nova Nano SEM 450, Thermo Fisher Scientific, Waltham, MA, USA). Briefly, the TiO2NPs were diluted to near transparency in Milli-Q water, plated on a smear-free coverslip and allowed to dry overnight in an oven at 37 °C. The coverslip was then coated with osmium tetraoxide for FESEM analysis.
Zeta potential, hydrodynamic size and PDI of TiO2NPs were analyzed by zeta sizer (Malvern Zetasizer Nano ZS, UK).
The binding properties of TiO2NPs to the T. citrinoviride extract were studied using Fourier transform infrared spectroscopy (FTIR) (Nicolet Impact 400 FTIR spectrophotometer, Nicolet Instrument Corp., Madison, WI). Briefly, the bio-transformed product present in the cell-free filtrate was freeze dried and diluted with potassium bromide in 1:100 ratio. All measurement was carried out in the range of 400–4000 cm−1 with a resolution of 4 cm−1. Similarly, the crystallinity of TiO2NPs was studied using powder X-ray diffraction (XRD, Bruker AXS Inc.).
Antibacterial activity of TiO2NPs
The P. aeruginosa clinical isolates (PA01 and PA02) were treated with different concentrations of TiO2NPs (0, 25, 50, 75 and 100 µg/mL). Briefly, overnight-grown bacterial suspension of PA01 and PA02 were added to fresh Muller Hinton broth (HiMedia, Mumbai, India) and the O. D. at 600 nm was adjusted to 0.1 before treating with TiO2NPs. The flasks with TiO2NPs-treated clinical isolates were kept overnight on shaker incubator (80 rpm) at 37 °C.
The SEM analysis of P. aeruginosa (PA01) treated with 100 µg/mL was performed as per the previously reported procedure [19]. The treated sample was subjected to SEM analysis after three different time points, i.e., 0, 30, and 60 min post-treatment. The sample for analysis was prepared as per the procedure described by Dosunmu et al. and the SEM images were taken (Nova Nano SEM 450, Thermo Fisher Scientific, Waltham, MA, USA) [20].
Antioxidant activity
The antioxidant activity of TiO2NPs was performed according to the procedure given by Santhoshkumar et al. (2014) [21]. Briefly, different concentrations of TiO2NPs (10, 20, 30, 40, 50, 75 and 100 µg/mL) and standard Gallic acid were taken in different test tubes. To this, 1 mL of freshly prepared DPPH (1 mM) (HiMedia, Mumbai, India), solubilized in methanol was added and vortexed thoroughly. Finally, the solution was incubated in dark for 30 min. The absorbance of stable (standard) DPPH was recorded at 517 nm. The DPPH (containing no sample) was used as a control and was prepared using the same procedure. The activity was expressed as the percentage (%) inhibition calculated using the equation of:
where Ac is the absorbance of the control (DPPH radical + methanol), and As is the absorbance of the sample (DPPH radical + TiO2NP/gallic acid).
Results and discussion
Identification of fungi and submerged culturing
Fungal colonies of T. citrinoviride were separated and identified based on the morphology (Fig. 2a) and reproductive characters with standard identification manual [22].
Physicochemical characterization of green synthesized TiO2NPs
The physicochemical characterization using UV–vis, FESEM, zeta sizer, XRD and FTIR revealed the details like size, shape, morphology, surface charge, crystallinity, stability and dispersivity of biogenic TiO2NPs. The strong absorbance recorded at 400 nm was considered as the initial sign for the successful synthesis of TiO2NPs.
FESEM of TiO2NPs synthesized from T. citrinoviride extract revealed the morphological details, predominantly comprising of irregular, triangular, pentagonal, spherical and rod-shaped particles with the size ranging between 10 and 400 nm (Fig. 3). We suppose that the size of TiO2NPs should be further modified and controlled by varying the concentration of T. citrinoviride extract. It will be interesting to see if monomorphic particles like spheres can be synthesized or isolated from the mixture for a particular application. For instance, spherical TiO2NPs of 25–200 nm are used in sunscreens to prevent erythema [23]. For NPs, the most common shape is spherical; however, pentagonal, triangular and rod shapes are more effective against bactericidal activity due to the exposed planes [24]. Furthermore, even if the NPs have identical surface areas, the shape of NPs is important as the planes having high atom density facets increase reactivity.
