Abstract
The present study aims to assess the physico-chemical parameters (pH, electrical conductivity, carbonate, bicarbonate, chloride, sulphate, nitrate, fluoride, calcium, magnesium, sodium, potassium, iron and total hardness) of the underground water in tehsil Bishnah, district Jammu, Jammu and Kashmir, India. The water samples were collected from tube wells and hand pumps in 26 chosen sites during the pre-monsoon period (May, June 2013). The results were compared with standard values of drinking water prescribed by IS: 10500, BIS and WHO. It was observed that certain parameters like electrical conductivity, bicarbonate, nitrate, total hardness, calcium, magnesium, sodium and iron as determined from the groundwater samples of tehsil Bishnah were above the limits set by IS: 10500, WHO and BIS at certain places. To analyse the data with statistical point of view the statistical parameters like mean, range, standard deviation, coefficient of variation, correlation coefficient, kurtosis, skewness were systematically calculated for each parameter. Also, single factor Anova tables, Piper diagram and Schoeller graph were prepared to signify the major results. Also, an account has been prepared to analyze the factors like sum of anions (meq/L), sum of cations (meq/L), calculated TDS (mg/L), dissolved minerals (mg/L) like halite (NaCl), sylvite (KCl), carbonate (CaCO3), dolomite (CaMg(CO3)2), anhydrite (CaSO4), permanent hardness, temporary hardness and alkalinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water plays a fundamental role in human life. Water supplies available for drinking and other domestic purposes must hold high degree of cleanliness. It should be free from chemical contamination and microorganisms [1–3]. Although groundwater is thought to be relatively clean and free from pollution than the surface water, however, the scientific research conducted globally during the last couple of decades has established that the ground water is also getting polluted radically because of increased human activities [4, 5]. Consequently, a number of cases of water borne diseases have been witnessed from time to time leading to health hazards [6, 7]. Therefore, basic monitoring on water quality is essential to observe the demand and pollution level of ground water.
For the present study, various samples of groundwater were collected from the different locations of tehsil Bishnah, dist. Jammu, Jammu and Kashmir, India and analysed for their physico-chemical parameters. The results were compared with drinking water specifications of WHO [8].
Material and Methods
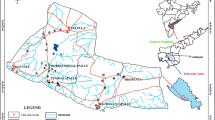
Study Area (Fig. 1)
District Jammu is located between 74°24′–75°18′ east longitude and 32°50′–33°30′ north latitude. Administratively the district has been divided into four tehsils, viz. Jammu, R. S. Pura, Akhnoor and Bishnah. Bishnah consist of 39 villages and 39 panchayats. Its coordinates are 32°37′0″N and 74°52′0″E in DMS (Degrees Minutes Seconds). It is located at an elevation of 294 m above sea level and its population amounts to 11,645.
Geography
In the area under investigation, the climate is typically monsoonal, though the region sufficiently harvests average 40–50 mm (1.6–2 in.) of rain per month between January and March. In the summer season, Bishnah is very hot and can reach up to 40 °C (104 °F) whilst in July and August, very heavy though erratic rainfall occurs with monthly extremes of up to 650 mm (25.5 in.). In September, rainfall declines, and by October conditions are hot but extremely dry, with minimal rainfall and temperature of around 29 °C (84 °F).
Sample Collection
The ground water samples were collected from tube wells and hand pumps from 26 chosen sites during the pre-monsoon period (May, June 2013).
Preparation of Water Samples
The samples were collected in precleaned, sterilized polyethylene bottles of one litre capacity without any air bubble as per standard procedure. Each sample bottle was clearly labelled and relevant details were recorded. The samples were kept in refrigerator, maintained at 4 °C and were analyzed within 12–24 h after collection.
Physico-chemical Analysis
Physico-chemical analysis was carried out for various water quality parameters such as pH, electrical conductivity (EC), carbonate (CO3 2−), bicarbonate (HCO3 −), chloride (Cl−), sulphate (SO4 2−), nitrate (NO3 −), fluoride (F−), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), iron (Fe), total hardness (TH) as per standard methods [5] (Table 1).
High purity certified analytical grade reagents, double distilled de-ionized water and borosil glassware were used.
