Abstract
In light of the recent evidences that pollinators have a significant implication for maintenance of pollen-mediated gene flow, the present study has been undertaken to investigate flowering phenology and visiting insect diversity and density in 9 different populations of Valeriana jatamansi. The performance of the V. jatamansi breeding system was also investigated at an experimental site. Field experiments were performed on control and visiting insect excluded plants (a total of 90 plants). Across populations, a total of 76 visiting insects belonging to 20 species were recorded. Comparison of visiting insects excluded plants and natural plants revealed 63.49% seed setting produced by natural-pollination, whereas only 37.51% of seeds were produced by self-pollination. Significantly higher seed viability (t = 4.284; p < 0.02) was recorded in seeds from control plants as compared to visiting insect excluded plants. Similarly, higher seed germination (38%) and seed weight were recorded in control plants as compared to visiting insect excluded plants. The total insect number (r = − 0.707) and species diversity (− 0.897) exhibited significantly (p < 0.05) negative relationship with altitude. A significant negative correlation existed between plant density and altitude (r = − 0.772; p < 0.05). The study provides further evidence that pollinators (and their diversity) are critical for sustaining genetic diversity and consequent adaptive capability in V. jatamansi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seeds enable plants to have an independent dispersal phase and to potentially increase the genetic diversity of a species, thus promoting adaptation to changing environments and the long-term sustainability of a plant species [1]. Seed and seedling life stages represent a reservoir of genetic variability in natural populations [2]. Pollination is the first step in the process of seed formation, and pollination failure results in the decline of genetic diversity and subsequently affects population status and species demography [3]. Recent studies have exhibited that the diversity of insect pollinators influences the reproduction and diversity of wild flowering plants [4]. In this context, pollinators have a substantial effect on the maintenance of pollen-mediated gene flow and thereby influence the genetic structure of plant populations. However, the diversity and density of the insect pollinators found to vary with altitude and habitats. Native pollinators are therefore vital for the maintenance of diversity within an ecosystem. Despite the importance of understanding plant–pollinator relationships, this knowledge is often lacking at ecosystem level. Further, landscapes with wide altitudinal ranges generally exhibit decreasing pollinator diversity, abundance and efficiency with increasing altitude [5]. Limited diversity and activity of insect pollinators may in turn affect plant reproductive success [6], but empirical evidence for this phenomenon in the Himalayan plants is lacking and Valeriana jatamansi is no exception.
Valeriana jatamansi Jones (Family—Valerianaceae), commonly known as Indian Valerian is a dioecious perennial herb with an approximate height of 15–45 cm, is known for its diverse medicinal uses [7,8,9]. This native Himalayan plant is distributed from Afghanistan to southwest China, India, Nepal, and Bhutan. The species is commonly found within an altitudinal range of 1000–3000 m asl [8, 9] and reproduces sexually (by seeds) and asexually (by rhizomes). Pollination in the species is reported to be facilitated by insects belonging to order Hymenoptera and Diptera [10]. While considering the relationship of altitude on pollinator density and diversity, few studies advocated that for identification and conservation of a population with higher diversity and density, and its role in maintaining genetic diversity, it is important to understand the effect of altitude on the pollinators assemblages. Such studies can support the species of local and national conservation value. The potentially far reaching effect of the altitude on pollinator diversity and reproductive success of a species is difficult to predict, especially when empirical data are limited. The present study was therefore designed to explore (1) the impacts of altitude on species diversity and the density of pollinating insects and (2) the effects of pollinator density and diversity on V. jatamansi reproduction by comparing naturally growing plants with plants lacking visiting insects.
Material and Methods
Target Species and Study Site
Uttarakhand is a state in the western Himalayan biogeographic province of India and broadly encompasses the study area. The study examined 9 populations [Katarmal, Daulaghat, Jyoli, Majkhali, Kalika, Bagari, Kausani, Barsoli, and Doonagiri] of V. jatamansi along an altitude range of 1150–2100 m asl in the Upper Kosi watershed of Almora, Uttarakhand during March and April 2011. Details of the population characteristics and geo-coordinates are provided in Table 1. Several parameters like flowering phenology and diversity of insect visitors (i.e., pollinators) to V. jatamansi were analyzed. The breeding system, seed viability and seed germination of V. jatamansi were evaluated at experimental sites (i.e., the Nature Interpretation and Learning Centre (NILC) and laboratories concerning Biodiversity Conservation and Management (BCM) at the G. B. Pant National Institute of Himalayan Environment and Sustainable Development (GBPNIHESD), Kosi-Katarmal, Almora, Uttarakhand.
