Abstract
In this study the flash pyrolysis of the cotton shell on the electrically heated fluidized bed has been conducted for producing cotton shell pyrolytic oil and with the presence of homogenous acid catalyst the cotton shell pyrolytic oil is converted into biofuel. Cotton shell was procured from resources available in India. The various physical properties such as density, viscosity, flash point and elemental analysis have been done for the bio oil. For the baseline data analysis, the same characteristics study has been carried out on the biofuel and an acceptable agreement is observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the world energy crisis in 1970s, energy from the biomass has been utilized. Due to the lower amount of sulfur and nitrogen contents in the biomass wastes, its energy utilization also creates less environmental pollution and health risk than other kind of fossil fuels. The energy recovery from a biomass involves chemical, biochemical and thermo chemical processes, depending on the nature of the source. Pyrolysis is thermal degradation either in the complete absence of oxidizing agent or with such a limited supply that gasification is also limited. Char, gas and liquid are obtained from the pyrolysis techniques [1]. The pyrolysis techniques seem to be a promising thermochemical conversion technology for the liquid oil production. The bio oil is obtained by the condensable gases. The carbon char from the process is used as a high grade carbon powder for industry applications [2]. Conventional pyrolysis, fast pyrolysis and flash pyrolysis are the subclasses of pyrolysis process. Slow pyrolysis processes are performed at a low heating rate and at long residence time. The longer residence times of the biomass cause secondary cracking of the primary products reducing the yield. In addition, a low heating rate and long residence time increases energy input. At present, the preferred technology for production of oily products is fast or flash pyrolysis at high temperatures with a very short residence time [3].
The fluidized bed technology is one of the efficient and economic methods of actuating flash pyrolysis of lignocellulosic biomass, as that provides rapid heat transfer, better control and suitable vapour residence time [4]. Wood remains the largest biomass source used for millennia for meeting numerous human needs including energy. In earlier days, the pyrolysis of wood wastes in a fixed bed reactor was performed for the pyrolysis oil production [5]. In the event of continuous development of a pyrolysis of lignocellulosic biomass, tree stumps, wood chips, shells, municipal wastes are pyrolysed. In this series the flash pyrolysis of cotton shell is carried out being the largest source of biomass for agricultural residues in India. Gossypium arboretum commonly called tree cotton is a species of cotton native to India. In India, the cotton crop occupies more than 9.2 million hectares and its share in the world total cotton area is 27 %. The country is divided into three main cotton growing zones, the northern G. hirsutum and G. arboreum zone in the States of Punjab, Rajasthan and Haryana, accounting for about 1.9 million hectares, the major central G. hirsutum, G. arboreum and G. herbaceum zone in the States of Gujarat, Madhya Pradesh and Maharashtra with 5.4 million hectares, the composite southern G. hirsutum, G. arboreum, G. herbaceum and G. barbadense zone in the states of Andhra Pradesh, Karnataka and Tamil Nadu accounting for about 1.8 million hectares. India’s cotton production is about 15.8 million bales. The seeds are removed from cotton and that is used to prepare highly viscous cotton seed oil which is used directly for energy purpose. But after elimination of cotton, and seeds, the shells are burned in open atmosphere due to its low bulk density, causing environmental pollution. So proper recycling is needed. The pyrolysis oil from biomass waste is found to be highly oxygenated compound and chemically unstable. Thus, the liquid products still need to be upgraded by lowering the oxygen content and removing residues [6].
Transesterification is the common process to upgrade the raw bio oil with an alcohol in the presence of suitable catalyst such as acid or alkaline catalysts [7]. Due to the low cost and better properties methanol is used for the transesterification process [8]. Potassium hydroxide is the effective alkaline catalyst in the transesterification process [9]. Nearly 85–90 % is the cost of raw materials [10, 11]. Therefore, production of biofuel from the low cost of feedstock is the possible solution. Most of the researches have been conducted on biofuel production [9] by using coconut, rapeseed, palm and tung oils. In the pyrolysis oil is upgraded as biofuel. Figure 1 shows the process flow chart of preparation of upgraded biofuel from cotton shell.
In the present investigation, cotton shell is pyrolyzed at the temperatures of 350, 400, 450, 500 and 550 °C in an electrically heated fluidized bed pyrolysis conditions.
