Abstract
Present study was undertaken to evaluate the acetamiprid-mediated toxicity and possible protective effect of curcumin in rats. Rats were divided into seven different groups. They received curcumin (100 mg/kg, b.wt. p.o.) and acetamiprid (26.25 and 105 mg/kg b.wt., p.o.) alone and in their combinations for period of 28 days. Lipid peroxidation and antioxidant enzyme activities were determined in liver, kidney and brain and cellular changes histopathologically. Study shows that administration of acetamiprid caused a significant induction in oxidative damage in liver, kidney and brain as evidenced by increased level of lipid peroxidation (LPO) and altered activities of reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) and glutathione reductase (GR). Co-administration of curcumin with acetamiprid significantly decreased LPO, caused marked restoration in the non-enzymatic (GSH) and enzymatic antioxidants system (CAT, SOD and, GR) and protected from acetamiprid-mediated structural alterations of liver, kidney and brain. The results of present study indicates that curcumin ameliorate the acetamiprid-induced toxic damage to vital organ mediated via oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetamiprid belongs to the neonicotinoid group of insecticide and is being used worldwide since 1990 [1]. The use of acetamiprid against a wide range of insects, both in agricultural and domestic purpose, is very much common. Acetamiprid is used against insects that have gained resistance to organophosphate, carbamate and synthetic pyrethroid [2]. It has significant toxicity to insects but low toxicity to mammals because of the stronger affinity for insect nicotinic acetylcholinic receptor (nAChR) than for mammalian nAChR [3]. It acts on the central nervous system causing irreversible blockade of the postsynaptic nicotinic acetylcholine. Although acetamiprid is considered safe for use in the vicinity of human and animals, but reports of headache, dizziness, nausea, vomiting and other symptoms after the inhalation of acetamiprid have been reported [4].
Many studies showed that pesticides induce oxidative stress, leads to generation of free radicals and alteration in antioxidants system. The free radical generation leads to DNA damage, protein degradation, LPO and finally culminating damage to various vital organs such as liver, kidney and brain resulted into the pesticides mediated toxicity [5,6,7]. It has been reported that acetamiprid exposure caused increase in LPO and decreased in activity of CAT, GSH-Px and T-SOD in may be responsible for oxidative stress [8]. Recently, it has been observed that, exposure of acetamiprid has immunotoxic effect [9], causes development neuro-toxicity [10], germ cell mutation [11], even in utero and lactational exposure induces abnormalities in socio-sexual and anxiety-related behaviors [12].
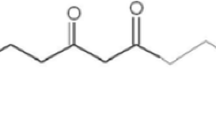
Consumption of certain vegetables and plant based antioxidants and related products and reduction of toxic effects mediated by various environmental toxicants has been very well documented [13]. Plant products are known to exert their protective effects via scavenging free radicals directly before they enter into peroxidation cycle, modulating detoxification of toxicant by forming complex with them and stimulating antioxidant defense system. Turmeric (Curcuma longa) and its active ingredient curcumin have been reported to scavenge free radicals such as reactive oxygen species, superoxide anion, the hydroxyl radical and nitrogen dioxide, induce detoxification enzymes and act as good antioxidants. It exerts protective effect in many conditions which includes heart diseases, liver damage, cerebral injury, neurodegenerative process, lung injury, cancer and, toxicants induced nephrotoxicity and reproductive toxicity [14,15,16]. It has been shown to exert protective effect against environmental contaminant by modulating the biochemical marker enzymes, LPO, and DNA damage [17, 18].
The toxic manifestation of acetamiprid exposure in terms of cellular damage and oxidative stress on different vital organs has not been systematically carried in past. However, no study has been reported related to the interaction between acetamiprid and curcumin. Therefore, to identify the potential modulatory role of the environmental toxicant acetamiprid, we evaluated the toxic effect of acetamiprid on oxidative stress and the structural alterations of liver, kidney and brain of rats, and then examined possible ameliorative effects of curcumin.