Statistical Analysis
To analyse the data with statistical point of view the statistical parameters like mean, range, standard deviation, coefficient of variation, correlation coefficient, kurtosis, skewness were systematically calculated for each parameter.
The standard formulae used in the calculation for statistical analysis are as follows:-
where x is the value of observation, n the no. of observations
where x is the value of parameter, n the no. of observations
Correlation coefficient
where x, y are values of array 1 and array 2 respectively, n is no. of observations.
Also, Single factor Anova tables, Piper diagram and Schoeller graph were prepared to signify the major results.
An account has been prepared to analyze the factors like sum of anions (meq/L), sum of cations (meq/L), calculated TDS (mg/L), dissolved minerals (mg/L) like halite (NaCl), sylvite (KCl), carbonate (CaCO3), dolomite (CaMg(CO3)2), anhydrite (CaSO4), permanent hardness, temporary hardness and alkalinity.
Dissolved minerals for the present water samples were calculated using the software.
Results and Discussion
The experimental values for all the physico-chemical parameters are presented in Table 2 and were compared with standards as prescribed by different agencies [4, 8]. The interpretation of data has been made with the help of statistical tools.
pH
pH serves as an index to denote the extent of pollution by acidic and alkaline waste and represents hydrogen ion activity in water. It affects equilibrium between most chemical species in water. The permissible range for pH is 6.5–8.5 [4] for potable drinking water. pH of the underground water tested (in 90 % samples) is in the range of 7.0–8.45 which is within the permissible limit.
Electrical Conductivity (EC)
It is a measure of the ability of an aqueous solution to carry an electric current. It depends on the presence of ions, on their total concentration, mobility and temperature of measurement. Higher value of conductivity shows higher concentration of dissolved ions [8]. Conductivity of water sample was found in the range of 470–1630 micromhos/cm2, which is quite high in some areas (Allah, Chak Chimna, Chak Jawahar Singh, Chak Ramdas, Doal, Fatehgarh, Karel Manhasan, Kathar, Khairi, Khojipur, Kotli Charkan, Pandhori Brahmna and Upper Kanhal) as compared to the WHO standards (0–800). Electrical conductivity is considered to be a rapid and good indicator of dissolved solids.
Carbonate (CO3 2−) and Bicarbonate (HCO3 −)
The stoichiometric or chemical equilibrium equation between bicarbonate and carbonate is \( {\text{HCO}}_{3}^{ - } = {\text{CO}}_{3}^{2 - } + {\text{H}}^{ + } \). Because bicarbonate and carbonate are on opposite sides of the equilibrium equation, they are not often detected in the same groundwater sample. However, they can occur simultaneously at defined temperature, pressure, and hydrogen ion concentrations (pH).
The carbonate concentration is zero at all places except at Doal, Joian and Khairi. Bicarbonate ranges from 134 to 775 mg/L in 98 % of the samples studied. The acceptable limit of carbonate (CO3 2−) and bicarbonate HCO3 − is 75 mg/L and 30–400 mg/L, respectively. The presence of high concentrations of HCO3 − can cause nutritional disturbances, such as reducing the availability of calcium and the uptake of iron.
Chloride (Cl−)
Chloride is an anion found in variable amount in groundwater. Chloride may be present naturally in groundwater and may also originate from diverse sources such as weathering and leaching of sedimentary rocks. The maximum permissible limit of chloride in potable water is 250 mg/L. In the analyzed water samples, the concentration of chloride varied from 7.1 to 82 mg/L which is well within the permissible limit.
Sulphate (SO4 2−)
Aquifers with a naturally occurring sulphate source, such as gypsum, have very high concentrations of sulphate as compared to other aquifers. Sulphate in groundwater may enter through weathering of sulphide bearing deposits. The acceptable limit of sulphate is 200 mg/L. The sulphate content in all the analyzed water samples varied from 0 to 125 mg/L.
Nitrate (NO3 −)
Natural nitrate levels in groundwater are generally very low (typically less than 10 mg/L), but nitrate concentrations grow due to human activities, such as agriculture, industry, domestic effluents and emissions from combustion engines.
