Abstract
The present work was undertaken to study the effects of 24-epibrassinolide (24-EBL) on percent germination, growth (root length, shoot length, fresh weight and dry weight), lipid peroxidation, sodium and potassium ion concentrations, proline content, total osmolyte content, level of antioxidants (ascorbic acid, tocopherol and glutathione) in 7-day old seedlings of Raphanus sativus exposed to cadmium and mercury toxicity. Results of present study revealed that growth of seedlings was enhanced with the treatment of 24-EBL. In addition, 24-EBL was proved effective to overcome cadmium and mercury stress by altering the level of ions, osmoprotectants and antioxidants of plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal toxicity has become a crucial environmental concern due to their potential adverse ecological effects. Certain heavy metals are required for the cellular functions, so these are considered as essential metals. Their doses beyond tolerable limits cause the production of reactive oxygen species (ROS). Because of many human activities, their concentration is exceeding in the environment [1]. Among various heavy metals cadmium and mercury are considered as very toxic to plants and animals. Cadmium is present as free hydrated ions or complexed by organic or inorganic ligands in the soil solution. Plants exposed to high concentrations of cadmium inhibit photosynthesis, nutrient and water uptake. Cadmium toxicity causes visible symptoms of injury which appear in terms of chlorosis, inhibition in growth, browning of root tips and finally the death of the plants [2]. Mercury is toxic to plants at very low concentrations [3]. It is a unique metal because of its existence in various forms like HgS, Hg2+, Hg0 and methyl-Hg. Evidences have shown that Hg2+ form of mercury can easily accumulate in higher and aquatic plants [4]. Visible injuries and physiological disorders are induced by high level of Hg2+ in plants [5]. It destroys the photosystem II, which further triggers the blockage in oxygen evolution. Hg gives rise to the production of ROS in the cells and also affects the cell growth and cell division. Usually it disturbs the reactivity with sulphydryl group of proteins, competence of ATP binding and cell permeability. It leads to the alteration in glutathione metabolism, which has major role in metal homeostasis [6].
In response to various abiotic stresses, plants possess various strategies to combat them like exogenous application of plant hormones overcome their deleterious effects. Brassinosteroids (BRs) are natural polyhydroxy steroids that are ubiquitously distributed phytohormones [7]. They have major influence on vegetative and reproductive development, seed germination and stress protection [8]. BRs promote photosynthetic capacity, cell elongation and fission at cellular level [9, 10]. BRs also play significant role as a stress protectant hormone [11].
Raphanus sativus is considered to be a hyperaccumulator of heavy metals. This plant is having economic as well as medicinal values [12]. Anthropogenic pollution caused due to heavy metals lead to entry of these metals into food chain through plants grown in the polluted environment.
The objective of present study was to examine the stress protective role of 24-epibrassinolide on growth, level of ions, osmoprotectants, photosynthetic pigments and antioxidant defence system of R. sativus L. exposed to cadmium and mercury.
Material and Methods
Disease free seeds of R. sativus L. var. Pusa chetaki were procured from Punjab Agricultural University, Ludhiana, Punjab. Surface sterilized seeds were given treatment of 0, 10−11, 10−9, 10−7 M concentrations of 24-EBL for 8 h, which was purchased from Sigma Aldrich, Ltd., New Delhi. 25 seeds were germinated in Whatman No.1 filter paper lined glass petriplates containing the 0.25 mM Cd + 0.25 mM Hg metal solution. Cd and Hg were given in the form of cadmium chloride (CdCl2) and mercury acetate (C4H6HgO4) respectively. Each petriplate was supplied with 3 ml of test solution on 1 day and 2 ml of test solution on alternate days, up to 7 days. Control seedlings were supplied with distilled water only. The experiment was conducted under controlled conditions (25 ± 0.5 °C, 16 h photoperiod). Three replicates of each sample were taken for analysis. These seedlings were harvested on 7th day to study the following parameters:
Percentage Germination
Percentage germination was calculated by placing the values in the following formula:
Growth Parameters
Fifteen seedlings were taken for the analysis of growth parameters. Root length, shoot length, fresh weight and dry weight were determined on 7 days old seedlings of R. sativus L. The seedlings were then placed in oven maintained at 80 °C for 24 h for taking its dry weight.
Lipid Peroxidation
Malondialdehyde (MDA) content was estimated by following the method of Heath and Packer [13].