Evaluation of Plant Variables
Plant variables, including the numbers of plants, inflorescences per plant and open flowers per plant were recorded in all populations. Survival of a plant in a new environment could only be interpreted by its fitness or the ability of forming seeds, which depend on flowering phenology, pollinators and its success [11]. No specific permissions were required to conduct this study at these locations, and this study did not examine endangered or protected species. The density of the target species was measured using a random quadrat approach [i.e., six random quadrats (1 × 1 m) for each population]. In order to determine the role of the species as available foraging resources, in each quadrat, the number of individuals, the number of inflorescences (i.e., branches with floral units)/individual and open flowers per branch with floral unit were counted for four randomly selected mature V. jatamansi plants.
Visiting Insect Diversity and Density
The protocol used in this study was defined under the global pollination project [12] with some modifications. One field plot (size 25 × 25 m) was identified per site, and data on visiting insect diversity was collected. Insect observations were recorded from 10:00 a.m. to 12:00 p.m. and from 2:00 to 4:00 p.m. on two different days (separated by 1 week). Each population was visited twice a day. A total of 8 transect walks (in 2 different days total 16 transact walk 5 min on each walk total 80 min per site) were performed to record visiting insect density and frequency in each population using a scan sampling method [12]. A total of 25 inflorescences with open floral units per experimental population were selected and used to screen visiting insect density. Scanning 25 inflorescences per round for target species provided reliable estimates of visiting insect density. As V. jatamansi produces large numbers of small flowers, the inflorescence was used as the flowering unit for visiting insect density assessment. Free-living flying insects were collected using an aerial net and were transferred into a jar where they were euthanized with ethyl acetate. Small insects were collected by hand using fine forceps. Visiting insect specimens were maintained for further identification and analysis.
Breeding System and Seed Viability
To understand the impact of insect visitors on V. jatamansi reproduction and subsequent seed production, experiments were conducted on naturally pollinated (control) plants and plants that lacked visiting insects. Two experimental plots (6 × 6 m each) with six randomly selected sub-plots (1 × 1 m each) were established in the Suryakunj Nature Interpretation and Learning Centre (NILC) of GBPNIHESD. Each sub-plot contained 15 mature plants. The female plants with pistillate flowers without an androecium and four white (large) or tinged with pink (small) petals were selected for detailed study [7, 13]. In both the experimental plots, three of the sub-plots were covered with insect netting to prevent insect movement; these sub-plots contained the pollinator-excluded plants. The remaining three sub-plots (without insect netting) served as a control. All plants (both the visiting insects excluded and control) were maintained until seeds were fully developed. Several phenological parameters, such as the number of inflorescences, open flowers, closed flowers (i.e., flowers not yet opened) and total flowers, were recorded weekly after the first flower was formed. At the end of the experiment, seeds were collected from each sub-plot, and their weights were measured using an electronic balance (Afcost, Japan).
Seeds collected from the control and visiting insects excluded sub-plots were dried at room temperature (22 ± 1 °C). Viability was tested for 25 seeds (in 4 replicates) from both types of sub-plot using the 2, 3, 5 triphenyl tetrazolium chloride (TTC) method with minor modifications [14]. Seeds were incubated in a 0.1% TTC solution (pH 6.5) for 24 h at 22 ± 1 °C (room temperature), and the stain intensity/pattern was examined under a microscope. Seeds with completely stained embryos were considered to be viable. Similarly, 300 seeds from both the control and visiting insect excluded sub-plots were randomly selected and incubated for 45 days in 6 petri dishes of 50 seeds each at room temperature (relative humidity 65 ± 5% and light intensity 42 µEm−2 s−1; inside culture tubes). Seed germination percentages were recorded for both control and pollinator-excluded plants.
