Abstract
Metal halide perovskite nanostructures have received much attention for many optoelectronic devices, such as solar cells, light-emitting diodes, photodetectors, and sensors due to their sharp emission spectra and adjustable wide optical bandgap. However, low emission efficiency and stability still impede their development and application. This paper reports a facile surface modification of the CsPbBr3 quantum dots (QDs) to enhance the optical performance using a silver thiocyanate (AgSCN) additive. Structural and chemical investigations show that the QD morphology and size do not change significantly, while the SCN– anion is incorporated into the CsPbBr3 QDs as a function of AgSCN loading. In addition, the SCN– anion interacts with Pb2+ in the CsPbBr3 QDs, which indicates that the SCN– anion fills out the Br– anion vacancy site or substitutes the Br- anion without significantly affecting the ligand configuration. Therefore, the photoluminescence (PL) intensity and stability of the AgSCN-treated CsPbBr3 QDs are improved compared to the pristine one, because the AgSCN plays a critical role in recovering the appropriate surface stoichiometry and eliminating the defective surface states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor quantum dots (QDs) have been achieved much progress because of their importance as a functional building block providing potential opportunities for optoelectronic device applications as well as for surveying novel quantum effects [1, 2]. Recently, metal halide perovskite QD structures have attracted considerable attention in display applications due to their superior properties, such as large exciton binding energy, band structure with defect tolerance for high photoluminescence (PL) efficiency, very narrow PL for high color purity, and wide range of band-gap energy controllability covering the whole visible wavelength spectral range [3,4,5,6,7,8,9]. Among the halide-based perovskite alloys, the cesium lead halide perovskite (CsPbX3; X = Cl, Br, and I) based QDs have been investigated widely because of a simple solution process as well as many advantages mentioned above [3,4,5]. Nevertheless, the intrinsically unstable nature of CsPbBr3 QD structures that deteriorate emission efficiency and stability due to the surface halide defects in air atmospheres are still considered to be a serious problem for practical applications [10,11,12,13,14,15]. Typically, the deterioration caused by moisture in the surface defects results in a decrease in exciton recombination efficiency and a reduction of emission characteristics [10,11,12,13,14,15]. Many approaches have been reported to circumvent these problems to improve the PL efficiency and stability of CsPbBr3 QDs, such as controlling the surface states, doping foreign elements, constructing core–shell structure, and inducing ligand exchange [16,17,18,19,20,21]. Among these methods, the ligand exchange strategy has provided an efficient and simple way to simultaneously improve the stability and luminescence efficiency. The surface passivation of CsPbBr3 QDs has been known to be very effective in enhancing the emission efficiency and stability of QD itself, because the existence of halide vacancy is one of the major factors restricting the performance of QD emitters. The exchanged ligands can passivate grain boundaries, which reduces the nonradiative recombination at the surfaces of the QDs and improves their electronic properties. Recently, it has been demonstrated that the surface defects of perovskite QDs are remarkably suppressed by introducing inorganic materials, such as ZnBr2, sodium thiocyanate (NaSCN), NH4SCN, Ga(NO3), and LiBr, revealing the important role of QD surface engineering [22,23,24,25,26,27]. In addition, the emission efficiency and stability of the CsPbBr3 nanostructures were improved by exchanging the organic ligand by the addition of the didodecyldimethyl-ammonium bromide (DDAB) [28]. The added DDAB eliminated the surface point defects by adding Br− anion and substituted the surface ligand with DDA+ anion. Therefore, the ligand exchange strategy is effective in improving the chemical stability as well as the emission efficiency and needs more investigations. In this study, we propose using a pseudo-halide-based ligand (silver thiocyanate, AgSCN) to enhance the PL efficiency and stability of CsPbBr3 QDs. A plausible enhancement mechanism is also discussed based on the comprehensive structural, chemical, and optical properties’ analysis.