The harvested plants of R. sativus were homogenized in 1 % trichloroacetic acid and centrifuged at 13,000 rpm for 20 min. Supernatant was collected and heated with thiobarbituric acid for 30 min at 95 °C. The absorbance was taken spectrophotometrically at 532 and 600 nm.
Sodium (Na) and Potassium (K) Ion Concentration
Determination of sodium and potassium ion concentration was done by flame photometre. Standards of sodium and potassium were prepared by using salts of sodium chloride (NaCl) and potassium chloride (KCl) respectively. Standardization of flame emission photometer was done by running standards and calibration curve was prepared.
Proline and Total Osmolyte Content
Proline was estimated by Bates et al. [14] method. The plant samples were homogenized in 3 % sulfosalicylic acid and then centrifuged at 10,000 rpm for 10 min. 2 ml of ninhydrin was added with 2 ml glacial acetic acid into 2 ml of supernatant and incubation was given at boiling temperature for 1 h. Extraction of mixture was done with toluene and proline was analyzed spectrophotometrically at 520 nm. Total osmolyte content was analyzed by using vapour pressure osmometer (Vapro 5600).
Antioxidants
One gram of fresh plant tissue was homogenized in pre-chilled pestle and mortar using 3 ml of tris buffer (50 mM, pH 10.0). The crushed material was then subjected to centrifugation using Eltek cooling centrifuge for 20 min at 13,000 rpm at a temperature of 4 °C. The supernatant from seedling extract was collected for the further analysis of antioxidants.
Ascorbic Acid (Vitamin C) Content
Ascorbic acid content was determined by following the method of Roe and Kuether [15]. 100 mg charcoal was added in the mixture containing 4 ml of double distilled water, 0.5 ml of plant extract and 0.5 ml of 50 % trichloroacetic acid (TCA). It was mixed well and filtered with Whatmann filter paper 1 .0.4 ml of 2,4-dinitrophenylhydrazine (DNPH) was added to filterate and mixture was incubated at 37 °C for 3 h by chilling on ice bath. 1.6 ml of cold H2SO4 (65 %) was added to it and kept at room temperature for 30 min. Absorbance was taken at 520 nm.
Tocopherol (Vitamin E) Content
Vitamin E was estimated by the method given by Martinek [16]. 0.5 ml of plant extract was mixed with 0.5 ml of absolute ethanol and 0.5 ml double distilled water and mixture was shaken to obtain divided protein precipitates. 0.5 ml of xylene was added to it and centrifugation tubes were shaken vigorously for 30 s followed by centrifugation at 3,000 rpm for 10 min. 0.5 ml xylene was mixed with 0.5 ml of 2,4,6-tripyridyl-S-triazine (TPTZ) reagent and absorbance was taken at 600 nm.
Glutathione (GSH) Content
The glutathione content was determined by the method given by Sedlak and Lindsay [17]. For glutathione estimation, 100 μl of plant extract was added to 1 ml of tris buffer. To it, 50 μl of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and 4 ml of absolute methanol were added and mixture was kept at room temperature for 15 min for incubation followed by centrifugation at 3,000 rpm for 15 min. The absorbance of supernatant was read at 412 nm.
Statistical Analysis
Results were analyzed by using two way ANOVA. Data was presented as mean ± SE.
Results and Discussion
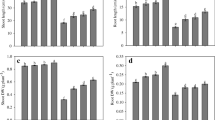
Percent germination enhanced with the treatment of EBL as compared to untreated control seedlings. Treatment of cadmium and mercury metals caused reduction in the percent germination (70 % ± 2.887) in comparison to control (75 % ± 0.001). Treatment of EBL alone enhanced the percent germination of seedlings. It was further significantly enhanced by 10−7 M EBL (83.33 % ± 1.667) along with metal in comparison to its control (70 % ± 2.887).