Statistical Analysis
Analysis of phenological parameters, including the numbers of plants, inflorescences per plant, and open flowers per plant, was conducted using Microsoft excel. The relationships between various parameters were statistically examined using SPSS software version 16. Significant differences in some parameters (i.e., seed setting, seed viability and seed germination) between control and visiting insect excluded sub-plots were assessed using t tests. Significant differences in plant variables were evaluated using Duncan’s multiple range test (DMRT, p < 0.05). Relationships between different parameters were determined by calculating Pearson correlation coefficients (r).
Results and Discussion
Average plant density of V. jatamansi was ranged from 12.17 ± 4.66 individuals per meter square in Bagari to 40.33 ± 4.98 individuals per meter square in the Katarmal population (Table 2). Significantly (p < 0.05) lower number of inflorescences (0.63 ± 0.20 inflorescences per plant) was observed in Barsoli than in the Kausani population (4.04 ± 0.50). Similarly, Daulaghat population had significantly more (63.83 ± 6.27; p < 0.05) open flowers per plant than the Katarmal population (7.92 ± 2.71). Over a range of altitudes, the highest plant density was recorded at 1200 m asl. Such successful adaptation to environmental changes fundamentally depends on amount of genetic diversity of a species. V. jatamansi has a relatively high level of genetic diversity and therefore has greater environmental adaptability. This adaptability is also demonstrated by the distribution of species, which extends from a warm temperate (< 1200 m asl) to an extremely cold temperate (> 2400 m asl) climate in the Himalaya. The high genetic diversity in V. jatamansi can be attributed to its breeding system, which includes both sexual and asexual modes of reproduction.
Among the 9 studied populations, a total of 76 insects from 20 different species [representing 13 diptera (65%), 2 hymenoptera (10%), 3 lepidoptera (15%) and 2 species belonging to other minor groups (10%)] were recorded on V. jatamansi flowers (Table 3; Supplementary Information Fig. S1). The maximum number of insects (13 insects from 7 species) was recorded in the Majkhali population, followed by the Katarmal population (11 insects from 8 species). The maximum number of insect species was recorded in the Katarmal population and the minimum number of insect species was recorded in Barsoli. The most common insect visitors of V. jatamansi were hoverflies and Bombyliidae species 1 (Family Diptera). Previous studies have reported higher genetic diversity of populations in grasslands may be attributed to greater sunlight exposure, which attracts more insects. Similar habitat effects on genetic diversity have been reported elsewhere [15]. Generally, out-crossing plant species have higher genetic variation within populations as compared to selfing species or species with mixed mating systems [16]. The high within-population genetic diversity [8, 17] indicates the out-crossing nature of the species. The high genetic differentiation and high gene flow (Nm 1.414) may have contributed to reduced genetic drift. The estimates of gene flow (Nm < 1) between populations suggest that it is insufficient to counter the effects of random genetic drift [18]. V. jatamansi flowers have remarkable and unique features (e.g., flower numbers, patterns of arrangement, inflorescences, etc.) making this species one of the most complex flowers in all of Valerinaceae. The flowers are tubular, hermaphrodite, and generally white or pink in color. Pollen grains are generally oblate spheroidal and have a colpus-shaped aperture with long, rounded acute ends. Although these features facilitate visits from small-bodied, long-tongued bees (e.g., Bombyliidae species 1), they represent a barrier for some larger visitors, such as bumble bees (not observed in this study). However, the shade-growing nature of this species may also limit the movement of diurnal species. In general, the present findings agree with Fenster et al. [19], who hypothesized that floral complexity may reflect the selection of narrower functional group of pollinators.