Material and Methods
Experimental Animal
Adult male wistar rats weighing 100–120 g were procured from the Laboratory Animal Resources Section, Indian Veterinary research Institute (IVRI, Izzatnagar. Prior to experiment, all the rats were kept at laboratory condition for a period of 7 days for acclimatization. All rats were housed having room temperature at 24 ± 1 °C with 55–60% relative humidity and a 12-h dark–light cycle. All rats were provided access to standard ration (Feed Unit, IVRI, Izzatnagar) and water ad libitum. All the experimental animals were kept and handled as per the guideline and permission of Institutional Animal Ethics Guidelines (IAEG) and Institutional Animal Ethics Committee (IAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and forests, Government of India.
Chemicals
Acetamiprid a technical grade (purity 96.8%) was a kind of gift received from M/s Gharda Chemicals, Pvt. Ltd. Mumbai, India. Curcumin was purchased from M/s Sigma Chemicals, USA. All other chemicals used were of analytical grade from Merck, Germany and India; Sigma Chemicals, USA and SRL Chemicals, India.
Dose Selection and Experimental Design
Acetamiprid doses were selected on the basis of LD50 of acetamiprid in rats. LD50 of acetamiprid was determined prior to start of experiment and was found to be 525 mg/kg body weight. Acetamiprid was administered at three different doses, low (1/20th and 1/5th of LD50 dose of AMI). An ameliorative effect of curcumin was examined against low and high dose of AMI. AMI and curcumin doses were prepared in corn oil as vehicle and administered by oral gavage for 28 days.
Total number of rats was randomly divided into eight groups comprising of six rats in each. Group I and II served as control and, vehicle control (corn oil; 1 ml/100 gm body weight, p.o.), respectively. Group III served as curcumin control (100 mg/kg b.wt. o.p.), while Group IV and V received AMI @ 26.25 and 105 mg/kg b.wt. p.o., respectively. Animals from Group VI and VII received AMI as in Group IV (26.25 mg/kg) and V (105 mg/kg) plus curcumin.
All the animals were observed twice daily for the development of any toxic clinical manifestations during the entire period of the study. Abnormalities such as alteration in locomotors activity, alertness, depression, feces consistency and body coat condition were monitored visually. Rats were sacrificed at the end of the last dose. Vital organ liver, kidney and brain were excised, washed with ice cold normal saline and used for oxidative stress assay and part of liver, kidney and brain were stored in 10% formal neutral buffers for histopathological examination.
Assessment of Oxidative Stress and Antioxidant Enzyme Activity
For estimations of oxidative stress-related biochemical parameters, 10% tissue homogenate of liver, kidney and brain were prepared in ice-cold phosphate buffer saline. Separately, 10% tissue homogenate was prepared using homogenizer (IKA, Germany) in 0.02 M EDTA for GSH estimation. Finally, samples were preserved in − 20 °C until assay. Tissue protein contents were determined by standard protocol using bovine serum albumin as standard.
Catalase Activity Assay
Catalase (CAT) activity was measured using spectrophotometer. Tissue homogenate was diluted with 50 mM phosphate buffer (50 mM KH2PO4, 50 mM Na2HPO4; pH 7.0). The exponential decrease of 10 mM hydrogen peroxide was measured at 240 nm in the presence of tissue homogenate. Reaction mixture without cell homogenate was used as tissue blank.
Superoxide Dismutase Activity Assay
The activity of superoxide dismutase (SOD) was measured as per the method of Madesh and Balasubramanian [19]. The final test reaction mixture contained 10 µl tissue homogenate (10%), 75 µl pyragallol (100 µM), 30 µl MTT (3-(4-5 dimethyl thiazol 2-xl) 2,5-diphenyl tetrazolium bromide; 1.25 mM), 0.65 ml phosphate buffer saline (NaCl: 8 g, KCl: 0.2 g, KH2PO4: 0.2 g and Na2HPO4: 0.94 g; pH 7.4). The reaction was terminated by the addition of 0.75 µl dimethyl sulfoxide, which helps to solubilize the formazan formed. In control reaction mixture, tissue homogenate was added after termination of reaction and in blank samples tissue homogenate was omitted. The absorbance was read at 570 nm against distilled water (blank). The activity was expressed as SOD Units/mg protein (one unit of SOD is the amount (in μg) of protein required to inhibit the MTT reduction by 50%). Y% = OD of Test × 100/OD of Control.
