Abstract
Sulphur is now recognized as the fourth major plant nutrient after N, P and K globally. Sulphur in soils comes from the sulphur containing minerals present in parent materials from which the soils are derived and from the plants and animals residues or from the external addition of elemental S or its minerals. Sulphur enters the biological systems from soil through microbial activities involving mineralization of organic matter, immobilization, oxidation and reduction. Plants take up sulphur only as SO =4 and reduce it to form S containing amino acids and other compounds. Amino acid cysteine is the source of sulphur for most other S-compounds in plants. Sulphur containing vitamin thiamine (Vitamin B1) is also synthesized only in plants and not in humans or other animals. Plants also produce vitamin biotin and a number of S-containing metabolites including glutathione, glucosinolates and alliin/allicin. Sulphur deficiency in wheat can lead to poor baking quality and in oilseeds it can lead to reduced oil content and yield. Sulphur is taken in as sulphur containing amino acids (SAAs) cysteine and methionine by human beings. The recommended dietary allowance for SAAs for humans is 14 mg kg−1 body weight. Lack of sulphur can lead to arthritis, muscle and joint stiffness, spondylitis, etc. Dietary supplements containing (chondroitin sulphate, glucosamine sulphate, methylsulfonylmethane etc.) can be beneficial in the treatment of joint diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulphur (S) with an atomic number 16 and an average atomic mass 32.06 occurs in group 16 and period 3 of the periodic table. It has an atomic configuration [Ne] 3s 23p 4. It expresses oxidation states of +4 in SO2, +6 in H2SO4, and −2 in H2S [1]. Sulphur is an essential macronutrient for microorganisms, plants, animals and humans. Proteins store most S. As regards to its abundance, in both plants and humans, S comes after K, Ca and P. In plants, K is present in the largest amount, while in humans, Ca is present in the largest amount.
Sulphur enters the biological systems from the S present in earth crust and soil. Soil is the top layer of earth crust, which has undergone weathering. It contains organic matter and is teeming with living organisms. All these three factors affect the availability of plant nutrients in soil [2]. S enters the plants as SO =4 , which has the capacity to reduce it to SH−or sulfide (S=). Amino acid cysteine is being the main organic molecule. The S containing amino acids (SAAs) as cysteine and methionine are the major forms in which S enters human beings either directly as plant food or indirectly as animal foods. Both cysteine and methionine are essential amino acids although human beings can synthesize some cysteine. From the biological system, S goes back to soil as crop residues, animal and human excreta completing the S nutrition cycle.
Sulphur in the Earth Crust
In the earth’s crust, S is present in very small amount as compared to iron (5.0 %), calcium (3.6 %), potassium (2.6 %) and magnesium (2.1 %) [3]. Sulphur in the earth crust occurs as elemental sulphur, sulphide and sulphate minerals. The sulphate minerals include anhydrite (CaSO4), gypsum (CaSO4·2H2O), barite (BaSO4), Kieserite (MgSO4·H2O) and epsonite (MgSO4·2H2O), while the sulfide minerals include pyrites (FeS2), chalcocite (Cu2S), bornite (Cu5FeS4), digenite (Cu9S5), tetrahydrite (Cu12Sb4S13), molybdenite (MoS2), sphalerite (ZnS), galena (PbS) etc. [4].
Elemental S is found near hot springs and volcanic regions in Indonesia, Chile, Japan and Italy [5]. The anaerobic bacteria acting on sulphate minerals, such as, gypsum in salt domes produce S. Such deposits in salt domes occur along the coast of Gulf of Mexico and evaporate in Europe. Fossil based S deposits from salt domes which have been recently the basis of commercial S production in USA, Russia, Turkmenistan and Ukraine [6]. However, today most elemental S is produced as a byproduct of natural gas and petroleum industry [7].