In the present study, root length and dry weight of 7-day old seedlings was enhanced due to treatment of EBL alone with respect to untreated control, whereas, shoot length and fresh weight were not significantly affected by EBL supplementation. Growth of radish was inhibited when subjected to metal stress (Table 1) in comparison to untreated control. Further, EBL supplementation enhanced the root length and shoot length (cm). 10−9 M EBL was the most effective concentration in improving the root length (2.97 ± 0.21) as compared to its respective control (2.1 ± 0.2). Similar trend was observed in shoot length, where 10−7 M EBL stimulated the length of shoot to the maximum (3.19 ± 0.17). Growth and development of plants are often affected by the different environmental challenges caused by biotic and abiotic stresses [18]. Heavy metal toxicity leads to adverse biological effects due to its non-biodegradable nature [19]. Heavy metals decrease soil fertility, induce toxicity in plants and infect the food chains, when accumulate in the soil [20]. In the present work, growth of radish seedlings was inhibited due to cadmium and mercury treatment which was improved by EBL supplementation. Sharma and Bhardwaj [21] reported that growth of mustard plant was enhanced under Cu stress, when seeds were given EBL treatment. It has been found that EBL blocked the metal uptake and accumulation in these plants. Radish seedings exposed to Cu toxicity also showed increased growth with EBL supplementation [22]. Ozdemir et al. [23] reported the similar results in barley seedlings, where BRs showed their stress ameliorative effects. Thus, treatment of EBL helped in promoting the growth in metal stressed seedlings in the present study.
Lipid peroxidation (µ mol g−1 FW) was significantly enhanced in the seedlings exposed to cadmium and mercury metals (13.97 ± 1.68) and treatment of EBL alone or in combination with metals reduced the MDA content (Table 2). Therefore, EBL supplementation helped in inhibiting the oxidative stress in terms of decreasing the lipid peroxidation.
Sodium and potassium ion concentration (ppm) was enhanced slightly in seedlings treated with EBL and decreased with metal treatment in comparison to untreated control. Further EBL supplementation enhanced the ion level in the metal stressed seedlings (Table 3). 10−9 M EBL was proved as most effective treatment in increasing the ion concentration in seedlings (5.83 ± 0.86 and 4.44 ± 0.43 respectively). Proline (µ mol g−1 FW) and total osmolyte content (m mol/Kg) was observed to increase with metal stress. EBL treatment alone or in combination with metals enhanced total osmolyte content, but proline content was not much affected (Table 2), however, 10−9 M EBL enhanced their content up to maximum (27.76 ± 2.74 and 183 ± 3.43). Lipid peroxidation was reduced by EBL along with accumulation of ions and certain osmoprotectants like proline or total osmolytes as they act as stress protectant [24] and scavenge the free radicals generated during the oxidative stress. Similar results were observed by Alaoui-Sosse et al. [25] in cucumber plant. Thus, in the present investigation EBL treatment helped in membrane stabilization, reduction of ion leakage and enhanced the level of osmoprotectants.
Decrease in antioxidant contents (mg/g FW) was observed in the seedlings treated with cadmium and mercury (Table 4). Supplementation of EBL alone enhanced ascorbic acid and tocopherol content, but glutathione content decreased. Seedlings subjected to metal stress showed reduction in antioxidant level. EBL when supplemented along with metal enhanced its level. 10−9 M EBL was found to be the most effective concentration for increasing the ascorbic acid and tocopherol level in the seedlings (3.07 ± 0.01 and 5.47 ± 0.14 respectively). However, glutathione content was enhanced to the maximum when 10−7 M EBL was supplemented along with the metals (5.53 ± 0.1). In the present study, level of antioxidant was altered by BRs under metal stress condition. These reports are in coherence with the findings of Pietrini et al. [26], in Phragmites australis under Cd metal stress. In the present work, there was an increase in glutathione, ascorbic acid and tocopherol content by the application of 24-EBL. The results are in coherence with the findings of Bajguz [27]. Similarly in R. sativus plants, Cr stress was mitigated with application of EBL by enhancing the level of antioxidants [28]. Bajguz [27] analyzed that BRs and its derivatives acted as a stimulator of phytochelatins under lead metal stress in Chlorella vulgaris which are derived from glutathione pool. Thus these antioxidants may help in detoxification of metal stress.
Conclusion
Cadmium and mercury toxicity retarded the growth and also caused alteration in the level of ions, osmoprotectants and antioxidants of radish seedlings, which is ameliorated by EBL supplementation. As 24-EBL stimulated the accumulation of antioxidants and osmoprotectants in plants, which further scavenged the free radicals generated during stressed conditions. They also helped in stabilization of membrane and prevented ion leakage and provided essential nutrients to plants necessary for their growth and other metabolic processes. Treatment of EBL also helped in inhibiting MDA content, which is the indicator of oxidative stress and thus overcome the oxidative stress generated by the heavy metals.