The hydrodynamic size distribution (Z-average) and zeta potential of the TiO2NPs were found to be 10–396 nm and 29.5 mV, respectively (Fig. 4a, b). The zeta potential close to + 30 mV suggests that the biogenic TiO2NPs are strong cationic and would remain stable in the suspension for a longer period. Further, the low polydispersity index (PDI) value of 0.327 indicated high particle homogeneity. The high zeta potential and low PDI assured stability of the TiO2NPs.
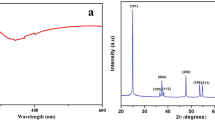
XRD of synthesized TiO2 showed numerous peaks (Fig. 4c) indicating the presence of two or more phases of TiO2 in the sample. The observed 2θ values were comparable with JCPDS card no. 21–1272 and 21–1276 of anatase and rutile TiO2. The peaks at 2θ—25.17, 37.70, 36.81, 39.17, 47.91, 55.01, 62.59, 68.92, 70.49 and 75.09 belong to the anatase phase (JCPDS card No. 21–1276); while, peaks at 2θ = 27.40, 35.91, 38.25, 41.19, 56.45, 63.93, and 69.91 belong to rutile phase of TiO2 (JCPDS, no. 21–1276). Apart from peaks of these two phases, extra peaks were not present in the XRD data indicating that a sample of TiO2 is pure. From XRD data, crystal parameters of both phases were calculated and they match the JCPDS of rutile (a = 4.6026 Å c = 2.9661 Å crystal volume = 62.83 Å3) and anatase phase (a = 3.7752 Å c = 9.5359 Å crystal volume = 135.91 Å3) of TiO2 [25, 26]. The crystallite size of both phases was calculated by Debye–Scherrer’s formula and it is found to be 53 and 66 nm, respectively, for anatase and rutile phase.
FTIR spectrum of synthesized TiO2NPs was analyzed in a range of 400–4000 cm−1 (Fig. 4d). It displays strong IR absorbance below 1200 cm−1, which belong to Ti-O-Ti stretching frequency. The stretching vibrations observed near 1400–1600 cm−1 and 3000–3100 cm−1 suggest the presence of aromatic group carbonyl groups like C=C and C–H, respectively. Peaks observed at 1670–1820 cm−1 can be attributed to the carbonyl group (C=O). Thus, the FTIR spectra confirmed the synthesis of TiO2NPs and the capping of functional groups from T. citrinoviride extract.
Antibacterial activity of TiO2NPs against P. aeruginosa
The antibacterial activity was aimed to analyze the concentration of TiO2NPs required to inhibit the growth of extremely drug-resistant P. aeruginosa clinical isolates (PA01 and PA02). The bacterial cultures grown overnight showed a decrease in cell density with increase in the concentration of TiO2NPs (Fig. 5). Both PA01 and PA02 strains showed a similar trend and no bacterial growth was observed at 100 µg/mL of TiO2NPs. The SEM analysis of bacterial culture (PA01) at three time points (0, 30 and 60 min) showed bacterial cell lysis after TiO2NPs treatment (Fig. 6).
When we talk about medicine, the indiscriminate use of antibiotics has generated the problem of antimicrobial resistance resulting in millions of fatalities every year. As a result, WHO has listed a few critical priority pathogens which require immediate attention [27]. One such pathogen is drug-resistant Pseudomonas aeruginosa that causes nosocomial infections, cystic fibrosis, pneumonia, sepsis and, therefore, is one of the reasons for mortality in terminally ill patients in ICUs. The biogenic TiO2NPs can become an attractive alternative to antibiotics or can be used synergistically to potentiate the ailing antibiotics [28].
