Abstract
The present investigation aims to develop a solid acid-type heterogeneous catalyst for the hydrolysis of biomass using coconut shell, an inexpensive and easily available material, as yet unexplored in this field. Catalyst characterization by scanning electron micrography, X-ray diffraction, FTIR spectroscopy and nitrogen adsorption has been carried out. Pretreated sawdust from Acacia nilotica heartwood and microcrystalline cellulose were hydrolyzed in aqueous medium using the developed catalyst. Maximum sugar yields of 91% and 93% were obtained on hydrolysis of pretreated sawdust and microcrystalline cellulose, respectively. The principal hydrolysis product was glucose with a selectivity of 98%. These results highlight the potential of the developed catalyst for use in industrial biomass saccharification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, the principal constituent of lignocellulosic biomass, is a biopolymer of glucose units connected by β-1,4-glycosidic linkages. Lignocellulosic biomass saccharification involves splitting of β-1,4-glycosidic bonds by acidic species which leads to the production of mono/oligosaccharides like glucose. Much of these sugars are fermentable by yeast to produce ethanol. This “bioethanol” can be blended with gasoline and used as vehicular fuel. The use of bioethanol-blended gasoline can cut down on carbon emissions and decrease fossil-fuel dependency. Conventionally, the hydrolysis process used liquid acid catalysts like HCl and H2SO4. The effectiveness of dilute H2SO4 as hydrolysis catalyst can be improved by sonication as demonstrated in the case of hydrolysis of pretreated rice straw [1]. These catalysts can achieve high conversions of cellulose to oligosaccharides, but they have a serious drawback—product isolation and catalyst recycle are extremely difficult leading to poor energy efficiency and process economics. Acids can also cause corrosion of the reactor. Hence, the present trend is to search for more efficient catalysts of heterogeneous nature (solid catalysts) which can be easily separated from the reaction mixture and reused.
Solid catalysts developed and investigated for this purpose have been of various types that include zeolites [2, 3], metal oxides [4], supported metal catalysts [5], polymer-based acids [6], Nafion [7, 8], activated carbon-based catalysts [9, 10], heteropolyacids [11, 12], silica–carbon nanocomposites [13], magnetic solid acids [14] and polymeric solid acids [15].
The catalytic hydrolysis of lignocellulosics to fermentable sugars is the foremost step for the production of bioethanol. These catalysts are being researched to make the industrial production of bioethanol significantly more economically viable. Solid acids, due to their considerably large specific surface areas and strong acidic sites, are expected to achieve excellent results in hydrolyzing cellulose and hemicellulose. Cellulose-mimetic catalysts and sulfonated activated carbon catalysts have a strong affinity to the carbon chains in cellulose and have achieved very high yields of sugars [16], comparable to that of mineral acids. However, mass-transfer limitations between insoluble cellulose and hemicellulose and these heterogeneous solid catalysts pose a significant challenge toward making these systems industrially viable [16]. Recent researches have focussed strongly on development of reusable solid acid catalysts for hydrolysis of cellulosic [17] and hemicellulosic [18] biomass.
Most of the research on solid catalysts for biomass hydrolysis has focused on the hydrolysis of cellulose, hemicellulose and starch but not on the hydrolysis of actual lignocellulosic feedstocks except for some like Jiang et al. [19] and Namchot et al. [20]. The present work highlights a procedure for developing an activated carbon-based solid acid catalyst from coconut shell, which is a highly abundant natural carbon source, not yet explored in this field. The work also focuses on the characterization of the catalyst with regard to its structure, functional groups, acidic nature and the influence of these properties on hydrolysis performance. The catalytic performance has been evaluated through a series of hydrolysis experiments conducted on pretreated Acacia nilotica sawdust, another abundant and, importantly, non-edible biomass source.
Materials and Methods
Materials
ZnCl2 (> 95%), NaOH (97% pure), H2SO4 (98%), HCl (assay 36.5–38%) and NaCl (99% pure) were purchased from Merck Chemicals. Coconut shell was obtained from locally available sources, and sawdust from A. nilotica heartwood was purchased from local saw-mills. Standard glucose, xylose, galactose and mannose were also purchased from Merck Chemicals.
The feedstock for the hydrolysis reaction was A. nilotica sawdust. Acacia nilotica heartwood composition is given in Table 1.
Alkaline pretreatment of the sawdust (with sodium hydroxide) was carried out according to a method published previously [21]. The pretreatment process resulted in 60% reduction of original lignin content.