Role of Microbes
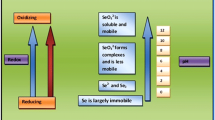
Microbes play an important role in releasing S from elemental S and sulfide minerals in the earth surface to soil. Only from sulphate minerals, S becomes readily available in soil and to plants, since plants take up sulphur only as SO =4 . Elemental S, sulfide S and thiosulfate S have to be first oxidized. Microorganisms from all the three domains, bacteria, arachaea and eukaryotes (fungi) are involved in the oxidation of sulphur; however, the major role is played by the bacteria, Thiobacillus sp. In the domain Archaea, aerobic oxidation of sulphur is restricted to the members of the Sulfolobales [8]. The fungi reported to be involved in the oxidation of elemental-S and thiosulphate are Alternari atenui, Aureobasidium pullulans and Epicoccum nigrum, and a range of Penicillium sp. Selecobasidium constrictum, Myrothesium circutum and Aspergillus sp. [9, 10]. The bacteria involved in the oxidation of sulphur can be classified into three groups as under [11, 12].
Chemolithoautotrophs
These bacteria obtain energy from oxidation of sulphur and carbon from carbon-dioxide for their growth and development. The examples are Thiobacillus thioparus, T. neapolitanus, T. denitificans, T. thiooxidans, T. ferrooxidans, T. halophilus and some species of Thiomicrospira.
Chemolithoheterotrophs
These bacteria obtain energy from oxidation of sulphur and carbon from organic molecules for their growth and development. The examples are Thiobacillus novellus, T. acidophilus, T. aquaesulis, Paracoccus dentrificans, P. versutus, Xanthobacter tagetidis, Thiospaera pantotroph and Thiomicrospira thasirae.
Chemolithomesoptrophs
These bacteria obtain energy from oxidation of sulphur and carbon from inorganic as well as organic molecules for their growth and development. The examples include Thiobacillus denitrificans and T. ferrooxidans.
There are a number of enzymes involved in sulphur oxidation. These include thiosulphate dehydrogenase, tetrathionate hydrolase, trithionate hydrolase and sulphur oxygenase [13, 14].
On the contrary, sulphates are reduced to H2S by S reducing organisms under anaerobic conditions such as those obtained in low-land rice paddies. H2S is responsible for the bad odour from paddy fields. Sulphate reducing bacteria reduce sulphate to obtain energy. Sixty genera containing 220 species of sulphate reducing bacteria are known [15]. The largest group (about 23 genera) includes Desulfpbacteriales, Desulfovibrionales and Syntrophobacterales [16]. The second largest group includes genera Desulfotomaculum, Desulfosporomusa and Desulfosporosium.
Sulphur in Soil and Crop Responses to S Fertilization
Sulphur in soil comes from the sulphur containing minerals present in parent materials in rocks from which the soils are derived and from the plant and animal residues or from the external addition of elemental S or its minerals. Total S content in soils is reported in the range of 30–400 mg kg−1 and about 50–90 % of it is bound to soil organic matter [17]. The C:N:S ratio in soil organic matter is about 100:8.9:1 [18]. Only a small fraction of total S present in soil is available to crops in a growing season and there are a number of soil tests for available S, such as 0.15 % CaCl2 extractable S [19]. For this procedure, the critical deficiency level is 10 mg S kg−1 soil [20].
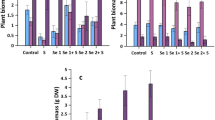
Sulphur is now recognized as the fourth major plant nutrient after N, P and K globally [21] and crop responses to S fertilization have been reported from Asia [22, 23], Africa [24], Europe [25], north America [21], south America [26] and Oceania [27]. Sulphur is now considered as an important component of balanced fertilization of crops [28, 29]. Crop response to sulphur in India is the highest for oilseeds (rapeseed/mustard, sunflower, safflower, groundnut, soybean etc.). Average S uptake (kg per metric ton of grain/seed) by crops is reported to be 9.9 for oilseeds, 7.6 for pulses or beans and 4.1 for the cereals [29]. Sulphur fertilization increases protein content in the grains of cereals [30] and oil content in seeds of oilseeds [31, 32]. The recent increase in S deficiency in soils of India and other Asian countries is due to use of high analysis fertilizers such as urea and diammonium phosphate (DAP) in place of sulphur containing ammonium sulphate and single superphosphate, use of high yielding varieties and hybrids of cereals and practicing intensive cropping systems such as rice–wheat [33]. Sulphur fertilizers in India include elemental S, gypsum, pyrites and bentonite S [30, 34]. In addition, S is also present in a number of nitrogen (ammonium sulphate, ammonium phosphate sulphate), phosphorus (single/ordinary super phosphate, triple/concentrated super phosphate), potassium fertilizers (potassium sulphate, potassium magnesium sulphate) and micronutrient fertilizers (zinc sulphate, copper sulphate, manganese sulphate) [18]. Application of nitrogen, phosphorus and potash increases the availability of applied S to the crops [35]. Also when elemental S or pyrites are used as a source of S, they should be applied on soil surface to permit their oxidation to sulphate [12, 36].