2 Methods
2.1 Selection of Pyrolysis Process Parameters
The yield products of the pyrolysis reaction are affected by the feedstock particle size, process temperature, feeding rate and sweep gas flow rate. Effect of particle size on the slow pyrolysis of rapeseed has been investigated in a Heinze retort in the range of 0.224–1.8 mm and for palm shell the particle size varied from 0.5 to 2 mm [12], In our study all the experiments are conducted at the particle size of 1 mm for the yield of maximum bio oil. The increase in the particle size enhances the solid yield in the process due to the larger temperature gradient inside the particles. Particle size plays an important role in the yield of char, oil and gas on the pyrolysis of bagasse and rice husk [13, 14]. The moisture content of the process feedstock affects the efficiency of the pyrolysis reaction and hence the quality of the bio-oil product. If it is too high, the moisture content in the bio-oil is also high, it decreases the caloric value of the oil. Therefore the moisture content in the feedstock should be minimum [3]. The nitrogen flow rate directly affects the residence time of the evolved gases produced from the pyrolysis reactions and minimizes the secondary decomposition of higher molecular weight products. However, it has been observed that the yield of the bio oil decreases when the nitrogen flow rate exceeds. This elevates the heat transfer rate and increased production of volatiles. At the same time, the vapor residence time is shortened leading to lower probability of the secondary tar cracking reaction. The suitable flow rate is 1.75 m3/h for higher yield. Pyrolysis temperature, greatly affects the type and amount of the reaction products; at a relatively low temperature, say below 350 °C, the main products are char, whereas at higher temperature exceeding 700 °C, most of the reaction products are non-condensable gases. So the suitable temperature for the pyrolysis process is in the range of 400–600 °C [15].
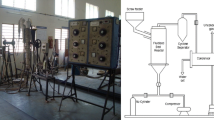
2.2 Experimental Reactor Setup
The devolatilization fraction samples are prepared from the flash pyrolysis of the cotton shell in an electrically heated fluidized—bed reactor. Pyrolysis experiments were carried out under nitrogen atmosphere at the temperatures of 350, 400, 450, 500 and 550 °C. The pyrolysis experiments conducted in the reactor is made up of stainless steel tube of internal diameter 50 mm. The reactor is filled with sand of particle size of 0.71 mm to a height of 30 cm supported with the perforated base for enabling fluidization of the cotton shell powder. The reactor is heated using 2 kW electrical heater with ammeter and voltmeter setup to measure the power input and is controlled by an autotransformer and temperature cutoff unit. The temperatures are measured with the help of thermocouple located at five different points along the reactor, and is well insulated with mineral wool and Chromel–Alumel. The screw feeder unit consisting of hopper is attached to a variable speed motor to vary the feed rate. The cotton shell particles are kept in the hopper and are fed into the reactor through screw feeder. Fluidization is first done by air till the reactor reaches the uniform temperature and then in inert atmosphere using nitrogen gas. The flow rate of the nitrogen was measured with the help of a rotameter. A distributor valve was utilized to separate the flow of air and nitrogen. The fluidizing gas velocity was approximately maintained two times greater than the minimum fluidization velocity of 0.11 m/s and the flow rate was maintained to 1.75 m3/h. The density of the oil is determined by weighing the known volume and its viscosity is measured using Brookfield LV-DV-II Pro viscometer, Middleboro, USA at a constant temperature. Pensky Martens closed cup apparatus is used to determine the flash point of the oil. The component analysis of the sample and oil is performed by Elementar Vario EL-III, Germany with He as a carrier gas with 0.05 % accuracy. The calorific value of the oil is determined using Parr-6772 calorimetric thermometer.
The emerging gas from the reaction on the fluidized bed pyrolyser first passes through the cyclone separator where the particulate matters are dropped and thereby preventing char from reaching the condensing unit. The condenser is a shell and tube type heat exchanger. The vapors and the gases are passed through a water cooled condenser to a series of ice cooled collectors maintained at 5 °C to trap the derived liquid oil. The char and other particulate matters are collected in a cyclone separator and weighed. The liquid phase condensed in the condenser is weighed. The gaseous phase is then calculated by the remaining material balance.
Higher heating values (HHV) of the biomass in this study were obtained using the equation [12].
where C, H, O are the percentages on dry basis of carbon, hydrogen and oxygen, respectively.
The proximate and ultimate analysis of the cotton shell biomass are given in Table 1. The biomass samples contain 62 % volatile matter and 25 % fixed carbon. The fractions of sulfur and nitrogen are very low and is environment-friendly than the conventional fossil fuel.