Experimental Procedure
Coconut shell was used as the carbon source for the synthesis of the catalyst. The composition of coconut shell by mass was determined according to published methods [22], and the results are given in Table 2.
The sizeable lignin content is very helpful, since the aromatic lignin components render a significant aromatic nature to the produced activated carbon which should theoretically assist in incorporating sulfonic acid groups to the activated carbon.
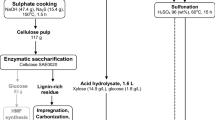
Carbonization of Coconut Shell
The coconut shells were ground to an average particle size of 100 mesh in a laboratory-scale ball mill. The shells were then chemically activated by a 24-h impregnation with 10% ZnCl2 solution. After subsequent filtration, the shells were dried at room temperature. They were then carbonized at 723 K in a muffle furnace for 1 h under a N2 flow rate of 40 ml min−1. The resulting mass was washed repeatedly with deionized water to remove chloride traces. After washing, the mass was air-dried overnight and then ground to powder size.
Sulfonation
The activated carbon powder was boiled in 98% sulfuric acid in a 500-ml borosilicate glass beaker at 403 K for 16 h with continuous magnetic stirring. The solid-to-liquid ratio was maintained at 1: 50. The reaction mass was cooled to room temperature and then diluted by adding deionized water. A black powdery mass precipitated which was filtered and washed repeatedly with deionized water to remove sulfate ion traces. Finally, the black mass was dried in a tray drier at 378 K for 5 h. This mass was the sulfonated activated carbon catalyst. Its average particle size was 100 mesh.
Catalyst Characterization
The crystalline structure and graphene content of the catalyst were analyzed by X-ray diffractometry (XRD) at a scan rate of 2°/min within a range of 2θ = 5°—90°. The functional groups in the developed catalyst were determined by Fourier transform infrared (FTIR) spectroscopy from 4000 to 500 cm−1. The specific surface areas of the activated carbon before sulfonation and the produced catalyst after sulfonation were determined by applying the BET isotherm to data from a N2 adsorption study carried out in a BET apparatus (Quantachrome Nova 1000 E, USA). Pore size and pore-size distribution analysis were carried out by mercury intrusion method in a Quantachrome Poremaster 60 instrument using a maximum pressure of 49,978 psi. The internal structure of the catalyst was studied by scanning electron micrography. The density (in mmol g−1 of catalyst) of sulfonic acid groups and total acid density were determined by titration methods [23].
Estimation of Catalyst Performance in the Hydrolysis of Acacia nilotica Heartwood
The developed catalyst was tested for the hydrolysis of pretreated A. nilotica sawdust. Required amounts of biomass and catalyst (as specified by the experimental design described in the following section) were taken in a 500-ml conical flask, with the addition of 200 ml deionized water to maintain an aqueous medium. The particle sizes of both the biomass and the catalyst were kept at 100 mesh to minimize the mass-transfer resistance and expose as much of the active sites of the catalyst to the biomass as possible. The flask was placed in an autoclave, and the reaction was carried out at temperatures specified by the design within a range of 363–413 K. The pH of the reaction mixture containing the catalyst was 1. A conical flask containing only the pretreated sawdust without the catalyst was also placed in the autoclave as a control sample. After the reaction, samples were collected and analyzed for total reducing sugars (TRS) by the DNS method [24]. In this method, 3, 4-dinitrosalicylic acid was mixed with required quantities of NaOH and potassium sodium tartrate to prepare the DNS reagent. Then, the hydrolysate samples were mixed with DNS reagent in the presence of 0.05 M acetate buffer (pH 4.8) in separate test tubes. The absorbance of the mixtures was then measured at 540 nm in a UV–Vis spectrophotometer (Hitachi U 4100) with 1-cm optical path. Reducing sugars reduce 3, 4-dinitrosalicylic acid to 3-amino-5-nitrosalicylic acid which results in a change in absorbance at 540 nm. Identification of the reducing sugars was carried out by HPLC using a Grace-Davison Prevail Carbohydrate ES 5 μ column and 75:25 (% v/v) acetonitrile–water as the mobile phase at a flow rate of 1.4 ml/minute, with a refractive index detector. Catalyst reusability studies were carried out by first filtering and weighing the residue (catalyst + biomass) remaining in the conical flasks. The residues were then transferred to fresh conical flasks and subjected to hydrolysis under the same experimental conditions. The resulting samples were analyzed by the DNS method. Microcrystalline cellulose (pure cellulose without lignin) hydrolysis was also carried out using the sulfonated solid catalyst for the determination of the resistive effect of lignin.