Sulphur in Plants
Important Components
Assimilation of sulphur by the plants is very important, because only through plants it enters the human beings. Sulphur is taken up by plants as SO =4 and is present as aqueous solutions in xylem tissue and in vacuoles of plant cells. In plant tissue, it occurs as SH− (thiol orsulfhydryl group) or S= (Sulfide) in various organic molecules including proteins. Many thiols have strong odors resembling that of garlic. Most S in plants is stored as proteins.
Sulphate in plants is changed to adenosine 5′phosphate (APS) catalyzed by ATP sulfurylase and later to 3′adenosine 5′phosphosulphate (PAPS), which is reduced to sulphite (SO =3 ) catalyzed by PAPS reductase and later to sulfide (S+) catalyzed by sulfide reductase. Sulphide so produced is then exchanged with acetyl group of O-acetylserine produced from serine with the help of serine acid transferase and produces cysteine [37]. Amino acid cysteine is the source of sulphur for most other S-compounds in plants. In the synthesis of another amino acid methionine, the main backbone is derived from aspartic acid and sulphur from cysteine or methanethiol or hydrogen sulfide and the three steps involved in the biosynthesis of methionine from homoserine are acylation, sulfurylation and methylation [36]. Methionine is the only amino acid with a thio-ether group (i.e. C–S–C) and in addition to structural role in proteins, methionine is best known for its role in the initiation of translation [38].
Sulphur containing thiamine (Vitamin B1) is also synthesized only in plants and not in human beings. Cysteine is the S donor in thiamine synthesis, which involves a number of S carrier proteins (ThiO, ThiG, ThiS, ThiF etc.) like cysteine desulfurase and a host of other enzymes. The thiazole [hydroxyethyl thiazole (HET-P)] [39] and pyrimidine [hydroxymethyl thiamine (HMP-PP)] [40] moieties of thiamine are synthesized separately and then are coupled to form thiamine monophosphate, which is further phosphorylated to thiamine pyrophosphate (Vitamin B1) [41]. In plants, thiamine is able to enhance tolerance to oxidation stress during different abiotic stress conditions [42]. It is also required for shoot meristem maintenance in maize [43]. Biotin (vitamin H or coenzyme R) is another S-containing vitamin. It has two moieties ureido and thiophene. Its precursors are alanine and S-containing pimeloyl-CoA [44].
Plants also produce a number of S-containing metabolites. One of the important metabolite is glutathione (GSH), a tripeptide (L-γ-glutamyl-L-cysteine-glycine) [45]. It is an important anti-oxidant in plants, animals, fungi and some bacteria and prevents damage to important cellular compounds caused by free radicals and peroxide [46]. Thiol groups in GSH are reducing agents and in the process GSH is oxidized to GSH-disulfide (GSSH) also called L-(−) GSH. It is later reduced to GSH by GSH-reductase using NADP as an electron donor [47]. Other secondary metabolites include glucosinolates (GSLs) in the members of the Brassicae family, such as, rapeseed-mustard, cabbage, broccoli, horse radish etc. and alliin/allicin in the members of Alliaceae family, such as, garlic, onion etc. [48]. These secondary metabolites are part of plant’s protection mechanism against diseases and pests, but they have been found to be useful for alleviating several diseases in humans as discussed later.