2.3 Bio Oil Upgradation
Generally without any upgrading the pyrolysis bio oil used as a fuel oil for combustion in a boiler directly. But the physical and chemical properties adversely affect the combustion properties. Commercial transesterification reactions are conducted with alkaline catalysts. It has been observed that sodium methoxide is more effective than sodium hydroxide. Sodium alkoxides are among the most efficient catalysts used for this purpose. However, due to its low cost, NaOH has been used in large-scale transesterification. The process is carried out in a 1000 ml round flask, equipped with a thermometer and magnetic stirring systems. For transesterification process 500 ml of cotton shell pyrolytic oil is heated up to 70 °C in a round flask to remove moisture and stirred strongly. The catalyst NaOH of 2.5 g is dissolved in methanol (99 % pure) in bi molar ratio, in a separate vessel and is continuously poured into the flask while stirring the mixture at 60 °C for 60 min at normal atmospheric pressure. The mixture is allowed to settle under gravity for 48 h in a separating funnel. However, it takes longer settling time for the separation because of a low temperature, lack of stirring and presence of low amounts of catalyst and methanol [16]. The products yields during this transesterification process are higher value biofuel and glycerin. Glycerin, excess alcohol, catalyst, impurities and other unreacted oil settle under the bottom layer. Biofuel, alcohol and some soap are formed at the upper layer of the mixture. 80–85 % pure glycerin is obtained by the evaporation of water and alcohol. For best economy and pollution prevention, the alcohol is fully recycled. Glycerol is an economical co-product that should be fully refined. The unreacted alcohol is removed by water wash with hot distilled water. Finally the remaining mixture is allowed to settle under gravity for 24 h. The separated biofuel is taken for characterization.
3 Results and Discussion
3.1 Effect of Temperature on Pyrolysis Product and Yield of Bio Oil
The product distributions from pyrolysis of cotton shell at the temperatures of 350, 400, 450, 500 and 550 °C are listed in Table 2. The product distributions of biomass material changed with increasing the temperature. By increasing the pyrolysis temperature from 400 to 450 °C, the bio-oil yield increased and the bio-char yield decreased. The results show that the liquid yield increases up to 51 wt% at 450 °C. At 350 °C the maximum bio oil yield is only 29.7 wt% due to incomplete decomposition of the biomass whereas the char yield is 35.1 wt%. When the temperature exceeds 550 °C the liquid and char yield decrease but it increases the gas yield. The secondary reaction of the liquid fraction of the volatile matter causes the decomposition of the char particles at higher temperature. In most cases, lower temperatures favor char yields due to the incomplete decomposition of the biomass. With increasing the pyrolysis temperature, both liquid and gaseous yields increase.
3.2 Characterization of Bio Oil
The bio oil derived from the flash pyrolysis of cotton shell has higher oxygenated compound, whereas the oxygen content is 50 %. The calorific value of the pyrolytic oil is as low as 19.3 MJ/kg and is lower compared with the fossil fuels. This is because of the moisture content and oxygenated compounds present in the biomass material. Thus we get the raw bio oil as a low grade fuel directly which is upgraded to high quality liquid fuel. The viscosity of the oil is found as 7.87 cSt at 40 °C. The flash point of the oil is 160 °C which indicates that it can be stored safely at room temperature. The density of the oil is 1005 kg/m3 and it has pH value of 3.3. The ash content of the oil is less than 0.1 %
Table 3 lists the physical and elemental analysis of the pyrolytic oil derived from the cotton shell.
3.3 Yield of Biofuel
In our experiment the maximum biofuel yield is about 85 % at a process temperature of 60 °C, 60 min and at 6:1 ratio of methoxide and oil. Transesterification is the process of exchanging the organic group R of an ester with the organic group of an alcohol, and takes more time and higher temperature for this replacement. At higher process time the yield of biofuel decreases. By increasing the time we do not get higher yield, which means no more argonic group R is exchanged at these conditions [17].
3.4 Biofuel Characterization
The elemental analysis of the biofuel is reported in Table 4. Physical properties of the upgraded biofuel including its density, viscosity, flash point are investigated. The density and kinematic viscosity of the biodiesel are 960 kg/m3 and 4.28 cSt at 30 °C. High viscosity of vegetable oil is a major problem in using it as fuel for diesel engines. Flash point and fire point specify the safety during transport, storage and handling. The flash point of the biofuel is 125 °C which is higher than the diesel fuel. The flash point may be reduced and it can be used as an alternative fuel by blending with diesel fuels in an internal combustion engines. Table 5 lists the physical properties of the upgraded biofuel. The oxygen content of the bio oil reduces from 50 to 31 % during upgradation and the hydrogen fraction increased from 11.24 to 11.71 %. The biofuel has the higher heating value of 28.8 MJ/kg.