Nitrates generally move relatively slow in soil and groundwater: there is a time lag of approximately 20 years between the pollution activity and the detection of the pollutant in groundwater. For this reason, it is predicted that current polluting activities will continue to affect nitrate concentrations for several decades. However, if the pressure in the aquifer is high, transport can be very rapid within the saturation zone [9].
The nitrate content in the study area varied in the range 0–45 mg/L which is higher than prescribed limit of 40 mg/L at selected places like Karel Manhasan.
Fluoride (F−)
Small concentration of fluoride in drinking water has beneficial effect on human health for preventing dental caries. Higher concentration of fluoride than that of 1.5 mg/L carries an increased risk of dental fluorosis and even higher concentration could lead to skeletal fluorosis [10].
Fluoride content of 95 % of the groundwater samples of the study area ranges from 0.0 to 0.93 mg/L which is well within the permissible limit.
Total Hardness (TH)
When water passes through or deposits over limestone the levels of Ca2+, Mg2+ and HCO3 − ions present in the water can greatly increase and cause the water to be classified as hard water. High levels of hard-water ions can cause scaly deposits in plumbing, appliances, and boilers. These two ions also combine chemically with soap molecules, resulting in decreased cleansing action. In the present study the total hardness values ranged from 205 to 585 mg/L. The moderately hard water has the range of 60–120 mg/L.
The high values of total hardness have an effect on the soil nature like lack of aeration and permeability of earth surface and it may indirectly affect the growth of plants and the general health of human society.
Calcium (Ca2+)
Calcium salts and calcium ions occur most commonly in nature. They may result from the leaching of soil and other natural sources or may come from man-made sources such as sewage and some industrial wastes. Calcium is usually one of the most important contributors to hardness. Human body requires approximately 0.7–2.0 g of calcium per day as a food element; excessive amounts can lead to the formation of kidney or gallbladder stones [11]. The desirable limit of calcium is 75 mg/L while in the present samples it ranged from 46 to 158 mg/L. Thus it was found higher than the desirable limit at many places like Arnia, Chak Chimna, Chak Jawahar Singh, Chak Ramdas, Fatehgarh, Joian, Karel Manhasan, Kathar, Kotli Charkan, Laswara, Marchopur, Pachel, Pandhori Brahmna and Upper Kanhal.
Magnesium (Mg2+)
The magnesium content in the study area varied in the range from 15 to 57 mg/L while the range prescribed by WHO is 30 mg/L as desirable limit to 100 mg/L as permissible limit in the absence of alternate source.
A major hypothesis that has emerged from studies in recent years is that magnesium, which together with calcium is the main determinant of water hardness, protects against death from ischemic heart disease [18]. There are a number of facts to support the hypothesis that magnesium deficiency can induce artery spasm, as has been shown in animal experiments [12, 13].
Epidemiological studies in the United States [14], Canada [15, 16], South Africa [17], Finland [18, 19], and Sweden [20] have shown an inverse correlation between magnesium in drinking water and mortality from ischemic heart disease.
It has been suggested that the incidence of sudden death from ischemic heart disease is higher when water magnesium level is lower, owing to an increased tendency to vasoconstriction [13, 14] or arrhythmias [17, 21–24].
Though it has also been suggested that at very high concentrations, magnesium salts have a laxative effect particularly when present as magnesium sulphate [25].
Sodium (Na+)
The sodium content of all groundwater samples of the study area ranged from 5.6 to 130 mg/L while the permissible limit is 50 mg/L as prescribed by BIS. At room temperature, the average permissible limit of sodium is about 200 mg/L according to WHO. Sodium is a principal chemical in body fluids, and it is not considered harmful at normal levels of intake from combined food and drinking water sources. However, increased intake of sodium in drinking water may be problematic for people with hypertension, heart disease or kidney problems that require them to follow a low sodium diet besides it also affects the palatability of water [26]. Higher content of sodium in irrigation water may cause salinity problems.
Potassium (K+)
Potassium, an important fertilizer, is strongly held by clay particles in soil. Therefore, leaching of potassium through the soil profile into ground water is important only on coarse-textured soils. Potassium is common in many rocks. Many of these rocks are relatively soluble and potassium concentrations in ground water increase with time [27].
Potassium is weakly hazardous in water, but it does spread rapidly, because of its relatively high mobility and low transformation potential.