2 Experiments and discussion
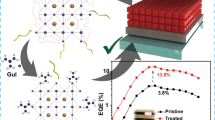
In this study, the pristine CsPbBr3 QDs were fabricated based on a ligand-assisted reprecipitation (LARP) method [6, 7]. After synthesizing the CsPbBr3 QDs, their surface was modified using AgSCN additives. Figure 1 shows a schematic diagram of the synthetic procedure and surface modification process of the CsPbBr3 QDs by the AgSCN additives. To synthesize the pristine CsPbBr3 QDs, 0.4 mmol of PbBr2 and CsBr was dissolved in 10 ml of N,N-dimethylformamide (DMF) solvent and stirred for 1 h at room temperature. And then, 600 μl of oleylamine (OAm) was added to the precursor solution. The mixed precursor solution was stirred for 1 h. The precursor solution was filtered through a 0.2 μm polytetrafluoroethylene syringe filter to remove the impurities and undissolved sources. To make a crude solution, 8 mL of oleic acid (OA) was added to 80 ml of a toluene solution and stirred vigorously at room temperature for 1 min. After rapidly injecting 4 ml of the precursor solution into the toluene solution containing OA, the mixed solution was stirred for 20 s to obtain the pristine CsPbBr3 QD solution. The synthesized CsPbBr3 QD solution showed strong green emission under UV light, as shown in Fig. 1. All the processes were conducted in a tightly controlled Ar-filled glove box to avoid oxygen and water contamination. The crude solution was then centrifuged at 12,000 rpm for 10 min to remove the large particles. Methyl acetate and ethyl acetate are added to the remained solution at a volume ratio of 1:1:1 and centrifuged again at 12,000 rpm for 10 min to remove the remaining impurities. Didodecyldimetyl ammonium bromide (DDAB, 99%) and OA were added to the precipitated CsPbBr3 QD solution, and the mixture was uniformly dispersed for 1 min. Finally, the pristine CsPbBr3 QD solution was obtained by dispersing the precipitates into the 800 μl of n-octane. The above procedures were carried out twice to obtain 1,600 μl of pristine CsPbBr3 QD solution. The AgSCN was dissolved into the diethyl sulfide with a 5, 10, 15, and 20 mg/ml concentration. 10 μl of the dissolved AgSCN solutions with different concentrations were added into the 300 μl of pristine CsPbBr3 QD solutions. These samples are labeled as AgSCN-5, -10, -15, and -20, respectively. Here, the AgSCN was added to the CsPbBr3 QDs to investigate the effect of surface passivation by the pseudo-halide SCN group. As a reference, 10 μl of diethyl sulfide was added into the CsPbBr3 QD solution to confirm the influence of the solvent itself, but there were no changes in the photoluminescence (PL) and UV–visible absorption spectra. The CsPbBr3 QD solutions were spin-coated on the sapphire and glass substrates to fabricate the thin films for the optical measurements. After spin-coating at 1,500 rpm for 60 s, the QD films were annealed at 100 °C for 10 min. The CsPbBr3 QD films showed uniform green emission on the sapphire substrate, as shown in Fig. 1.
The optical properties of the CsPbBr3 QDs were examined using PL and UV–visible spectrophotometer (Agilent 8453). PL spectra were measured at room temperature using a 405 nm diode laser and MAYA 2000 spectrophotometer. Structural properties of the CsPbBr3 QDs were performed by X-ray diffraction (XRD, Rigaku D/Max-2500 diffractometer equipped with a Cu Kα source) and transmission electron microscopy (TEM; JEOL-2010). Elemental analysis of the QDs was carried out using a TEM equipped with an energy-dispersive X-ray spectrometer (EDS). The chemical bonding states of the CsPbBr3 QDs were investigated by a Fourier-transform infrared (FTIR, Thermo scientific, NICOLET iS10) spectroscopy.
Figure 2 presents the optical properties of the CsPbBr3 QDs by the post-treatment of AgSCN as a surface source. To investigate the effect of AgSCN addition on the optical properties of the CsPbBr3 QDs, UV–visible absorption spectroscopy was measured, as shown in Fig. 2a. By adding an AgSCN source, the CsPbBr3 QDs became more bright under 395 nm UV lamp illumination, while the green emission color remained constant, as shown in the inset of Fig. 2. This was also confirmed by the UV–visible absorption spectra, which showed no change in the shape of spectra and absorption edges even with a variation of the AgSCN content. The shape of the absorption spectra was similar for all the QDs with a steep absorption edge and sharp exciton absorption peak at about 498 nm. The band-gap energy of pristine CsPbBr3 QDs estimated from the absorption edge was about 2.40 eV (516 nm), which is slightly larger than its bulk band-gap energy of 2.32 eV [29]. The increased band-gap energy of pristine CsPbBr3 QDs may be due to the quantum confinement effect [9, 10, 16, 17, 30]. As the AgSCN is added, the main absorption increases at a similar wavelength to the pristine one. In addition, it should be noted that all QDs showed the absorption peak corresponding to the exciton state even with increasing the AgSCN content. This indicates the uniform size distribution. To evaluate the optical emission properties of the AgSCN-treated CsPbBr3 QD films, steady-state PL spectra were measured at room temperature, as shown in Fig. 2b. The PL spectra of the CsPbBr3 QDs exhibited similar symmetric emission peaks centered around 512 nm while showing different emission intensity and peak positions with increasing the AgSCN. As shown in Fig. 2c, the PL intensity increased and the emission peak position showed a red-shift with increasing the AgSCN content. This would be attributed to the slight modification of the electron energy band structure after the AgSCN-treatment. The chalcogen p-states in the pseudo-halide SCN are located in higher energy states than that of Br, such that they dominate the valence band maximum [31]. This would induce a slight reduction in the band-gap energy of the AgSCN-treated CsPbBr QDs. It should be noted that the PL intensity of the AgSCN-treated CsPbBr3 QDs showed comparable performance to the DDAB-treated one [28].