Experimental Design for the Hydrolysis Runs
A central composite design scheme was used for the experimental design. The aim was to determine the influence of two factors: reaction temperature and catalyst/biomass ratio on the response, i.e., total sugar yield. From a previous set of experiments with reaction times varying between 30 min and 2 h within the same temperature range, it was observed that reaction time of 1 h was the optimum time for hydrolysis reaction because, at reaction durations of 1.5 h and 2 h, the sugar yield decreased, which can be attributed to the degradation of sugars to 5-HMF [25]. Accordingly, time was not selected as a factor for determination of the effects of process parameters on sugar yield and the reactions were carried out for 1 h under varying temperatures and catalyst/biomass ratio as specified by the design. The catalyst/biomass ratio was varied within a range of 0.79:1 to 2.21:1.
Results and Discussion
Catalyst Characterization
The SO3H density in the catalyst was estimated to be 1.27 mmol g−1, and the total acidity was 10 mmol g−1 which indicates significant acidity due to the presence of other acidic groups like carboxylic acids.
The specific surface area of the developed catalyst was 10.162 m2 g−1. This value is higher than previous values for sulfonated carbon catalysts reported in the literature [9, 20]. The specific surface area of the activated carbon before sulfonation was 744.8 m2 g−1. The reduction in surface area post-sulfonation is in accordance with previous works [9, 20]. This reduction happens because strong mineral acids like concentrated sulfuric acid degrade the porous activated carbon structure to some extent.
The XRD pattern of the developed catalyst is shown in Fig. 1.
The peaks (broad, weak) between 20° and 30° and between 40°—50° correspond to an amorphous structure composed of randomly oriented aromatic graphene-like sheets [9]. The graphene sheet content in the developed catalyst was estimated according to a published method [26] and was found to be 39%.
The FTIR spectrum of the sulfonated activated carbon catalyst is shown in Fig. 2.
The peaks corresponding to 1027 cm−1 and 889 cm−1 indicate the presence of the SO3H group. The presence of large amounts of sulfonic acid groups is highly beneficial as these groups attack the intermolecular hydrogen bonds and the β-1,4 glycosidic oxygen between glucose units in the cellulose chain resulting in the formation of free glucose [15]. The peaks at 1703 and 1383 cm−1 indicate C=O stretching and carboxylic OH bending vibrations, respectively, confirming the presence of the COOH group. The peak at 3530 cm−1 indicates phenolic OH stretching vibration. Phenolic OH and COOH groups play an important role in the hydrolysis reaction since they can adsorb cellulose and hemicellulose chains in solution on to the catalyst surface via hydrogen bonding between these groups and the glycosidic oxygen atom [16, 20]. This facilitates greater availability of hydrolyzable polysaccharides in close contact with the SO3H groups on the surface, thereby increasing catalytic activity. The peak at 812 cm−1can be attributed to aromatic C–H bending and peaks at 1515 and 1643 cm−1 point to aromatic C=C bending. The catalyst can thus be said to possess a significant aromatic nature. The graphene structure of the catalyst also facilitates effective adsorption of cellulose and hemicellulose on to the catalyst surface.
Figure 3 shows the scanning electron micrograph of the catalyst.
The porosity of the catalyst is quite high, with the average pore diameter ranging from 10 to 20 μ. This is also confirmed by the pore-size distribution analysis in Fig. 4.
Table 3 gives an overall summary of the catalyst physical properties.
Performance Evaluation of Catalyst
The results of the hydrolysis experiments carried out for 1 h following the central composite design are given in Table 4.
From HPLC analysis of the hydrolysates, it was observed that there was a strong peak at around 6.8 min corresponding to glucose and another very small peak at around 5.1 min corresponding to xylose. Calculation of percentages gave around 98% glucose on average as the principal component of the TRS in all the hydrolysates. Xylose percentage in the TRS was significantly small in comparison. This can be attributed to two possible reasons:
- (1)
The percentage of cellulose in the original biomass (40%) is considerably more than that of hemicellulose (30%).
- (2)
The highly acidic nature of the catalyst which in the aqueous medium resulted in a pH of 1.