SOD = mg of protein in 0.01 ml homogenate × 50 × Dilution factor/Y%.
GSH Assay
GSH content was estimated by the method of Sedlak and Lindsay [20] by estimating free-SH groups, using DTNB (dithiobis-nitro-benzoic-acid). The absorbance of reactant mixture was read at 412 nm within 5 min. Reagent blank contained no sample and sample blank was without DNTB. The concentration of GSH was calculated using the extinction coefficient 13,000/M/cm and the results were expressed as mM of GSH per gm protein.
Glutathione Reductase Activity Assay
Glutathione reductase (GR) activity was assayed as described below. Three ml of reaction mixture contained 2.6 ml phosphate buffer (0.12 M, pH 7.2), 0.1 ml EDTA (15 mM) and 0.1 ml GSSG (65.3 mM). To this 10 μl of homogenate was added and then volume was made up to 2.95 ml with distilled water. After incubation at room temperature for 5 min, 0.05 ml of NADPH (9.6 mM) was added. The decrease in OD was immediately recorded for 3 min. The enzyme activity was expressed as μmoles of NADPH oxidized to NADP/mg of protein/min using the molar extinction coefficient of 6200/M/cm at 340 nm.
Lipid Peroxidation
LPO was evaluated in terms of malondialdehyde (MDA) production. One ml of 10% tissue homogenate was incubated at 37 ± 0.5 °C for 2 h. To each sample, 250 μl of 10% trichloroacetic acid was added. The mixture was thoroughly mixed and centrifuged at 720 g for 10 min. To 250 μl of supernatant, an equal volume of 0.67% thiobarbituric acid was added and kept in boiling water bath for 10 min, cooled and diluted with 250 µl distilled water. The absorbance was read at 535 nm. The amount of LPO was expressed as nanomoles (nM) of MDA formed per g protein.
Histopathology
Representative pieces of liver, kidney and brain were collected and fixed in 10% neutral buffered-formalin. Samples were processed as per the standard laboratory procedure for hematoxylin–eosin staining. All the sections of the liver, kidney and brain samples were examined for structural histopathological alterations under light microscope (Olympus, BH-2, Tokyo, Japan).
Statistical Analysis
All the data were expressed as Mean ± SE. The data were analyzed by using Microsoft Excel and ‘SPSS statistics 7.5’ software. Data were compared by employing one way ANOVA followed by Duncan multiple comparison as post hoc test. A p value of < 0.05 was considered as statistically significant.
Results and Discussion
Effect on Life-in Parameters
Administration of AMI, curcumin and their combination did not produce any mortality in any of the groups. Mild depression, abnormal gait and rough body coat were observed in AMI alone treated groups. Control and AMI plus curcumin treated rats exhibited normal activities and behavior during the observational period. Significantly lower (p < 0.05) body weights were recorded in AMI treated rats when compared with curcumin control at the end of the experiment (Table 1). Curcumin co-treated groups showed significant improvement in body weight as compared to AMI alone treated rats.
Effect on LPO and GSH Content
The malondialdehyde (MDA) level in liver, kidney and brain tissues were significantly (p < 0.05) higher in AMI alone treated rats as compared to control with dose dependent manner (Table 2). When curcumin was given along with AMI caused significant reduction (p < 0.05) in LPO as compared to AMI alone treated animals. Further, AMI alone treatments significantly (p < 0.05) reduced the levels of GSH in liver, kidney and brain as compared to control (Table 2), while, AMI plus curcumin treatment significantly improved GSH level in all tissues.