a UV–Vis absorption spectra of CsPbBr3 QD solutions with the variation of AgSCN additive content. The emission images of the QD solutions were taken under excitation by a commercial UV lamp. b PL spectra and emission images of CsPbBr3 QD films coated on sapphire substrate with the variation of AgSCN additive content. The PL was taken under excitation by a 405 nm laser. c Variation of the PL emission peak emission energy and integrated intensity of the CsPbBr3 QD films with the variation of AgSCN content
Figure 3 shows the TEM images of the pristine and AgSCN-treated CsPbBr3 QDs. The pristine CsPbBr3 QDs show a uniform cuboidal shape with an average particle size of 10.2 nm. The CsPbBr3 QDs exhibit well-defined cuboidal shape in the high-resolution-TEM (HR-TEM) images, indicating high crystalline quality. The AgSCN-treated CsPbBr3 QDs showed a similar particle shape and size to the pristine one. In addition, no significant change in QD morphology was observed and the well-defined lattice fringes were maintained even with increased AgSCN contents (Fig. 3b–e). The size of CsPbBr3 QDs was in the range between 10 and 11 nm independent of the variation of the AgSCN content, as shown in Fig. 3f. This suggests that the AgSCN additive did not affect the crystallographic structure or crystallinity of CsPbBr3 QDs. To understand the elemental distribution as a function of AgSCN loading, EDS analysis was performed on the AgSCN-10 and -20 samples. As shown in Fig. 3g, h, the EDS results confirm the homogeneous presence of Cs, Pb, and Br in AgSCN-treated CsPbBr3 QDs. It should be noted that the N and S elements were also identified, but there is no evidence indicating the presence of Ag element (the arrows shown in the spectra) with the AgSCN content added up to 20 mg/ml. Therefore, we believe during AgSCN loading that only the SCN anion would substitute the Br lattice site or fill out its vacancy site. To preserve the tolerance and octahedral factor of the perovskite structure, the Ag elements are expelled from the QDs, because the ionic radius of Ag+ (126 pm) is much different from those of Cs+ (167 pm) and Pb2+ (119 pm).
TEM image of synthesized CsPbBr3 QDs; a pristine CsPbBr3 QDs, b 5 mg/mL of AgSCN-treated, c 10 mg/mL of AgSCN-treated, d 15 mg/mL of AgSCN-treated, and e 20 mg/mL of AgSCN-treated CsPbBr3 QDs. f Variation of the average size of CsPbBr3 QDs with the variation of AgSCN content. EDS spectra of g 10 mg/mL of AgSCN-treated and h 20 mg/mL of AgSCN-treated CsPbBr3 QDs
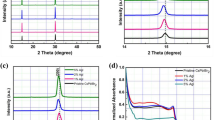
The structural evolution of the AgSCN-treated CsPbBr3 QDs was investigated by XRD, as shown in Fig. 4a. The XRD patterns of the CsPbBr3 QDs were assigned to the cubic phase (JCPDS No. 18-0346), and the crystal structure atomic model is shown in the inset of Fig. 4a. The pristine CsPbBr3 and AgSCN-treated CsPbBr3 QDs showed an identical cubic crystal structure without showing any trace of a secondary phase. This indicates that the CsPbBr3 QDs maintained a cubic structure even after the AgSCN-treatment. Even as the AgSCN content was increased from 0 to 20 mg/ml, no additional peak appeared. This reveals an absence of any secondary phase or alloys corresponding to the Ag- or SCN-related phases. However, the diffraction peaks were shifted to a higher angle side as the AgSCN content was increased from 0 to 15 mg/ml. Figure 4b, c shows the variation of the (200) peak position and full-width at half-maximum (FWHM) of (200) peak according to the AgSCN content. This variation was attributed to the smaller ionic radius of SCN– than that of Br–. An SCN– moiety is a pseudo-halide with a linear shape and an atomic radius of 137 pm, smaller than Br– (196 pm) [32]. As the SCN– is incorporated by substituting into the Br− lattice sites or its vacancy site, the unit cell of the AgSCN-treated CsPbBr3 QDs would be shrunk compared with that of the pristine one. This is confirmed from the shifted diffraction peak to the higher angle side, as expected by Bragg’s law. As shown in Fig. 4c, the FWHM of (200) peak decreases with increasing the AgSCN content up to 15 mg/ml, while it increases as the AgSCN content increases further to 20 mg/ml. This indicates that the crystallinity of the CsPbBr3 is improved