Vital functions of potassium include its role in nerve stimulus, muscle contractions, blood pressure regulation and protein dissolution. It protects the heart and arteries, and may even prevent cardiovascular disease. The intake of a number of potassium compounds may be particularly harmful at high doses [28].
In the present samples the range of potassium varied in between 0.8 and 166 mg/L.
Iron (Fe)
Iron in rural groundwater supplies is a common problem: its concentration level ranges from 0 to 50 mg/L, while WHO recommended level is <0.3 mg/L. Iron and manganese occur naturally in water, especially groundwater. Neither of the elements causes adverse health effects; they are, in fact, essential to the human diet. However, water containing excessive amounts of iron and manganese can stain clothes, discolour plumbing fixtures, and sometimes add a “rusty” taste and look to the water. In the present samples the concentration ranges from 0 to 5.32 mg/L which is higher to the desirable limit at certain places.
Long term consumption of drinking water with high concentration of iron may lead to liver diseases [29].
Statistical Analysis
The statistical results related to mean, standard deviation and coefficient of variation are given in Table 3, Anova tests are depicted in Table 4 and correlation coefficient is shown in Table 5.
An account has been prepared (Table 6) to analyze the factors like sum of anions (meq/L), sum of cations (meq/L) and ion balance.
Also, the major ionic species obtained from the physico-chemical analysis are projected graphically on the modified Trilinear Piper diagram (Fig 2). The water-types interpreted from Trilinear Piper diagrams are summarized in Table 2.
Figure 3 represents the groundwater types in the Schoeller diagram. The Schoeller diagram [30] is used to study the comparative changes in the concentrations and ratios of water quality parameters for different samples.
Figure 4 represents the variation in different parameters at 26 stations.
Conclusion
In the present study it was observed that some physico-chemical parameters like electrical conductivity, bicarbonate, nitrate, total hardness, calcium, magnesium, sodium and iron as determined from the groundwater samples of tehsil Bishnah were above the limits set by IS: 10500, WHO and BIS at certain places. The people residing in these areas are therefore at high impending threat of contracting ailments/diseases related to higher level of such contaminants present in water.
In conclusion, it is essential to apply tough preventive measures in the area concerned to save groundwater from further deterioration and regular supervision and assessment of the groundwater should be done periodically. The present paper forms a part of a major project, the studies undertaken during the post-monsoon period reveal that a better water quality was found in the post-monsoon season than that of pre-monsoon season, because of water recharging due to rains.
Since, agriculture is the main source of economy of people in the area under study, it is evident that except for water lost through evapotranspiration, agricultural water is recycled back to surface water and/or groundwater. However, agriculture is both source and victim of water pollution. Therefore, appropriate steps must be taken to ensure that agricultural activities do not adversely affect the water quality in the area so that subsequent use of water for different purposes is not impaired.
References
Food and Agriculture Organization (FAO) (1997) Chemical analysis manual for food and water, 5th edn. FAO, ROME, 1, pp 20–26
Lamikanra A (1999) Essential microbiology for students and practitioners of pharmacy, medicine and microbiology, 2nd edn. Amkra books, Lagos, p 406
Omezuruike OI, Damilola AO, Adeola OT, Fajobi Enobong A, Shittu BO (2008) Microbiological and physico-chemical analysis of different water samples used for domestic purposes in Abeokuta and Ojota, Lagos State, Nigeria. Afr J Biotechnol 7(5):617–621
ISI (1983) Indian standard specification for drinking water, IS 10500. ISI, New Delhi
APHA (1998) Standard methods for the examination of water and waste water. American Public Health Association, Washington
WHO (1984) Guidelines for drinking water quality: recommendations, vol 1. WHO, Geneva