Reports on chemically synthesized TiO2NPs suggest that the concentration of biogenic TiO2NPs (100 µg/mL) required to inhibit the growth of XDR P. aeruginosa is 3.5-fold less than the chemically synthesized TiO2NPs (350 µg/mL) [28, 29]. This suggests the potency of biogenic TiO2NPs over chemically synthesized ones. However, the TiO2NPs have shown varying toxicity to human cell types [30], and therefore, the complete toxicity assessment of T. citrinoviride TiO2NPs on different cell types is recommended before it is considered for human use.
TiO2NPs synthesized by both biogenic and chemical means have shown antibacterial properties [7, 31]. The bactericidal effect of TiO2NPs is generally attributed to the ROS-mediated decomposition of bacterial cell wall and membrane. Due to the presence of hydroxyl group, TiO2NPs can dissolve the bacterial outer-membrane causing electrolytic leakage [32]. Apart from ROS, the shape of TiO2NPs (e.g., pentagonal, triangular and rod) may have also played a major role towards their antibacterial potential. The NPs with corners and planes are more surface reactivity as compared to spherical and irregular NPs [24]. However, the other plausible mechanisms that may have resulted in the inhibition of bacterial growth are also reported elsewhere [33]. But looking at the overall outcome, these TiO2NPs can be tested against other bacterial pathogens and even in combination with antibiotics for their synergistic antimicrobial potential.
Antioxidant potential
The DPPH (α, α-diphenyl-β-picrylhydrazyl) is the most stable free radical that can be used to quantify the ROS scavenging activity of antioxidant compounds towards it. The anomalous electron of the nitrogen atom in the DPPH is reduced by accepting the hydrogen atom from antioxidants to the resultant hydrazine [34]. The T. citrinoviride extract and the TiO2NPs showed better antioxidant activity as compared to gallic acid standard (Fig. 7). The DPPH scavenging activity of TiO2NPs was found to increase in a dose-dependent manner.
Conclusion
The present work demonstrated the possibility of using T. citrinoviride for the biogenic synthesis of TiO2NPs. The physicochemical characterization revealed that the TiO2NPs are polymorphic and stable. Further through the basic antibacterial studies, we demonstrated that the biogenic TiO2NPs can inhibit the growth of extremely drug-resistant P. aeruginosa clinical isolates in a concentration and time-dependent manner. The TiO2NPs also showed better antioxidant potential as compared to the T. citrinoviride extract and gallic acid. Our finding provides an idea about the excellent prospects for Trichoderma sp. to produce TiO2NPs using green synthesis approach in the coming years. Owing to the ubiquitous nature, ease of laboratory culturing and rapid growth, Trichoderma genus can become a preferred choice for synthesizing biogenic TiO2NPs. Thus, the outcome would benefit mainly the researchers working at the interface of nanotechnology and mycology, pharmaceutical industries with a focus on repurposing NPs as antibiotics, wastewater treatment plants, skincare products and other allied nano-based industries.
References
Singh, J., Dutta, T., Kim, K.H., Rawat, M., Samddar, P., Kumar, P.: “Green” synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnol. (2018). https://doi.org/10.1186/s12951-018-0408-4
Raveendran, P., Fu, J., Wallen, S.L.: Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 125, 13940–13941 (2003). https://doi.org/10.1021/ja029267j
Feroze, N., Arshad, B., Younas, M., Afridi, M.I., Saqib, S., Ayaz, A.: Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 83, 72–80 (2020). https://doi.org/10.1002/jemt.23390