which potentially may be due to fewer microstructural defects after incorporating a small amount of SCN– anions [32,33,34]. Because the size of QDs was not affected significantly as shown in Fig. 3, the decrease of the FWHM would not be attributed to the variation in the size of crystalline domains which can be expected from the Scherrer equation. This indicates that the decrease of the FWHM of diffraction peak would be due to the improved crystalline quality by eliminating the microstructural defects after incorporating a small amount of SCN– anions. This improved stability of CsPbBr3 is attributed to stronger chemical bonding between SCN– and Pb2+ than that between Br– and Pb2+ [32]. Therefore, the AgSCN can improve the stability of CsPbBr3 QDs by eliminating the Br-related vacant sites or substituting the Br lattice sites, resulting in improved PL efficiency.
a Normalized XRD patterns of the CsPbBr3 QDs with the variation of AgSCN content. The inset shows the crystal structure of the cubic CsPbBr3 phase. b Variation of (200) peak of the CsPbBr3 QDs with the variation of AgSCN content. c The variation of (200) diffraction peak position and its FWHM with the variation of AgSCN content
To investigate the modification of surface functional groups on the CsPbBr3 QDs, Fourier-transform infrared (FTIR) spectroscopy was measured, as shown in Fig. 5. The peaks at 1378–1461 and 2856–2957 cm−1 correspond to the functional groups of COO and C–H-stretching modes, respectively. The C–H-stretching modes are corresponding to the carboxyl acid of the OA ligand. The broad peaks at 2065–2087 cm−1 found for the AgSCN-treated QDs correspond to the SCN functional group [33]. This broad peak is consistent with the C≡N bond of a thiocyanate bound to lead with a Pb−S bond. The position of this peak exhibits a distinctive shift depending on the identity of the element to which the thiocyanate is bound [34]. This indicates that the SCN interacts with Pb in the CsPbBr3 QDs by filling out the Br vacancy site or substituting the Br anion. The SCN-related peak intensity increases as the AgSCN content increases from 0 to 20 mg/ml, while the peaks corresponding to the C–H-stretching mode remain constant. This indicates that the addition of AgSCN does not affect the bonding states of pristine passivation ligands. Therefore, the AgSCN-treatment would passivate the CsPbBr3 QD surface without significantly affecting the original aliphatic ligand configuration.
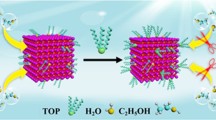
To investigate the emission stability of the CsPbBr3 QDs, the PL was measured for the QD thin films after 2 and 3 weeks stored in air ambient. Figure 6a shows the PL spectra measured 3 weeks later and variation of the normalized PL intensity with time. All the CsPbBr3 QDs show degradation of PL intensity with increasing the aging time in air ambient. Much has been reported about the degradation of PL efficiency of perovskite materials. Two main degradation mechanisms of perovskite nanostructures have been reported as oxidation and surface defect formation. The oxidation of metal cations in perovskite crystal structure occurs as the electrons have been captured by oxygen molecules [11, 12]. To circumvent this problem, modifying the surface of perovskite QDs or the strong steric hindrance of surface ligands on the QD surface are the two main approaches for improving their stability to oxygen. In addition, the perovskite structure can be easily degraded in a humid environment, because the perovskite materials exhibit ionic bonding [13, 14, 35]. Due to moisture induction, surface atoms fall off to cause surface defects, and it is also easy to cause agglomeration, which ultimately reduces quantum yield and affects the luminous performance [15]. Here, the AgSCN-treatment circumvents these problems by passivating anion vacancy sites and removing excess Pb atoms on the surface of CsPbBr3 QDs as shown in the schematic (Fig. 6b). In this way, the AgSCN-treatment plays a critical role in improving the optical performances by recovering the appropriate surface stoichiometry and eliminating the surface defects. Therefore, the AgSCN-treatment is effective in improving the emission stability as well as in enhancing the PL efficiency.