Sulphur Deficiency Symptoms in Plants
Since S and N are components of proteins, therefore their deficiency symptoms in plants are similar, that is, yellowing of leaves. However, since S is less mobile in plants, its deficiency symptoms first appear on the younger leaves as a contrast to N deficiency symptoms which first appear on lower leaves [49]. In rapeseed/mustard, young leaves of S deficient plants become pale and chlorotic and are cupped. In most other crops interveinal yellowing of young leaves is observed, which under extreme deficiency may get bleached in some crops [50]. The general critical level for severe deficiency in leaves is 0.15 %, while the sufficiency level is 0.45 % on dry matter basis [50].
Sulphur Fertilization and Crop Quality
Sulphur deficiency in plants reduces not only yield but also reduces the quality of the produce. Zhao et al. [51] reported that when S is deficient, it reduces S-containing amino acids in wheat, which create an imbalance between high molecular weight (HMW) and low molecular weight (LMW) glutenin sub-units [52] reducing bread making quality of wheat. Randall et al. [53] reported that when S concentration in wheat grain is below 1.2 mg g−1 and N:S ratio is above 17:1, the grain is deficient in S and both yield and quality are affected. Unger et al. [54] also observed that a high N:S in wheat grain was associated with low loaf height and volume in bread. In rice also Shivay et al. [55] found that application of 45 kg S/ha reduced N:S ratio in grain from 9.77 to 7.79.
Sulphur has received special attention in oilseed crops and a number of reports are available on the increase in seed yield and oil content in rapeseed/mustard [56–58], groundnut [31, 59], sunflower [32] and soybean [60].
Sulphur in Humans
Sulphur is an abundant element in human body and along with nitrogen and phosphorus it forms a triad of elements needed for most of the body tissue. As regards the amounts of minerals, it comes only after calcium and phosphorus. Human proteins contain 3–6 % sulphur containing amino acids (SAAs) [61]. Although some cysteine is synthesized in human body, most sulphur is taken in as SAAs cysteine and methionine. Recommended dietary allowance (RDA) for sulphur containing amino acids (SAAs) (cysteine and methionine) is 14 mg kg−1 body weight. Thus, a person weighing 70 kg would need around 1.1 g SAAs and a safe intake is about 2 g SAAs per day [62].
Disulfide bonds in S-compounds are involved in the function of connective tissue and provide flexibility in tissue. Lack of sulphur can lead to tendonitis, arthritis, bursitis, muscle and joint stiffness, spondylitis, fibrosis and sclerosis etc. Dietary supplements containing sulphur (chondroitin sulphate, glucosamine sulphate, Methylsulfonylmethane etc.) can be beneficial in the treatment of joint diseases [63].
Glutathione (GSH) is an important detoxifier in liver and takes care of free oxygen radicals and peroxides [64, 65]. GSH is also the way sulphur is stored in liver. GSH has also a role in biosynthesis of prostglandines (PGS), which are associated with key processes in inflammation.
Cysteine deficiency can lead to a number of disorders in humans and its derivative acetylcystein or N-acetylcystein is used as a nutritional supplement to cure paracetamol toxicity [66], prevention of acute renal failure [67] and progressive interstitial lung disease [68, 69] and treatment of psychiatric disorders [70].
Sulphur is involved in the formation of collagen and keratin. Sulphur deficiency can lead to hair, skin and nail problems. Lack of methionine leads to build up hydrogen peroxide in hair follicles and a gradual loss of hair colour [71]. An important metabolite of methionine S-adenosylmethionine (SAMe) is involved in many metabolic processes in humans [72]. SAMe nutritional supplements are used for curing depression [73] and osteoarthritis [74]. However, too much methionine can be harmful and methionine restriction without energy restriction may extend life span in rats [75]. Methionine restriction is also reported to inhibit age related processes in rats [76].