Generally, in case of animal-pollinated plants, pollinator limitation can commonly cause failure either through lack of pollinator activity driven by environmental conditions, such as low temperature [20], or species-specific pollination dependent plants. Pollen transfer of both wind- and animal-dispersed pollen typically exhibits a leptokurtic pattern, with successful plant pollination occurring at more frequently at closer distances within relatively continuous flower populations. Actual pollination movement by bees and flies between plants is generally at short distances (< 20 m) [21]. Reports also indicate that small populations in habitats with rich floral diversity can be buffered against pollinator loss [22]. Alternatively, impacts of pollinator loss will only be apparent once a minimum threshold is reached below, which pollination levels show a marked decline. To pollinate the opposite sex, V. jatamansi has pierced stamens to successfully reproduce sexually. Although wind plays an important role in V. jatamansi pollination, it is not an efficient method of pollen dispersal because high numbers of pollen grains are lost during the process [23]. A pollinator’s effectiveness is determined by its frequency and ability to impact the fruit and seed sets [24]. Members of a pollinator functional group can vary in their pollinator effectiveness and morphology [25]. In this context, this study suggests that hoverflies are important to V. jatamansi reproductive success and are likely this species’ most reliable pollinator. The reproduction and pollination mechanisms employed by V. jatamansi are complex and exhibit significant adaptations to their environment. There were only a few species of pollinators identified for V. jatamansi, potentially because various other plants, such as Berberis asiatica (Berbediaceae), Pyrus pashia (Rosaceae), Pyracantha crenulata (Rosaceae) and Zanthoxyllum armatum (Rutaceae), were simultaneously blooming. V. jatamansi and several of these species share visits from hoverflies and other pollinating species. However, visitation rate alone may not be a good indicator of reproductive success [26]. With respect to pollinator efficiency, solitary bees carried and deposited more pollen after a single visit than did hoverflies. Similarly, other studies report that the duration of visits from solitary bees is longer and therefore increases pollen deposition on stigmas [27].
A greater number of inflorescences per plant were recorded in visiting insect-excluded sub-plots (4.71 ± 0.76–6.51 ± 2.19) as compared to control sub-plots (4.11 ± 0.16–6.47 ± 0.53; Fig. S1. 1 A, B, C, D, E). Similarly, there were more open flowers (7.24 ± 3.21–96.73 ± 15.50) and closed flowers (63.57 ± 16.72–163.48 ± 42.82) in visiting insect excluded plants than in control plants (open flowers: 1.64 ± 0.87–77.93 ± 12.34; closed flowers: 49.47 ± 10.60–149.13 ± 19.46). However, control sub-plots produced a greater total number of flowers as compared to visiting insect excluded sub-plots. On all observation days, the percent of flowering plants in control sub-plots (1.00 ± 0.54–44.52 ± 4.05) was always greater than that of visiting insect excluded sub-plots (2.51 ± 1.00–44.29 ± 19.04). Although variations were recorded in the naturally pollinated and pollinator excluded plant in various attributes. However, fell down of the flowers and lower values of studied attributes from naturally pollinated plants due to rainfall during the period of experimentation is one of the main cause of variations. Moreover, in case of insect excluded plants, the flowers were preserved by insect net. A plant’s breeding system can influence the effects of pollinators, such that pollinator failure is less severe in self-incompatible and autogamous species [28]. The balance between sexual reproduction via seeds and vegetative propagation via rhizomes appears to have greatly influenced the spatial genetic structure of V. jatamansi. Flexibility in pollination system enables plants to be able to set seed when either pollinator group is scare or absent [29]. The present results are in agreement with an earlier study, which indicated that this species reproduces both sexually and asexually [10]. This species is reported to have stylar movements that promote self-pollination in adverse conditions [10].
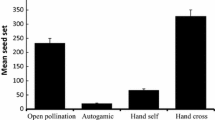
It is interesting to note that 63.49% of seeds (1.377 ± 0.42 g) were produced by natural pollination (i.e., in naturally growing plants) and only 37.51% (0.792 ± 0.26 g) seeds were produced by self-pollination (i.e., in pollinator-excluded plants; Fig. 1a). However, this difference was not significant between control and visiting insect excluded sub-plots (t = 1.233; p < 0.343). A total of 64% of seeds were viable in control plants as compared to 45% viability in visiting insect excluded sub-plots (Fig. 1b; Supplementary Information Fig. S2). Similarly, seeds from control sub-plots exhibited greater seed germination (38%) as compared to those of visiting insect excluded sub-plots (18.6%; Fig. 1b). Results of the present study clearly reveal that cross pollination (in naturally growing plants) promoted reproduction. These findings are substantiated by the fact that lower altitude populations (from 1201 to 1500 m) had higher visiting insect density and diversity and also had comparatively greater genetic diversity which finally contributed to the pollination success at lower altitude populations. As observed in the garden experiment, cross pollination also facilitated better seed setting, viability and germination. Conversely, reproductive assurance and species survival in adverse conditions in high altitude areas (i.e., thermal stress, high wind speed, etc.) are promoted by stylar movement that facilitates selfing and, as a result, reduces V. jatamansi genetic diversity [10].