Jain CK, Bhatia KKS, Vijay T (1995) Ground water quality monitoring and evaluation in and around Kakinada, Andhra Pradesh, technical report, CS (AR) 172, National Institute of Hydrology, Roorkee, 1994–1995
Wightman WE, Jalinoos F, Sirles P, Hanna K (2003) Application of geophysical methods to highway related problems. Federal Highway Administration, Central Federal Lands Highway Division, Lakewood, Publication No. FHWA-IF-04-021
Spalding RF, Exner ME (1993) Occurrence of nitrate in groundwater—a review. J Environ Qual 22:392–402
Vyas HV, Sawant VA (2008) Seasonal variations in drinking water quality of some bore well waters in urban area of Kolhapur city. Nat Environ Pollut Technol 7(2):261–266
Stringer MD, Soloway RD, Taylor DR, Riyad K, Toogood G (2007) Calcium carbonate gallstones in children. J Pediatr Surg 42(10):1677–1682
Rubenowitz E, Gösta A, Ragnar R (1996) Magnesium in drinking water and death from acute myocardial infarction. Am J Epidemiol 143(5):456–462
Altura BM, Altura BT, Carella A (1981) Hypomagnesemia and vasoconstriction: possible relationship to etiology of sudden death ischemic heart disease and hypertensive vascular disease. Artery 9:212–231
Turlapaty PDMV, Altura BM (1980) Magnesium deficiency produced spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science 208:198–200
Schroeder HA (1960) Relationship between mortality from cardiovascular disease and treated water supplies. Variations in states and 163 largest municipalities of the United States. J Am Med Assoc 172:1902–1908
Anderson TW, Le Riche WH (1971) Sudden death from ischemic heart disease in Ontario and its correlation with water hardness and other factors. Can Med Assoc J 105:155–160
Allen HAJ (1972) An investigation of water hardness, calcium and magnesium in relation to mortality in Ontario. Ph.D. thesis, University of Waterloo, Ontario
Leary WP, Reyes AJ, Lockett CJ (1983) Magnesium and deaths ascribed to ischaemic heart disease in South Africa. S Afr Med J 64:775–776
Punsar S, Karvonen MJ (1979) Drinking water quality and sudden death: observations from West and East Finland. Cardiology 64:24–34
Luoma H, Aromaa A, Helminen S (1983) Risk of myocardial infarction in Finnish men in relation to fluoride, magnesium and calcium concentration in drinking water. Acta Med Scand 213:171–176
Rylander R, Bonevik H, Rubenowitz E (1991) Magnesium and calcium in drinking water and cardiovascular mortality. Scand J Work Environ Health 17:91–94
Sjögren A, Edvinsson L, Fallgren B (1989) Magnesium deficiency in coronary artery disease and cardiac arrhythmias. J Int Med 226:213–222
Seelig MS, Heggtveit HA (1974) Magnesium interrelationships in ischemic heart disease: a review. Am J Clin Nutr 27:59–79
Anderson TW (1977) Water hardness, magnesium and ischemic heart disease. N S Med Bull 56:58–61
Karunakaran K, Thamilarasu P, Sharmi R (2009) Statistical study on physico-chemical characteristics of groundwater in and around Namakkal, Tamil Nadu, India. E-J Chem 6(3):909–914
Chandetrik R, Sharma A (2011) Assessment of drinking water quality: a case study of Ambala cantonment area, Haryana, India. Int J Environ Sci 2(2):933–945
Ground Water Monitoring and Assessment Program (1999) Environmental outcomes division. In: Sodium and potassium in Minnesota’s ground water. Minnesota Pollution Control Agency, Groundwater Monitoring and Assessment Program
IOM (2004) Dietary reference intakes for water, potassium, sodium, chloride, and sulphate. Prepared by the Institute of Medicine. National Academies Press, Washington
Gyamfi ET, Ackah M, Amin AK, Hsnson JK, Kpattah L, Enti-Brown S, Adjei-Kyereme Y, Nyarko ES (2012) Chemical analysis of potable water samples from selected suburbs of Accra, Ghana. Proc Int Acad Ecol Environ Sci 2(2):118–127
Schoeller H (1965) Geochemistry of ground water. An international guide for research and practice, UNESCO, 15, pp 1–18
Acknowledgments
The author is grateful to the Jammu and Kashmir State Council for Science and Technology, Department of Science and Technology, Jammu and Kashmir for providing the financial assistance for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khanna, P. Physico-chemical Parameters of Groundwater of Bishnah, District Jammu, Jammu and Kashmir, India. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 85, 121–130 (2015). https://doi.org/10.1007/s40011-014-0345-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-014-0345-4