Thiamine deficiency in humans disturbs the central nervous and circulatory systems and causes beriberi [77], Wernicke–Korsokof syndrome (alcohol abuse syndrome) [78] and bilateral visual loss or impaired colour perception [79]. Excess sulphur from humans and animals is excreted as sulphate. Small amounts of sulphur enter human and animals as a number of sulpha drugs, but discussion on these is beyond the scope of this review.
Conclusion
Sulphur is an essential element for plants and humans, where most of it is present as sulphur containing amino acids (SAAs) cysteine and methionine. Although humans can synthesize some cysteine (hence semi essential), most of it and all methionine (essential, because humans cannot synthesize it) must be obtained from plant or animal foods. Sulphur deficiency leads to a number of ailments including joint stiffness and arthritis. RDA for SAAs in humans is 14 mg per kg body weight. Plants can synthesize both these SAAs, but should be able to get enough of S as sulphate from the soil. Adequate S fertilization of soils is the key for adequate S nutrition of the plants and humans. Sulphur fertilizers in India include elemental S, gypsum, pyrites and bentonite S. In addition S is also present in a number of nitrogen (ammonium sulphate, ammonium phosphate sulphate), phosphorus (single/ordinary super phosphate, triple/concentrated super phosphate), potassium (Potassium sulphate, potassium magnesium sulphate) and micronutrient fertilizers (zinc sulphate, copper sulphate, manganese sulphate).
References
Rao CNR (1999) Understanding chemistry. University Press (India) Ltd., Hyderabad
Tandon HLS (2014) History of soil fertility. In: Prasad R, Kumar D, Rana DS, Shivay YS, Tewatia RK (eds) Textbook of plant nutrient management. Indian Society of Agronomy, New Delhi, pp 1–22
Tarbuck EJ, Lutgens FK, Tasa DG (2014) Earth sciences, 14th edn. Printice-Hall, New Jersey
Klein C, Cornelius SH Jr (1986) Manual of mineralogy, 20th edn. Wiley, New York
Rickwood PC (1981) The longest crystals. Am Mineral 66(9/10):885–907
Nehb W, Vydrak K (2006) Sulfur. In: Ullmann’s encyclopedia of industrial chemistry. Wiley, New York
Eow JS (2002) Recovery of sulphur from acid gas: a review of the technology. Environ Prog 21(3):143–162
Setter KO, Fiala G, Huber G, Huber H, Segerer A (1990) Hyperthermophilc microorganisms. FEMS Microbiol Rev 75:117–124
Wainwright M (1978) A modified sulphur medium for the isolation of sulphur oxidizing fungi. Plant Soil 49(1):191–193
Shinde DB, Patil PL, Patil BR (1996) Potential use of sulphur oxidizing microorganisms as soil inoculants. Crop Res 11:291–295
Aragono M (1991) Aerobic chemolithoautotrophic bacteria. In: Christjansson JK (ed) Thermophilic bacteria. CRC Press, Boca Raton, pp 7–103
Vidyalakshmi R, Paranthaman R, Bhakyaraj R (2009) Sulphur oxidizing bacteria and pulse nutrition—a review. World J Agric Sci 5(3):270–278
Friedrich CG, Rother D, Bardischewsky F, Quentmeier A, Fischer J (2001) Oxidation of reduced inorganic compounds by bacteria: emergence of a common mechanism. Appl Environ Microbiol 67(7):2873–2882
Keppler U, Bennet B, Rethmeier J, Schwarz G, Deutzmann R, McEwan AG, Dahl C (2000) Sulfit: cytochrome c oxidoreductase from Thiobacillus novellus, purification, characterization and molecular biology of a heterdimeric member of sulfite oxidase family. J Biol Chem 275(18):13202–13212
Barton LL, Fauque AJ (2008) Biochemistry, physiology and biotechnology of sulphate reducing bacteria. Adv Appl Microbiol 68(Ch 2):41–98
Muzer G, Stams AJ (2008) The ecology of biotechnology of sulphate-reducing bacteria. Nat Rev Micriobiol 6:441–454