4 Conclusion
The cotton shell pyrolytic oil has been obtained from the flash pyrolysis of cotton shell in an electrically heated fluidized bed reactor in nitrogen atmosphere. In this study the pyrolysis temperature is found to be the significant factor to determine the pyrolysis products. The gas yield increases as the pyrolysis temperature increases. The raw pyrolysis oil is a bio fuel and it has been upgraded to high quality liquid fuel by transesterification process. The result also shows that alkaline catalyzed transesterification for upgradation of bio oil may be obtained in large scale. The viscosity and density of the biofuel is comparable to petro-diesel and flash point indicates that it can be stored safely at room temperature.
References
Onay O, Kockar OM (2003) Slow fast and flash pyrolysis of rapeseed. Renew Energy 28:2417–2433
Park HJ, Park Y-K, Kim JS (2008) Influence of reaction condition and the char separation system on the production of bio-oil from radiata pine sawdust by fast pyrolysis. Fuel Process Technol 89:797–802
Madhu P, Kanagasabapathy H, Neethi MI (2014) Cotton shell utilization as a source of biomass energy for bio-oil by flash pyrolysis on electrically heated fluidized bed reactor. J Mater Cycles Waste Manag. doi:10.1007/s10163-014-0318-y
Lv PM, Xiong ZH, Chang J, Wu CZ, Chen Y, Zhu JX (2004) An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour Technol 95:95–101
Girods P, Rogaume Y, Dufour A, Rogaume C, Zoulalian A (2008) Low-temperature pyrolysis of wood waste containing urea-formaldehyde resin. Renew Energy 33:648–654
Tsai WT, Lee MK, Chang YM (2007) Fast pyrolysis of rice husk: product yields and compositions. Bioresour Technol 98:22–28
Bouaid A, Martinez M, Aracil J (2009) Production of biodiesel from bioethanol and Brassica carinata oil: oxidation stability study. Bioresour Technol 100:2234–2239
Berrios M, Gutiérrez MC, Martín MA, Martín A (2009) Application of the factorial design of experiments to biodiesel production from lard. Fuel Process Technol 90:1447–1451
Fangrui M, Milford AH (1999) Biodiesel production: a review. Bioresour Technol 70:1–15
Demirbas A (2009) Political, economic and environmental impacts of biodiesels: a review. Appl Energy 86:108–117
Lin L, Dong Y, Sumpun C, Saritporn V (2009) Biodiesel production from crude rice bran oil and properties as fuel. Appl Energy 86:681–688
Abnisa F, Wan Daud WMA, Husin WNW, Sahu JN (2011) Utilization possibilities of palm shell as a source of biomass energy in Malaysia by producing bio-oil in pyrolysis process. Biomass Bioenergy 35:1863–1872
Madhu P, Neethi MI, Kanagasabapathy H (2014) Parametric analysis of cotton shell and Palmyra Palm Fruit Bunch for bio oil in fixed bed pyrolysis system. Int J Appl Environ Sci 9(5):2427–2436
Natarajan E, Ganapathy SE (2009) Pyrolysis of rice husk in a fixed bed reactor. World Acad Sci Eng Technol 56:504–508
Park Y-K, Yoo ML, Lee HW, Park SH, Jung S-C, Park S-S, Kim S-C (2012) Effects of operation conditions on pyrolysis characteristics of agricultural residues. Renew Energy 42:125–130
Leung DYC (2006) Guo Y (2006) Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process Technol 87:883–890
Eman NA, Cadence IT (2013) Characterization of biodiesel production from palm oil via base catalyzed transesterification. Proc Eng 53:7–12
Acknowledgments
The authors thank the School of Mechanical Sciences, Karunya University, Coimbatore, Tamilnadu for their support that made this work possible by providing their facilities and Ms. G. Periyanayagi of Ayya Nadar Janaki Ammal College, Sivakasi, Tamilnadu for her extended support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhu, P., Neethi Manickam, I. & Kanagasabapathy, H. Production and Upgradation of Cotton Shell Pyrolytic Oil for Biofuel from Flash Pyrolysis by Fluidized Bed Reactor. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 85, 457–462 (2015). https://doi.org/10.1007/s40010-015-0220-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-015-0220-6