Effects on Enzymatic Antioxidant Enzyme
CAT activity significantly (p < 0.05) decreased in AMI alone treated rats in liver, kidney and brain tissues. Curcumin co-treatment caused significant (p < 0.05) increase in the CAT activities in all the tissues (Table 3). AMI treatment alone caused significant dose dependant decrease in SOD activities in all the tissues as compared to animals of control group. A significant (p < 0.05) restoration of SOD activities in liver and kidney were observed in curcumin plus AMI treated animals. However, in brain there was partial restoration of SOD activity (Table 3). Significantly decreased levels of GR were noticed in AMI alone treated rats (Table 3). Significant restoration of GR was observed in liver and kidney and non–significant restoration (p > 0.05) was noticed in brain of AMI plus curcumin treated groups.
Histopathological Changes
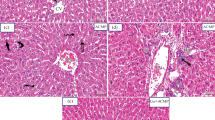
Liver from low dose AMI exposed liver showed mild degenerative changes in hepatocytes (Fig. 1a), while, high dose AMI exposed animals showed sinusoidal dilatation and moderate to severe degenerative changes, karyomegaly and individual cell necrosis (Fig. 1b). Curcumin co-treatment in AMI treated animals were significantly protected from AMI mediated structural alterations. Liver showed almost restored structure with normally arranged hepatocytes in low dose AMI plus curcumin treated animal (Fig. 1c), while, hepatocytes with mild degenerative changes were noticed in high dose AMI plus curcumin treatment (Fig. 1d). The kidney showed mild congestion at low dose exposed AMI animals (Fig. 2a), whereas, dilation of tubules, degenerative, desquamated lining epithelium and mild glomerular edema along with interstitial congestion was noticed in of rat treated with high dose of AMI (Fig. 2b). Kidney from low dose AMI plus curcumin treatment showed almost normal appearance with normally arranged glomeruli and tubules (Fig. 2c), whereas, mild degenerative congestion in high dose AMI plus curcumin treatment was noticed (Fig. 2d). In brain, mild degenerative changes were noticed at lower dose of AMI (Fig. 3a), while, neuronal degeneration and congestion of blood vessels were observed in high dose AMI treatment animals (Fig. 3b). Low dose AMI plus curcumin treatment resulted in normal appearance structural alteration in brain (Fig. 3c), while, moderate restored structure with mild degeneration observed in high dose AMI plus curcumin treatment (Fig. 3d).
Photomicrograph of liver of rats at the end of 28 days of exposure. a Liver showing moderate degenerative changes (→) and individual cell necrosis in low dose of AMI treatment. b Liver section showing sinusoidal dilatation (∆), moderate to severe degeneration (→) and necrosis of hepatocytes and karyomegaly (<) in high dose AMI treatment (Inset X400). c Liver showing almost restored liver structure with normally arranged hepatocytes in low dose AMI plus Cur treatment. d Liver showing hepatocytes arranged normally with mild degenerative changes in high dose AMI plus Cur treatment. Magnification: a, b, c and d, X100
Photomicrograph of kidney of rats at the end of 28 days of exposure. a Kidney showing dilated tubules (∆), degenerative and desquamated mild glomerular edema along with interstitial congestion (→) in low dose of AMI treatment. b Kidney showing dilated tubules (∆), degenerative and desquamated lining epithelium with cast formation at some places and mild glomerular edema along with interstitial congestion (→) high dose AMI treatment. c Kidney showing almost normal appearance with normally arranged glomeruli and tubules in low dose AMI plus Cur treatment. d Kidney showing restored structure with mild degeneration congestion (→) in high dose AMI plus Cur treatment. Magnification: a, b, c and d, X100
Photomicrograph of brain of rats at the end of 28 days of exposure. a Brain showing mild neuronal degeneration (→) in low dose AMI treatment. b Brain showing severe neuronal degeneration and congestion (∆) of blood vessels in high dose AMI treatment in high dose AMI treatment. c Brain showing almost normal appearance structure in low dose AMI plus Cur treatment. d Brain showing (ACE restored structure with mild degeneration in high dose AMI plus Cur treatment. Magnification: a, b, c and d, X100
Discussion
Results of the present study revealed decrease in body weight in AMI treated animals. It may be attributed to the effect of insecticide on gastrointestinal tract resulting in decreased appetite and absorption of nutrients from gut. Administration of AMI in male mice caused significant decrease in body weight gain [8]. Since weight gain in animals serves as an index of growth rate [21], however decrease in weight gain indicated toxic effect of AMI. Histopathological observation showed sinusoidal dilatation and moderate to severe degenerative changes in hepatocytes, karyomegaly and individual cell necrosis in liver, indicating that metabolic activity of animal was altered with exposed AMI resulting in decrease in feed intake and decrease in body weight gain. Similarly, when rats were exposed to imidacloprid, resulted in decrease in body and organ weight [17], mild focal necrosis with swollen cellular nuclei and cytoplasmic lesions in liver, and dilation tubules, degenerative/desquamated lining epithelium and mild glomerular edema along with interstitial congestion in kidney [22]. Further, mild to severe degenerative changes with congestion of blood vessel was noticed in brain of AMI treated rats in the present study. Similar results have been reported with thiacloprid and imidacloprid in poultry and rats [16, 23].