References
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr J Bot 76:167–179
Kovalchuk O, Titov V, Hohn B (2001) A sensitive transgenic plant system to detect toxic inorganic compounds in the environment. Nat Biotechnol 19(6):568–572
Chen J, Yang ZM (2012) Mercury toxicity, molecular response and tolerance in higher plants. Biometals 25(5):847–857
Israr M, Sahi S, Datta R, Sarkar D (2006) Bioaccumulation and physiological effects of mercury in Sesbania drummonii. Chemosphere 65:591–598
Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM (2007) Metabolic adaptations to mercury-induced oxidative stress roots of Medicago sativa L. J Inorg Biochem 101:1–9
Seth CS, Remans T, Keunen E, Jozefczak M, Gielen H, Opdenakker K, Weyens N, Vangronsveld J, Cuypers A (2012) Phytoextraction of toxic metals: a central role for glutathione. Plant Cell Environ 35:334–346
Kandelinskaya OL, Topunov AF, Grishchenko ER (2007) Biochemical Aspects of Growth-Stimulating Effects of Steroid Phytohormones on Lupine Plants. Appl Biochem Microbiol 43(3):324–331
Ryu H, Cho H, Kim K, Hwang I (2010) Phosphorylation dependent nucleocytoplasmic shuttling of BES1 is a key regulatory event in brassinosteroid signaling. Mol Cells 29(3):283–290
Khripach VA, Zhabinskii VN, De-Groot AE (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447
Yu JQ, Huang LF, Hu WH et al (2004) A role for brassinosteroids in regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143
Kartal G, Temel A, Arican E, Gozukirmizi N (2009) Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regul 58:261–267
Benzarti S, Mohri S, Ono Y (2008) Plant response to heavy metal toxicity: comparative study between the hyperaccumulator Thlaspi caerulescens (ecotype Ganges) and nonaccumulator plants: lettuce, radish and alfalfa. Environ Toxicol 23(5):607–616
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Bates LS, Waldren RP, Tear ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Roe JH, Kuether CA (1943) The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenyl hydrazine derivative of dehydroascorbic acid. J Biol Chem 147:399–407
Martinek RG (1964) Method for the determination of vitamin E (total tocopherols) in serum. Clin Chem 10:1078–1086
Sedlak J, Lindsay RH (1968) Estimation of total, proteinbound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Aldoobie NF, Beltagi MS (2013) Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. Afr J Biotechnol 12:4614–4622
Jaleel CA, Jayakumar K, Xing ZC, Azooz MM (2009) Antioxidant potentials protect Vigna radiata (L.) Wilczek plants from soil cobalt stress and improve growth and pigment composition. Plant Omics 2:120–126
Gaur N, Flora G, Yadav M, Tiwari A (2014) A review with recent advancements on bioremediation-based abolition of heavy metals. Environ Sci 16:180–193
Sharma P, Bhardwaj R (2007) Effects of 24-Epibrassinolide on growth and metal uptake in Brassica juncea L. under copper metal stress. Acta Physiol Plant 29:259–263
Choudhary SP, Bhardwaj R, Gupta BD, Dutt P, Gupta RK, Biondi S, Kanwar M (2010) Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiol Plant 140:280–296
Ozdemir F, Bor M, Demiral T, Turkan I (2004) Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42:203–211
Sharma SS, Dietz KJ (2008) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–49
Alaoui-Sosse B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Pietrini F, Iannelli MA, Pasqualini S, Massacci A (2003) Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol 133:829–837
Bajguz A (2000) Blockage of heavy metal accumulation in Chlorella vulgaris cells by 24-epibrassinolide. Plant Physiol Biochem 38:797–801
Choudhary SP, Kanwar M, Bhardwaj R, Yu JQ, Tran LP (2012) Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 7:e33210
Acknowledgments
Authors are also thankful to Department of Botanical and Environmental Sciences, Guru Nanak Dev University- Amritsar (India) for providing laboratory facilities for this work.
Conflict of interest
Authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapoor, D., Rattan, A., Gautam, V. et al. Alleviation of Cadmium and Mercury Stress by Supplementation of Steroid Hormone to Raphanus sativus Seedlings. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 86, 661–666 (2016). https://doi.org/10.1007/s40011-015-0501-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0501-5