Across populations, insect species density and diversity exhibited significantly (p < 0.05) negative relationships with altitude (r = − 0.707 and r = − 0.897, respectively; Table 4; Fig. 2). Similarly, a significantly negative correlation existed between plant density and altitude (r = − 0.772; p < 0.05). V. jatamansi density across populations was positively related to total visiting insect number (r = 0.779) and insect species diversity (r = 0.860). Observations of flowering phenology at the experimental sites revealed that the number of inflorescences in both visiting insect excluded sub-plots and control sub-plots increased over time [(weekly interval) (R2 = 0.66, visiting insect excluded; R2 = 0.75, control)]. Similarly, the number of open flowers [R2 = 0.88 (visiting insect excluded); R2 = 0.77 (control)] and the flowering percent [R2 = 0.90 (visiting insect excluded); R2 = 0.96 (control)] increased over time (Supplementary Information Fig. S3). The present study confirmed these trends by reporting reduced insect density and diversity at greater altitudes. At low altitude populations, this species invariably exhibited profuse flowering that attracted large numbers of visiting insects, which facilitate cross pollination and gene flow among individuals. On high altitude mountain ridges, there were lower numbers of visiting insects, which may demarcate plant habitats, limit gene flow among plant populations, and reduce colonization at new sites [30]. Other studies generally suggest that there is a decline in the abundance, efficiency, and diversity of visiting insects as altitude increases [5]. Similarly, studies have shown that the diversity of insect pollinator influences the reproduction and diversity of wild flowering plants [4].
Conclusions
The study concludes that cross pollination contributes considerably more to breeding system of this species and therefore, the diversity and density of pollinators has direct bearing on defining level of genetic diversity. The study further validates the earlier report [9, 17], which showed decline in V. jatamansi genetic diversity with increasing altitude is a consequence of lower visiting insect diversity and density with increasing altitude. However, the role of other factors like low plant density, smaller plant population size, and recent fragmentation at high altitudinal area cannot be ignored. This study can aid the design for the effective conservation of V. jatamansi for its sustainable utilization. Further, the decreasing diversity of the pollinators with the increasing altitude suggests that the plants should cultivate more on the lower altitude for obtaining maximum productivity of the species.
References
Ackerman JD, Sabat A, Zimmerman JK (1996) Seedling establishment in an epiphytic orchid: an experimental study of seed limitation. Oecologia 106:192–198
Alvarez-Buylla ER, Chaos A, Pnero D, Garay A (1996) Demographic genetics of a pioneer tropical tree species: patch dynamics, seed dispersal and seed banks. Evolution 50:1155–1166
Bond WJ (1994) Do mutualism matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philos Trans R Soc B Biol Sci 344:83–90
Biesmeijer JC et al (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Totland O (1993) Pollination in alpine Norway: flowering phenology, insect visitors, and visitation rates in two plant communities. Can J Bot 71:1072–1079
Hermansen TD, Minchinton TE, Ayre DJ (2017) Habitat fragmentation leads to reduced pollinator visitation, fruit production and recruitment in urban mangrove forests. Oecologia 185:221–231
Prakash V (1999) Indian Valerianaceae a monograph on medicinally important family, vol 1–2. Scientific Publishers, Jodhpur
Jugran AK, Bhatt ID, Rawal RS, Nandi SK, Pande V (2013) Patterns of morphological and genetic diversity of Valeriana jatamansi Jones in different habitats and altitudinal range of West Himalaya, India. Flora 208:13–21
Jugran A, Rawat S, Dauthal P, Mondal S, Bhatt ID, Rawal RS (2013) Association of ISSR markers with some biochemical traits of Valeriana jatamansi Jones. Ind Crops Prod 44:671–676