GSH is the nonprotein thiol in organisms responsible for intracellular protection against toxic compounds generated reactive oxygen species intermediates and other form of free radicals [24]. Additionally, it has direct free radical scavenger activity; provides substrate for glutathione peroxidase and glutathione-S-transferase. SOD and CAT enzymes help in scavenging superoxide ions and hydroxyl ions, respectively [25]. In the present study, AMI significantly suppressed GSH concentration, increased SOD and decreased catalase activities in the liver, kidney and brain in acetamiprid exposed rats. Similarly, decrease in CAT, GSH-Px and T-SOD activities in testes with oral exposure of AMI in male mice has been reported [8]. Increase in the level of MDA in the hepatic, renal and brain tissues indicates the induction of LPO, which could lead to the loss of membrane structure and function [8]. Earlier studies with sister compound, imidacloprid, indicate that when administered orally to wistar rats resulted in significant alteration in GSH content, CAT, SOD, GST activities and induction of LPO in brain and testes along with histopathological alterations [16]. CAT and SOD constitute a mutually protective set of enzymes and this cooperative interaction is distinct from that which derives from prevention of OH− produced in the iron-catalyzed interaction of O2 with H2O2. SOD catalyzes the dismutation of O2 radical anions to H2O2 and O2 [26]. CAT is an antioxidant enzyme and decrease in its activity implies Fenton reaction mediated conversion of more H2O2 to the ultimate toxicant, the OH− and prevents lipid membrane [27]. The efforts of the endogenous antioxidant enzymes to remove the continuously generated free radicals initially increase due to an induction but in chronic exposure enzyme depletion results, subsequently leading to oxidative damage [28]. This indicates that AMI has deleterious effect on endogenous antioxidant system through oxidative stress/ORS generation.
Curcumin, a phenolic compound, exhibits protective effects against oxidative damage and it is considered to be a potent cancer chemopreventive agent [29]. In present study, rats that received curcumin along with acetamiprid showed increase in body weight as compared to acetamiprid alone treated rats which indicate that curcumin has appetite inducer and anti-stress effect. This finding is in agreement with observation of increased weight gain of rats following treatment with curcumin pre-treated with arsenic [30]. Pre-treatment of curcumin with cypermethrin prevented histological alteration in liver, kidney and brain of male rats [31]. Further, our previous study on curcumin oral administration in imidacloprid treated rats showed significant protection in terms of improvement in weight gain, normal activity and restoration of functional activity of hepatic enzyme system [17]. Results of the present study revealed that administration of ACE plus curcumin resulted in suppressed LPO, and enhanced enzymatic and nonenzymatic antioxidants status in liver, kidney, and brain in rats. These results are in accordance with previous workers [16, 32], who demonstrated that curcumin alone significantly decreased LPO in tissue. Curcumin possesses distinct structural motifs that are responsible for its antioxidant activity. The presence of electron donating groups like phenolic hydroxyl groups and a β-diketone structure is responsible for the free radical scavenging activity and inhibiting LPO [33]. Curcumin has been shown to exert protective effect by modulating the anti-oxidant bio-marker such as SOD, CAT, GPx and GSH and LPO and augmenting antioxidant defense system [34]. Elevation of GSH levels in the tissue by curcumin as observed in the present study is in agreement with earlier findings. Curcumin treatment caused elevated GSH level in the tissue exposed with cadmium and arsenic; however, cypermethrin and imidacloprid induced decrease in the levels of GSH in liver, kidneys and brain of rats [16, 31, 35]. In the present study, levels of CAT, SOD, GSH and GR enzymes in liver, kidney and brain were improved by curcumin treatment in AMI treated animals. Hence, the preventive effect of curcumin on oxidative stress biochemical markers and microscopic alterations could be responsible for the better observed physio-chemical outcomes in liver, kidney and brain.