Kadam, V.V., Ettiyappan, J.P., Mohan Balakrishnan, R.: Mechanistic insight into the endophytic fungus mediated synthesis of protein capped ZnO nanoparticles. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 243, 214–221 (2019). https://doi.org/10.1016/j.mseb.2019.04.017
Naimi-Shamel, N., Pourali, P., Dolatabadi, S.: Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J. Mycol. Med. 29, 7–13 (2019). https://doi.org/10.1016/j.mycmed.2019.01.005
Gopinath, K., Karthika, V., Sundaravadivelan, C., Gowri, S., Arumugam, A.: Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J. Nanostruct. Chem. 5, 295–303 (2015). https://doi.org/10.1007/s40097-015-0161-2
Subhapriya, S., Gomathipriya, P.: Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 116, 215–220 (2018). https://doi.org/10.1016/j.micpath.2018.01.027
Cuevas, R., Durán, N., Diez, M.C., Tortella, G.R., Rubilar, O.: Extracellular biosynthesis of copper and copper oxide nanoparticles by stereum hirsutum, a native white-rot fungus from chilean forests. J. Nanomater. 2015, 1–7 (2015). https://doi.org/10.1155/2015/789089
Salvadori, M.R., Ando, R.A., Oller Do Nascimento, C.A., Corrêa, B.: Bioremediation from wastewater and extracellular synthesis of copper nanoparticles by the fungus Trichoderma koningiopsis. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst Environ. Eng. 49, 1286–1295 (2014). https://doi.org/10.1080/10934529.2014.910067
Devi, T.P., Kulanthaivel, S., Kamil, D., Borah, J.L., Prabhakaran, N., Srinivasa, N.: Biosynthesis of silver nanoparticles from Trichoderma species. (2013)
Ahluwalia, V., Kumar, J., Sisodia, R., Shakil, N.A., Walia, S.: Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crops Prod. 55, 202–206 (2014). https://doi.org/10.1016/j.indcrop.2014.01.026
Elgorban, A.M., Al-Rahmah, A.N., Sayed, S.R., Hirad, A., Mostafa, A.A.-F., Bahkali, A.H.: Antimicrobial activity and green synthesis of silver nanoparticles using Trichoderma viride. Biotechnol. Biotechnol. Equip. 30, 299–304 (2016). https://doi.org/10.1080/13102818.2015.1133255
Tripathi, R.M., Gupta, R.K., Singh, P., Bhadwal, A.S., Shrivastav, A., Kumar, N., Shrivastav, B.R.: Ultra-sensitive detection of mercury(II) ions in water sample using gold nanoparticles synthesized by Trichoderma harzianum and their mechanistic approach. Sensors Actuators B Chem. 204, 637–646 (2014). https://doi.org/10.1016/j.snb.2014.08.015
Joost, U., Juganson, K., Visnapuu, M., Mortimer, M., Kahru, A., Nõmmiste, E., Joost, U., Kisand, V., Ivask, A.: Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 142, 178–185 (2015). https://doi.org/10.1016/j.jphotobiol.2014.12.010
Nasrollahzadeh, M., Sajadi, S.M.: Synthesis and characterization of titanium dioxide nanoparticles using Euphorbia heteradena Jaub root extract and evaluation of their stability. Ceram. Int. 41, 14435–14439 (2015). https://doi.org/10.1016/j.ceramint.2015.07.079
Hajar, O.S., Abd Salam, N.R., Zainal, N., Kadir, B.R., Talib, R.A.: Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. J. Photoenergy Int (2014). https://doi.org/10.1155/2014/945930
Singh, A.K., Rathod, V.J., Singh, D., Ninganagouda, S., Kulkarni, P., Mathew, J., Haq, M. ul: Bioactive Silver Nanoparticles from Endophytic Fungus Fusarium sp. Isolated from an Ethanomedicinal Plant Withania somnifera (Ashwagandha) and its Antibacterial Activity, (2015)
Weinstein, M.P., Lewis, J.S.: The clinical and laboratory standards institute subcommittee on Antimicrobial susceptibility testing: background, organization, functions, and processes. J. Clin. Microbiol. (2020). https://doi.org/10.1128/JCM.01864-19
Arya, S.S., Sharma, M.M., Das, R.K., Rookes, J., Cahill, D., Lenka, S.K.: Vanillin mediated green synthesis and application of gold nanoparticles for reversal of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates. Heliyon. 5, e02021 (2019). https://doi.org/10.1016/j.heliyon.2019.e02021