The higher percentage of cellulose could have resulted in the production of significantly more glucose than xylose, especially since the protonic concentration was high enough to attack most of the glycosidic linkages in cellulose. The highly acidic nature of the catalyst (pH 1 in aqueous medium) meant that the availability of protons was very high in the reaction medium, which translates into a greater probability of attack of the glycosidic linkages by the protons. The effective pretreatment process adopted which resulted in the removal of a significant amount of lignin encapsulation (60%) also could have contributed to this high glucose yield by exposing much of the cellulose to attack by the protons coming from the numerous acidic sites of the catalyst.
As evident from Table 4, a maximum TRS yield of 91% was obtained at 140 °C (413 K) for a catalyst/biomass ratio of 2:1 in 1 h. Under the same experimental conditions, hydrolysis of microcrystalline cellulose resulted in a TRS yield of 93% which constituted mainly of glucose (99%) as expected. This indicates that the residual lignin in the sawdust after pretreatment played a minor role in creating a barrier for cellulose and hemicellulose hydrolysis, pointing to the effectiveness of the pretreatment process.
Catalyst reusability experiments were carried out for 1 h because it was found to be the optimum reaction time. The TRS yields for these runs are given in Table 5.
As evident from Table 4, TRS yield is significantly higher than those reported in previous studies [9, 13, 20, 27]. The sugar yields for the control batch (catalyst not present) were negligibly low under the same experimental conditions. The developed catalyst can thus be reported to have very good catalytic properties. Leaching of the SO3H groups into the aqueous medium is a limiting factor for catalytic activity which led to reduced sugar yields on reuse. High temperatures favor leaching which is indicated by larger reduction in TRS yield at higher temperatures. In comparison with the catalyst developed by Kitano et al. [10], the present catalyst has a lower specific surface area. The lower area will hinder the adsorption of large hydrophobic molecules; hence, for hydrophobic acid-catalyzed reactions, e.g., esterification, the developed catalyst will not be very effective. But smaller hydrophilic molecules (e.g., cellulose and hemicellulose) are easily adsorbed and stabilized onto the catalyst surface as explained in Sect. 3.1, and can thus be readily attacked by H+ ions from neighboring sulfonic acid groups. This makes the developed catalyst extremely effective for hydrophilic reactions like biomass hydrolysis.
Effects of Process Parameters on Sugar Yield
The dependence of the yield of reducing sugars on temperature and catalyst/biomass ratio has been determined by response surface analysis. The sugar yield was found to be linearly dependent on temperature within the experimental temperature range. The dependence on catalyst/biomass ratio was also linear. The dependence has been expressed in terms of the following model:
where t = reaction temperature (°C) and c/b = catalyst/biomass ratio.
The ANOVA for the model is shown in Table 6. The model was statistically significant (p value < 0.0001 at 95% confidence interval). It is evident that the effect of temperature on the sugar yield was slightly more significant (p value < 0.0001) than that of catalyst/biomass ratio (p value 0.0002).
The correlation between predicted and experimental values of sugar yield is shown in Fig. 5.
The standard deviation on replicating the hydrolysis experiments thrice was 2.6. The coefficient of determination (R2) for the model was 0.9634, and adjusted R2 was 0.956. This indicates that changes in the response (sugar yield) can be quite accurately predicted from changes in the factors tested (reaction temperature and catalyst/biomass ratio). Hence, the model can be effectively used for estimating sugar yield under the present experimental conditions. The response surface for sugar yield is shown in Fig. 6.
Conclusions
A solid acid catalyst for lignocellulosic biomass hydrolysis has been developed from a cheap and easily available carbon source, i.e., coconut shell. The catalyst has been used for hydrolysis of actual pretreated biomass (viz. A. nilotica heartwood) and not pure molecules like cellulose, hemicellulose and starch. High TRS yield (91%) and glucose selectivity (> 98%) have been obtained at moderate temperatures (413 K) and reaction time (1 h). The developed catalyst also has good reusability, resulting in slightly lower TRS yields on reuse. The findings support the applicability of the catalyst in batch biomass hydrolysis units in the industry. Further research is needed for enhancing the catalytic activity (similar yields with lesser catalyst) and designing continuous catalytic reactors for large-scale hydrolysis of biomass using this catalyst.