Conclusion
The finding indicates that curcumin could be beneficial in countering the acetamiprid-mediated oxidative stress at physiological, biochemical and microscopic levels. The anti-oxidative potential of curcumin might be playing key role in the observed protective outcome against AMI-mediated toxic manifestation. Since curcumin is a natural polyphenol without any reported side effects, it could be used as a safe alternative agent for amelioration of AMI-associated oxidative stress.
References
Brunet JL, Badio A, Belzunces LP (2005) In vivo metabolic fate of [14C]-acetamiprid in six biological compartments of the honeybee, Apes mellifera L. Pest Manag 61:742–748
Si SS, Liu XM, Wu RF (2005) Efficacy of several insecticides on the control of myzus Persicae and Lipaphis erysimi. Pestic Sci Adm 26:12
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48:339–364
Chen LM, Liu HS, Chen N, Du SM (2007) The determination of acetamiprid residues in serum by gas chromatography. Chin J Ind Hyg Occup Dis 5:191–192
Abdollahi M, Ranjbar A, Shadnia A, Nikfar S, Rezaie A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 10:141–147
Lopez O, Herandez AF, Rodrigo L, Gil F, Pea G, Serrano JL, Parron T, Villanueva E, Pla A (2007) Changees in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicol Lett 171:146–153
Khan SM, Sobti RC, Kataria L (2005) Pesticide-induced alteration in mice hepato-oxidative status and protective effects of black tea extract. Clin Chem Acta 358:131–138
Jiao-jiao Z, Yi W, Hai-yang X, Meng-xue L, Wen-hao L, Kai-ge M, Xian-zhong W, Jia-hau Z (2011) Oxidative stress: role in acetamiprid-induced impairment of the male mice reproductive system. Agric Sci China 10:786–796
Shakthi Devan RK, Prabu PC, Panchapakesan S (2015) Immunotoxicity assessment of sub-chronic oral administration of acetamiprid in wistar rats. Drug Chem Toxicol 38(3):328–336
Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M, Kawano H (2012) Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS ONE 7(2):e32432
Rasgele PG (2014) Abnormal sperm morphology in mouse germ cells after short-term exposures toacetamiprid, propineb, and their mixture. Arh Hig Rada Toksikol 65(1):47–56
Sano K, Isobe T, Yang J, Win-Shwe TT, Yoshikane M, Nakayama SF, Kawashima T, Suzuki G, Hashimoto S, Nohara K, Tohyama C, Maekawa F (2016) In utero and lactational exposure to acetamiprid induces abnormalities in socio-sexual and anxiety-related behaviors of male mice. Front Neurosci 10:228. https://doi.org/10.3389/fnins.2016.00228
Nandi P, Talukder G, Sharma A (1997) Dietary factor in cancer chemoprevention. Nucleus 40:128–144
Maheshwari RK, Singh KA, Gaddipati J, Srimal CR (2006) Multiple biological activity of curcumin: a short review. Life Sci 78:2081–2087
Oguz A, Kapan M, Onder A, Kilic E, Gumus M, Basarali MK, Firat U, Boyuk A, Buyukbas S (2013) The effects of curcumin on the liver and remote organs after hepatic ischemia reperfusion injury formed with Pringle manoeuvre in rats. Eur Rev Med Pharmacol Sci 17:457–466
Lonare M, Kumar M, Raut S, Badgujar P, Doltade S, Telang A (2014) Evaluation of imidacloprid-induced neurotoxicity in male rats: a protective effect of curcumin. Neurochem Int 78:122–129
Lonare M, Kumar M, Raut S, More A, Doltade S, Badgujar P, Telang A (2015) Evaluation of ameliorative effect of curcumin on imidacloprid-induced male reproductive toxicity in wistar rats. Environ Toxicol 31(10):1250–1263