Pandey DK, Tiwari KN, Tiwari RK (1989) Different forms of sulphur in alluvial soils. J Indian Soc Soil Sci 37:161–164
Prasad R (2007) Crop nutrition-principles and practices. New Vishal Publications, New Delhi
Williams CH, Steinberg A (1959) Soil sulphur fractions as chemical indices of available sulphur in Australian soils. Aust J Agric Res 10:340–342
Shukla LM (2001) Evaluation of soil test methods for sulphur in soils of India. Fertil News 46(10):55–58
Jez J (ed) (2008) Sulfur—a missing link between soils, crops and nutrition. Am Soc Agron Mon 50, Madison, WI, American Society of Agronomy
Meng C, Lu X, Cao Z, Hu Z (2004) Effect of sulphur fertilization on yield of rice and oil rape and the critical value of soil and available sulphur. Plant Nutr Fertil Sci 10:218–220 (in Chinese)
Tewatia RK, Yadav DS, Shivay YS (2014) Sulphur management. In: Prasad R, Kumar D, Rana DS, Shivay YS, Tewatia RK (eds) Textbook of plant nutrient management. Indian Society of Agronomy, New Delhi, pp 144–156
Friesen DK (1991) Fate and efficiency of sulphur fertilizer applied to food crops in West Africa. Fertil Res 29:35–44
Mathot GC, Thelier-Huche L, Lambert R (2009) Sulphur and nitrogen content as sulphur deficiency indicators for grains. Eur J Agron 30:172–176
Raun WR, Barreto HJ (1992) Maize grain yield response to sulphur fertilization in Central America. Sulphur Agric 16:26–29
Anderson GC, Pevrill KI, Brennan RF (2013) Soil-sulphur—crop response calibration relationships and criteria for field crops grown in Australia. Crop Pasture Sci 64(5):523–530
Singh MV (2001) Importance of sulphur in balanced fertilizers use in India. Fertil News 46(10):13–35
Tandon HLS (2011) Sulphur in soils, crops and fertilizers. FDCO, New Delhi
Shivay YS, Prasad R, Pal M (2014) Effects of levels and sources of sulfur on yield, sulfur and nitrogen concentration and uptake and S-use efficiency in basmati rice. Commu Soil Sci Plant Anal 45(18):2468–2479
Maity SK, Giri G (2003) Influence of P and S fertilization on productivity and oil yield of groundnut and sunflower in intercropping with simultaneous and staggered planting. Indian J Agron 48(4):267–270
Sarkar RK, Mallick RB (2009) Effect of nitrogen, sulphur and foliar spray of nitrate salts on performance of spring sunflower (Healianthus annus). Indian J Agric Sci 79(12):986–990
Prasad R (2005) Rice–wheat cropping systems. Adv Agron 86:255–339
Tiwari KN, Dwivedi BS, Pathak AN (1984) Evaluation of iron pyrites as sulphur fertilizer. Fertil Res 5(3):235–243
Tandon HLS, Messick DL (2007) Practical sulphur guide (revised). The Sulphur Institute, Washington, DC
Prasad R, Power JF (1997) Soil fertility management for sustainable agriculture. CRC-Lewis, Boca Raton
Hofgen R, Keft O, Willmitzer L, Hesse H (2001) Manipulation of thiol content in plants. Amino Acids 20(3):291–293
Ferala MP, Patrick WM (2014) Bacterial methionine biosynthesis. Microbiology 160(8):1571–1584
Dorrestein PC, Zhai H, McLafferly FW, Begley TP (2004) The biosynthesis of thiazole moiety of thiamine: the sulfur transfer mediated by the sulfur carrier protein. Chem Biol 11(10):1373–1381
Lawhoen BG, Mehl RA, Begley TP (2004) Biosynthesis of thiamine pyrimidine: the reconstitution of a remarkable arrangement reaction. Org Biomol Chem 2(17):2538–2546
Begley TP, Downs DM, Ealick SE, McLafferty FW, van Loon AP, Taylor S et al (1999) Thiamine biosynthesis in prokaryotes. Arch Microbiol 171(5):293–300
Tunc-Ozdemir M, Miller G, Song L, Sodek A, Kousseritzky S, Misra AN, Mittler R, Shintani D (2009) Thiamine confers enhanced tolerance to oxidation stress in Arabidopsis. Plant Physiol 151(1):421–432