Khajuria A, Verma S, Sharma P (2011) Stylar movement in Valeriana wallichii DC.—a contrivance for reproductive assurance and species survival. Curr Sci 100:1143–1144
Primack RB (1987) Relationships among flowers, fruits and seeds. Ann Rev Ecol Syst 18:409–430
FAO (2011) Pollination services for sustainable agriculture: field manuals. Protocol to detect and assess pollination deficits in crops: a hand book for its use
Nawchoo AI, Rather MA, Ganie HA, Jan RT (2012) Need for unprecedented impetus for monitoring and conservation of Valeriana jatamansi, a valuable medicinal plant of Kashmir Himalaya. Agric Sci Res J 2:369–373
Towill LE, Mazu P (1975) Studies on the reduction of 2,3,5-triphenyltetrazolium chloride as a viability assay for plant tissue cultures. Can J Bot 53:1097–1102
Jugran A, Bhatt ID, Rawat S, Giri L, Rawal RS, Dhar U (2011) Genetic diversity and differentiation in Hedychium spicatum Buch. Ham. ex. D. Don—a high value medicinal plant of Indian Himalaya. Biochem Genet 49:806–818
Nybom H (2004) Comparison of different nuclear DNA markers for estimating genetic diversity in plants. Mol Ecol 13:1143–1155
Jugran AK, Bhatt ID, Mondal S, Rawal RS, Nandi SK (2015) Genetic diversity of Valeriana jatamansi across habitat types and altitudinal range using nuclear and chloroplast microsatellite markers. Curr Sci 109:1273–1282
Real LA (1994) Ecological genetics. Princeton University Press, Princeton
Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD (2004) Pollination syndromes and floral specialization. Ann Rev Ecol Evol Syst 35:375–403
Wada N (1999) Factors affecting the seed-setting success of Dryas octopetala in front of Broggerbeen (Brogger Glacier) in the high Arctic, Ny-Alesund, Svalbard. Polar Res 18:261–268
Kunin WE (1993) Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology 74:2145–2160
Blaauw BR, Isaacs R (2014) Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl Ecol 15:701–711
Friedman J, Barrett SCH (2011) The evolution of ovule number and flower size in wind-pollinated plants. Am Nat 177:246–257
Stebbins GL (1957) Self-fertilization and population variability in higher plants. Am Nat 41:337–354
Thompson JD (2003) Behavioural effects of pesticides in bees- their potential for use in risk assessment. Ecotoxicology 12:317–330
Bauer AA, Clayton MK, Brunet J (2017) Floral traits influencing plant attractiveness to three bee species: consequences for plant reproductive success. Am J Bot 104:772–781
Harder L (1990) Pollen removal by bumblebees and its implications for pollen dispersal. Ecology 71:1110–1125
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520
Muchhala N (2003) Exploring the boundary between pollination syndromes: bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuiflora (Campanulaceae). Oecologia 134:373–380
Theurillant JP, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109
Acknowledgements
The authors thank Dr. P. P. Dhyani, Director, GBPNIHESD for use of the facilities and encouragement and they also thank Mr. Tarun Belwal for helping to perform the seed germination experiments and colleagues of the Biodiversity Conservation and Management Theme for their assistance during this study. Partial funding support from SERB, New Delhi (DST No: SB/YS/LS-162/262) and GEF/UNEP/FAO Global pollination project (GPP) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to publish this manuscript in PNASIB.
Additional information
Significance Statement
Insect diversity and density on V. jatamansi populations decreased with increasing altitude which may play role in decreasing genetic diversity of this species. These finding have significance with conservation and breeding of V. jatamansi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jugran, A.K., Joshi, R.K., Bhatt, I.D. et al. The Relationship of Visiting Insect Diversity and Density of Valeriana jatamansi with Increasing Altitude in Western Himalaya. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 371–378 (2019). https://doi.org/10.1007/s40011-017-0954-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0954-9