Tokac M, Taner G, Aydın S, Ozkardes AB, Deundar HZ, Taslipinar MY, Arikok AT, Kilic M, Basaran AA, Basaran N (2013) Protective effects of curcumin against oxidative stress parameters and DNA damage in the livers and kidneys of rats with biliary obstruction. Food Chem Toxicol 61:28–35
Madesh J, Balasubramanian KA (1998) Microtitre plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophy 35:184–188
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Palani V, Senthikumaran RK, Govindawamy S (1999) Biochemical evaluation of antitumor effect of Muthu marunthu (a herbal formulation) on experimental fibro-sarcoma in rats. J Ethnopharmacol 65:257–265
Bhardwaj S, Srivastava MK, Kapoor U, Srivastava LP (2010) A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem Toxicol 48:1185–1190
Goyal S, Sandhu HS, Brar RS (2010) Histopathological alterations induced after oral sub-acute thiacloprid toxicity in Gallus domesticus. Vet Arhiv 80:673–682
Anderson ME, Luo JL (1998) Glutathione therapy: from prodrugs to genes. Semin Liver Dis 18:415–424
Parke DV, Piotrowski JK (1996) Glutathione: its role in detoxication of reactive oxygen species and environmental chemicals. Toxicol 4:1–13
Okado-Matsumoto A, Fridovich IA (2001) Assay of superoxide dismutase: cautions relevant to the use of cytochrome c, a sulfonated tetrazolium, and cyanide. Anal Biochem 298:337–342
Dinkova-Kostova AT (2002) Protection against cancer by plant phenyl propenoids: induction of mammalian anticarcinogenic enzymes. Mini Rev Med Chem 2:595–610
Kalra J, Mantha SV, Prasad K (1994) Oxygen free radicals: key factors in clinical diseases. Lab Med Int 11(13–19):14
Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E (2005) Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223:181–190
Fatma MED, Mokhtar IY, Fatma MER (2009) Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol 47:249–254
Sankar P, Telang AG, Manimaran A (2012) Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp Toxicol Pathol 64:487–493
Pari L, Amali DR (2005) Protective role of tetrahydrocurcumin (THC) an activeprinciple of turmeric on chloroquine induced hepatotoxicity in rats. J Pharmacol Pharm Sci 8:115–123
Dinkova-Kostova AT, Talalay P (1999) Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogen 20:911–914
Kalpana C, Menon VP (2004) Curcumin ameliorates oxidative stress during-nicotine induced lung toxicity in Wistar rats. Ital J Biochem 53(2):82–86
Tirkey N, Kaur G, Vij G, Chopra K (2005) Curcumin, a diferuloylmethane attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol 5:15–23
Acknowledgements
The authors are thankful to the Director, Indian Veterinary Research Institute, Izzatnagar, Bareilly and Indian Council of Agriculture and Research, New Delhi, India for providing Junior Research Fellowship for research. They also acknowledge M/S Gharda Chemicals, Mumbai, India for generously providing technical grade acetamiprid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Significance statement Acetamiprid exposure has toxic effect on liver, kidney and brain in terms of induction of oxidative damage through LPO and altered antioxidant defence system, while, co-administration of curcumin reversed this toxic effect. This indicates that the curcumin would be helpful in counteracting toxic effect of acetamiprid on vital organ.
Rights and permissions
About this article
Cite this article
Doltade, S., Lonare, M., Raut, S. et al. Evaluation of Acetamiprid Mediated Oxidative Stress and Pathological Changes in Male Rats: Ameliorative Effect of Curcumin. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 191–199 (2019). https://doi.org/10.1007/s40011-017-0934-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0934-0