Woodward JB, Abeydeara ND, Paul D, Phillips K, Rapala-Kozik M, Freeling M, Begley TP, Ealick SE, McSteen P, Scanlon MJ (2010) A maize thiamine autotroph is defective in short meristem maintenance. Plant Cell 22(10):3305–3317
Marquet A, Bui BT, Florentin D (2001) Biosynthesis of biotin and lipic acid. Vitam Horm 61:51–101
Chakravarthi S, Jesop CE, Bulleid NJ (2006) The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidation stress. EMBO Rep 7(3):271–275
Pompella A, Visvikis A, Paolicchi A, Tata V, Casinin AF (2003) The changing faces of glutathione—a cellular protagonist. Biochem Pharmacol 66(8):1499–1503
Cuoto N, Malys N, Gaskell S, Barber J (2013) Partition and turnover of glutathione reductase from Sacchromyces cerevisiae: a proteomic approach. J Proteome Res 12(6):2885–2894
Prasad R (2014) Major sulphur compounds in plants and their role in human nutrition and health. Proc Indian Natl Sci Acad 80(5):1045–1054
Prasad R, Tiwari KN, Biswas BC (2003) Students guide to fertilizers and their efficient use. Potash & Phosphate Institute of Canada-India Programme, Gurgaon
Tiwari KN, Gupta BR (2006) Sulphur for sustainable high yield agriculture in Uttar Pradesh. Indian J Fertil 1(11):37–52
Zhao FJ, Hawkesford MJ, McGarth SP (1999) Sulphur assimilation and effects on yield and quality in wheat. J Cereal Sci 30(1):1–14
Gupta RB, Khan K, MacRitchie F (1993) Biochemical basis of flour properties in bread wheat, I. Effect of variation in the quantitysize distribution of polymeric proteins. J Cereal Sci 18(1):23–41
Randall PJ, Spencer K, Freney JR (1981) Sulphur and nitrogen effects on wheat. I. Concentration of sulphur and nitrogen in grain in relation to the yield response. Aust J Agric Res 32:203–212
Unger C, Flatyen DN, Grant C, Lukow OM (2002) Impact of sulphur fertilization on spring wheat bread making quality. In: Proceedings of great plains soil fertility conference, Denver, CO, USA, 5–6 March, 2002, pp 107–120. www.ppi.org
Shivay YS, Prasad R, Pal M (2014) Effects of levels and sources of sulfur on yield, sulfur and nitrogen concentration and uptake and S-use efficiency in basmati rice. Commu Soil Sci Plant Anal 45(18):2468–2479
Gupta AK, C. Jain NK (2006) Delineating sulphur deficiency in soils and response studied in principal crop sequences of semi-arid eastern plains of Rajasthan. In: Proceedings of TSI/FAI/IFA symposium-cum-workshop on sulphur, New Delhi, 4–5 October, 2006, pp 91–115
Kumar S, Singh B, Rajput AL (2001) Response of Indian mustard to sources and levels of sulphur. Indian J Agron 46(3):528–532
Sen P, Hansda S, Roy S (2006) Sulphur in balanced fertilization in urdbean-mustard cropping system in soils of Murshidabad district of West Bengal. In: Proceeding of TSI/FAI/IFA symposium-cum-workshop on SULPHUR, New Delhi, 4–5 October 2006, pp 209–217
Reddy KS, Requeeba M. (2000) Sulphur in balanced fertilization in red soils of Tirupathi in Andhra Pradesh. In: Proceedings of TSI/FAI/IFA symposium-cum-workshop on sulphur, New Delhi, 4–5 October, 2006, pp 119–122
Aulakh MS, Pasricha NS (1997) Role of balanced fertilization in oilseed-based cropping system. Fertil News 42(4):101–111
Nimni ME, Han B, Corodoba F (2007) Are we getting enough sulfur in our diet. Nutr Metab (Lond) 4:24–32
Rose WC, Wixom RL (1955) The amino acid requirements of man. XIII. The sparing effect of cysteine on the methionine requirement. J Biol Chem 216:753–773
Calamia V, Ruiz-Romero C, Rocha B, Fernández-Puente P, Mateos J, Montell E, Vergés J, Blanco FJ (2010) Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther 12(4):R138. doi:10.1186/ar3077