3 Conclusions
In summary, we report the enhancement of PL efficiency and stability of green light-emitting CsPbBr3 QDs can be realized by pseudo-halide-based ligand passivation. As the AgSCN content increased, no significant change in QD morphology and size was observed by TEM investigations. However, the XRD and EDS results showed that the SCN was incorporated into the CsPbBr3 QDs, while the crystalline structure was maintained consistently even with increasing the AgSCN content. Furthermore, the FTIR results show that the SCN interacts with Pb in the CsPbBr3 QDs, which indicates that the SCN fills out the Br vacancy site or substitutes the Br anion without significantly affecting the ligand configuration. As a result, the PL intensity and stability of the AgSCN-treated CsPbBr3 QDs are improved compared to the pristine one. Based on the structural and chemical investigations about the AgSCN-treated CsPbBr3 QDs, plausible emission efficiency enhancement mechanisms were discussed. We found that the AgSCN passivation plays a critical role in recovering the appropriate surface stoichiometry as well as eliminating the defective surface sites, which underpins the improved optical performances of CsPbBr3 QDs. This enhancement strategy can be applied to various QD materials to improve their optical performances for optoelectronic device applications.
References
C.R. Kagan, L.C. Bassett, C.B. Murray, S.M. Thompson, Chem. Rev. 121, 3186–3233 (2021)
G.H. Nam, I.K. Park, J. Korean Phys. Soc. 66, 785–789 (2015)
L. Protesescu, S. Yakunin, M.I. Bodnarchuk, F. Krieg, R. Caputo, C.H. Hendon, R.X. Yang, A. Walsh, M.V. Kovalenko, Nano Lett. 15, 3692–3696 (2015)
D.K. Sharma, S. Hirata, M. Vacha, Nat. Commun. 10, 4499 (2019)
H. Mashiyama, Y. Kurihara, T. Azetsu, J. Korean Phys. Soc. 32, 156 (1998)
S.B. Cho, J.I. Sohn, S.S. Lee, S.G. Moon, B. Hou, I.K. Park, J. Mater. Chem. C 9, 7027–7034 (2021)
S.G. Moon, S.B. Cho, K.K. Kim, I.K. Park, J. Alloys Comp. 858, 157643 (2021)
S.B. Cho, J.W. Jung, Y.S. Kim, C.H. Cho, I.K. Park, CrystEngComm 23, 2746–2755 (2021)
B. Li, M. Lu, J. Feng, J. Zhang, P.M. Smowton, J.I. Sohn, I.K. Park, H. Zhong, B. Hou, J. Mater. Chem. C 8, 10676 (2020)
Z. Shi, S. Li, Y. Li, H. Ji, X. Li, D. Wu, T. Xu, Y. Chen, Y. Tian, Y. Zhang, C. Shan, G. Du, ACS Nano 12, 1462–1472 (2018)
N. Aristidou, I. Sanchez-Molina, T. Chotchuangchutchaval, M. Brown, L. Martinez, T. Rath, S.A. Haque, Angew. Chem. Int. Ed. 54, 8208–8212 (2015)
M. Lorenzon, L. Sortino, Q. Akkerman, S. Accornero, J. Pedrini, M. Prato, V. Pinchetti, F. Meinardi, L. Manna, S. Brovelli, Nano Lett. 17, 3844–3853 (2017)
S. Pathak, A. Sepe, A. Sadhanala, F. Deschler, A. Haghighirad, N. Sakai, K.C. Goedel, S.D. Stranks, N. Noel, M. Price, ACS Nano 9, 2311–2320 (2015)
G.E. Eperon, S.N. Habisreutinger, T. Leijtens, B.J. Bruijnaers, J.J. van Franeker, D.W. DeQuilettes, S. Pathak, R.J. Sutton, G. Grancini, D.S. Ginger, ACS Nano 9, 9380–9393 (2015)