Abbreviations
- t:
-
Reaction temperature (°C)
- c/b:
-
Catalyst/biomass ratio
References
S. Bhattacharya, S. Dutta, S. Datta, C. Bhattacharjee, J. Inst. Eng. India Ser. E 93(1), 37 (2012)
Z. Zhang, Z.K. Zhao, Carbohydr. Res. 344, 2069 (2009)
H. Cai, C. Li, A. Wang, G. Xu, T. Zhang, Appl. Catal. B 123–124, 333 (2012)
A. Takagaki, C. Tagusagawa, K. Domen, Chem. Commun. 42, 5363 (2008)
H. Kobayashi, T. Komanoya, K. Hara, A. Fukuoka, ChemSusChem 3, 440 (2010)
R. Rinaldi, R. Palkovits, F. Schüth, Agnew. Chem. Int. Ed. 47, 8047 (2008)
S. Kim, A. Dwiatmoko, J.W. Choi, Y. Suh, D.J. Suh, M. Oh, Bioresour. Technol. 101, 8273 (2010)
J. Hegner, K.C. Pereira, B. DeBoef, L.B. Lucht, Tetrahedron Lett. 51, 2356 (2010)
S. Saganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi, M. Hara, J. Am. Chem. Soc. 130, 12787 (2008)
M. Kitano, K. Arai, A. Kodama, T. Kousaka, K. Nakajima, S. Hayashi, M. Hara, Catal. Lett. 131, 242 (2009)
K. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya, A. Satsuma, Green Chem. 11, 1627 (2009)
J. Tian, J. Wang, S. Zhao, C. Jiang, X. Jhang, X. Wang, Cellulose 17, 587 (2010)
S. Van de Vyver, L. Peng, J. Geboers, H. Schepers, F. de Clippel, C.J. Gommes, B. Goderis, P.A. Jacobs, B.F. Sels, Green Chem. 12, 1560 (2010)
D. Lai, L. Deng, J. Li, B. Liao, Q. Guo, Y. Fu, ChemSusChem 4, 55 (2011)
A. Vu, R. Wickramasinghe, X. Qian, Ind. Eng. Chem. Res. 57, 4514 (2018)
Y.B. Huang, Y. Fu, Green Chem. 15, 1095 (2013)
M. Goswami, S. Meena, S. Navatha, K.N. Prasanna Rani, A. Pandey, R.K. Sukumaran, R.B.N. Prasad, B.L.A. Prabhavathi Devi, Biores. Technol. 188, 99 (2015)
X. Li, F. Shu, C. He, S. Liu, N. Leksawasdi, Q. Wang, W. Qi, MdA Alam, Z. Yuan, Y. Gao, RSC Adv. 8, 10922 (2018)
Y. Jiang, X. Li, X. Wang, L. Meng, H. Wang, G. Peng, X. Wang, X. Mu, Green Chem. 14, 2162 (2012)
W. Namchot, N. Panyacharay, W. Jonglertjunya, C. Sakdaronnarong, Fuel 116, 608 (2014)
A. Mallick, S.N. Ash, D.K. Mahapatra, J. Inst. Eng. India Ser. E 97(1), 39 (2016)
R. Child, S. Ramanathan, J. Am. Chem. Soc. 60(6), 1506 (1938)
D. Lee, Molecules 18, 8168 (2013)
G.I. Miller, Anal. Chem. 31(3), 426 (1959)
W. Qi, S.P. Zhang, Q.L. Xu, Z.W. Ren, Y.J. Yan, Chin. J. Process Eng. 8(6), 1132 (2008)
Y.J. Liou, W.J. Huang, J. Mater. Sci. Technol. 29(5), 406 (2013)
P.D. Cara, M. Pagliaro, A. Bmekawy, D.R. Brown, P. Verschuren, N.R. Shiju, G. Rothenberg, Catal. Sci. Technol 3, 2057 (2013)
Acknowledgements
The authors express their heartfelt thanks to the laboratory assistants of the Department of Chemical Engineering, University of Calcutta, India, for their cooperation in carrying out the work. The authors also gratefully thank the SEM section of the Department of Metallurgical Engineering and Material Science, Jadavpur University, India, for their cooperation. The authors would also like to thank the Department of Polymer Science and Technology, University of Calcutta, for providing the facilities for XRD and FTIR. Last, but not the least, the authors would like to thank the authorities of the University of Calcutta, for providing the financial support for this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mallick, A., Mukhopadhyay, M. & Ash, S. Synthesis, Characterization and Performance Evaluation of a Solid Acid Catalyst Prepared from Coconut Shell for Hydrolyzing Pretreated Acacia nilotica Heartwood. J. Inst. Eng. India Ser. E 101, 69–76 (2020). https://doi.org/10.1007/s40034-019-00153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40034-019-00153-1