Townsend DM, Tew KD, Tariero H (2004) Sulfur containing amino acids and human disease. Biomed Pharmacother 58(1):47–55
Dorge W, Bretkreutz R (2000) Glutathione and immune function. Proc Nutr Soc 59(4):595–600
Green JL, Mead KJ, Reynolds KM, Albert D (2013) Oral and intraveinous acetylcystein for treatment of acetaminophen toxicity: a systematic review and meta-analysis. West J Emerg Med 14(3):218–226
Tapel M, van der Giet M, Schwartzfeld C, Laufer U, Lierman D, Zidek W (2000) Prevention of radiographic-contrast-agent induced reductions in renal function by acetylcystein. N Engl J Med 343(2):180–184
Pool J, Black PN (2001) Oral mucolytic drugs foe exacerbations of chronic obstructive pulmonary disease: systematic review. Brit Med J 322(7297):1271–1274
Stey C, Bachman S, Medici TC, Tramer MR (2000) The effect of oral N-acetylcystein in chronic bronchitis—a quantitative systematic review. Eur Respir J 16(2):253–262
Bark M, Malhi GS, Gay LJ, Dean OM (2013) The promise of N-acetylcystein in neuropsychiatry. Trends Pharmacol Sci 34(3):166–173
Wood JM, Decker H, Chavan B, Rokos H, Spencer JD, Hess S, Thornton MJ, Paus R, Schallreuter KU (2009) H2O2 mediated stress affects human hair colour by blunting methionine sulfoxide repair. FASEB J 23(7):2065–2070
Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasarathi K, McCann PP (1996) S-adenosylmethionine and methylation. FASEB J 10(3):471–480
Kagan BL, Sultzer DL, Rosenlicht N, Gerner RM (1990) Oral S-adenosylmethionine depression: a randomized double blind placebo-controlled trial. Am J Psychiatry 147(5):591–595
Di Padova C (1987) S-adenosylmethionine and treatment of osteoarthritis—review of the clinical studies. Am J Med 83(5A):60–65
Miller RA, Buehrer G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M (2005) Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4(3):119–125
Richie JP Jr, Lentzinger Y, Parthasarthy S, Malloy V, Orentreich N, Zimmerman JA (2005) Methionine restriction increases blood glutathione and longevity in F334 rats. FASEB J 8(15):1302–1307
Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds) (2006) Modern nutrition in health and disease, 10th edn. Lippincott Williams, Baltimore
Krill JJ (1996) Neuropathy of thiamine deficiency disorders. Metab Brain Dis 11(1):9–17
Spinazzi M, Angelini C, Patrini C (2010) Sub-acute sensory ataxia and optic neuropathy with thiamine deficiency. Nat Rev Neuro 16(5):288–293
Acknowledgments
The authors are grateful to the director of the institute and head, Division of Agronomy, Indian Agricultural Research Institute, New Delhi, India, for providing the necessary facilities to write this review. Professor Rajendra Prasad is also grateful to the director, Indian Agricultural Research Institute, for awarding him an Adjunct Professor position.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Both the authors declare that they do not have any conflict of interests for the publication of this review in the Proceedings of the National Academy of Sciences, Biological Sciences.
Additional information
Disclaimer The observations made on nutritional supplements and other sulphur compounds are for the general information of the reader and before using these they are advised to consult the nutrition and medical experts.
Rights and permissions
About this article
Cite this article
Prasad, R., Shivay, Y.S. Sulphur in Soil, Plant and Human Nutrition. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 429–434 (2018). https://doi.org/10.1007/s40011-016-0769-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0769-0