L. Zhang, M.G. Ju, W. Liang, Phys. Chem. Chem. Phys. 18, 23174–23183 (2016)
C. Zheng, C. Bi, F. Huang, D. Binks, J. Tian, A.C.S. Appl, Mater. Interfaces 11, 25410–25416 (2019)
M. Zirak, E. Moyen, H. Alehdaghi, A. Kanwat, W.C. Choi, J. Jang, A.C.S. Appl, Nano Mater. 2, 5655–5662 (2019)
M. Zhang, Z.Q. Tian, D.L. Zhu, H. He, S.W. Guo, Z.L. Chen, D.W. Pang, New J. Chem. 42, 9496–9500 (2018)
F. Boussoufi, M. Pousthomis, A. Kuntzmann, M. D’Amico, G. Patriarche, B. Dubertret, A.C.S. Appl, Nano Mater. 4, 7502–7512 (2021)
L. Xu, J. Li, T. Fang, Y. Zhao, S. Yuan, Y. Dong, J. Song, Nanoscale Adv. 1, 980–988 (2019)
H. Yang, W. Yin, W. Dong, L. Gao, C.H. Tan, W. Li, X. Zhang, J. Zhang, J. Mater. Chem. C 8, 14439–14445 (2020)
S. Sun, M. Lu, J. Guo, F. Zhang, P. Lu, Y. Fu, X. Bai, Z. Shi, Z. Wu, W.W. Yu, Y. Zhang, Chem. Eng. J. 433, 133556 (2022)
T. Wu, J. Li, Y. Zou, H. Xu, K. Wen, S. Wan, S. Bai, T. Song, J.A. McLeod, S. Duhm, F. Gao, B. Sun, Angew. Chem. Int. Ed. 59, 4099–4105 (2020)
J. Wang, Y. Xu, S. Zou, C. Pang, R. Cao, Z. Pan, C. Guo, S. Hu, J. Liu, Z. Xie, Z. Gong, J. Mater. Chem. C 9, 11324–11330 (2021)
M. Lu, J. Guo, P. Lu, L. Zhang, Y. Zhang, Q. Dai, Y. Hu, V.L. Colvin, W.W. Yu, J. Phys. Chem. C 123, 22787–22792 (2019)
D. Yoo, J.Y. Woo, Y. Kim, S.W. Kim, S.-H. Wei, S. Jeong, Y.-H. Kim, J. Phys. Chem. Lett. 11, 652–658 (2020)
J.B. Cho, S.B. Cho, I.K. Park, J. Alloys Comp. 891, 161996 (2022)
J. Pan, L. Quan, Y. Zhao, W. Peng, B. Murali, S.P. Sarmah, M. Yuan, L. Sinatra, N.M. Alyami, J. Liu, E. Yassitepe, Z. Yang, O. Voznyy, R. Comin, M.N. Hedhili, O.F. Mohammed, Z.H. Lu, D. Kim, E.H. Sargent, O.M. Bakr, Adv. Mater. 28, 8718 (2016)
M. Sebastian, J.A. Peters, C.C. Stoumpos, J. Im, S.S. Kostina, Z. Liu, M.G. Kanatzidis, A.J. Freeman, B.W. Wessels, Phys. Rev. B 92, 235210 (2015)
L.E. Brus, J. Chem. Phys. 80, 4403 (1984)
S. Thapa, G.C. Adhikari, H. Zhu, P. Zhu, J. All. Compd. 860, 158501 (2021)
P. Suksaengrat, N. Faibut, A. Chompoosor, J. Mater. Sci. Mater. Electron. 32, 1557–1569 (2021)
M. Hou, A. Yu, R. Lu, J. Raman Spectrosc. 48, 108–112 (2016)
B.A. Koscher, J.K. Swabeck, N.D. Bronstein, A.P. Alivisatos, J. Am. Chem. Soc. 139, 6566–6569 (2017)
S. Zhan, X.-B. Fan, J. Zhang, J. Yang, S.Y. Bang, S.D. Han, D.-W. Shin, S. Lee, H.W. Choi, X. Wang, B. Hou, L.G. Occhipinti, J.M. Kim, J. Mater. Chem. C 8, 16001–16009 (2020)
Acknowledgements
This study was supported by the Research program funded by the Seoultech (Seoul National University of Science & Technology)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, HY., Cho, SB., Hou, B. et al. Silver thiocyanate treatment-induced enhancement of photoluminescence efficiency of CsPbBr3 perovskite quantum dots. J. Korean Phys. Soc. 81, 150–157 (2022). https://doi.org/10.1007/s40042-022-00501-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40042-022-00501-2