Dosunmu, E., Chaudhari, A.A., Singh, S.R., Dennis, V.A., Pillai, S.R.: Silver-coated carbon nanotubes downregulate the expression of Pseudomonas aeruginosa virulence genes: a potential mechanism for their antimicrobial effect. Int. J. Nanomed. 10, 5025–5034 (2015). https://doi.org/10.2147/IJN.S85219
Santhoshkumar, T., Rahuman, A.A., Jayaseelan, C., Rajakumar, G., Marimuthu, S., Kirthi, A.V., Velayutham, K., Thomas, J., Venkatesan, J., Kim, S.K.: Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 7(12), 968–976 (2014)
Siddiquee, S.: Practical handbook of the biology and molecular diversity of trichoderma species from tropical regions. Springer International Publishing, Cham (2017)
Popov, A.P., Lademann, J., Priezzhev, A.V., Myllylä, R.: Effect of size of TiO[sub 2] nanoparticles embedded into stratum corneum on ultraviolet-A and ultraviolet-B sun-blocking properties of the skin. J. Biomed. Opt. 10, 064037 (2005). https://doi.org/10.1117/1.2138017
Slavin, Y.N., Asnis, J., Häfeli, U.O., Bach, H.: Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol (2017). https://doi.org/10.1186/s12951-017-0308-z
Wang, Y., Li, L., Huang, X., Li, Q., Li, G.: New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 5, 34302–34313 (2015). https://doi.org/10.1039/c4ra17076h
Li, W., Liang, R., Hu, A., Huang, Z., Zhou, Y.N.: Generation of oxygen vacancies in visible light activated one-dimensional iodine TiO2 photocatalysts. RSC Adv. 4, 36959–36966 (2014). https://doi.org/10.1039/c4ra04768k
Beyer, P., Paulin, S.: Priority pathogens and the antibiotic pipeline: an update (2020). https://www.who.int/bulletin/volumes/98/3/20-251751/en/. Accessed 9 Aug 2020
Lee, N.Y., Ko, W.C., Hsueh, P.R.: Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Pharmacol Front (2019). https://doi.org/10.3389/fphar.2019.01153
Skocaj, M., Filipic, M., Petkovic, J., Novak, S.: Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 45(4), 227–247 (2011)
Arora, B., Murar, M., Dhumale, V.: Antimicrobial potential of TiO 2 nanoparticles against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 10, 819–827 (2015). https://doi.org/10.1080/17458080.2014.902544
Grace, V.M., Peedikayil, J.N., Narayanan, P.M., Vani, C., Sevanan, M.: In vitro study on the efficacy of zinc oxide and titanium dioxide nanoparticles against metallo beta-lactamase and biofilm producing Pseudomonas aeruginosa. J. Appl. Pharm. Sci. 4, 041–046 (2014). https://doi.org/10.7324/JAPS.2014.40707
Ahmed, F.Y., Aly, U.F., Abd El-Baky, R.M.: Waly NGFM (2020) Comparative study of antibacterial effects of titanium dioxide nanoparticles alone and in combination with antibiotics on MDR pseudomonas aeruginosa strains. Int. J. Nanomedicine. 15, 3393–3404 (2020). https://doi.org/10.2147/IJN.S246310
Rajakumar, G., Rahuman, A.A., Roopan, S.M., Khanna, V.G., Elango, G., Kamaraj, C., Zahir, A.A., Velayutham, K.: Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria . Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 91, 23–29 (2012). https://doi.org/10.1016/j.saa.2012.01.011
Liu, W., Bertrand, M., Chaneac, C., Achouak, W.: TiO2 nanoparticles alter iron homeostasis in: Pseudomonas brassicacearum as revealed by PrrF sRNA modulation. Environ. Sci. Nano. 3, 1473–1482 (2016). https://doi.org/10.1039/c6en00316h
Acknowledgment
The authors thank Dr. Renu Bharadwaj, Head of Department, Microbiology, B. J. Govt. Medical College, Pune – 411001, India for providing P. aeruginosa clinical isolates.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arya, S., Sonawane, H., Math, S. et al. Biogenic titanium nanoparticles (TiO2NPs) from Tricoderma citrinoviride extract: synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int Nano Lett 11, 35–42 (2021). https://doi.org/10.1007/s40089-020-00320-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-020-00320-y