Abstract
A genetic linkage map of Brassica carinata (BBCC; 2n = 34) has been constructed with 23 RAPD, 29 ISSR and 17 SSR markers using an F2 hybrid population of 150 individuals. The mapping population was developed by selfing single F1 plant following hybridization between a resynthesized B. carinata genotype, Ar29 and natural B. carinata cv. PC5. The segregation of each marker and linkage analysis was performed using the program MapMaker v3.0. The 69 mapped markers were aligned into 17 major linkage groups and a subgroup 5A at LOD value of 3.0. The total length of the map was 2,166 cM, with an average marker interval of 31.39 cM. To the best of our knowledge, this is the first report on genetic linkage mapping of B. carinata and the map developed in the present study will be highly useful for anchoring of more loci in future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethiopian mustard (Brassica carinata A. Braun) is an amphidiploid species (BBCC; 2n = 34), believed to have originated in Ethiopian plateau from interspecific hybridization between two diploid progenitor species Brassica nigra (BB; 2n = 16) and Brassica oleracea (CC; 2n = 18) [1]. Current work on the crop suggests that it has a great potential as an oilseed crop for many regions, especially those with semi-arid or mediterranean climates, and can withstand wide range of biotic stresses, more effectively than conventional Brassica oilseed crops [2–4]. Wide scale adoption of the crop has, however, been hampered by its late maturity, poor harvest index, low oil content and unattractive grain colour, besides poor oil and meal quality [5]. In this sense, it is imperative to exploit the primary gene pool available in the country of its origin or introgress desirable alleles from related Brassica digenomics for the improvement of this crop species. However, exploitation of these genetic resources, through conventional plant breeding so far has not been very encouraging and only limited selection advances for yield and component traits have yet been achieved [6]. Under these circumstances, DNA markers-based genetic linkage maps would provide great potential for increasing the speed and precision of cultivar improvement programmes of B. carinata.
The members of the Triangle of U species [7] have been subjected to extensive genetic mapping using molecular markers to identify loci associated with various qualitative and quantitative traits of agronomic interest [8–15]. However, most studies have focused on more economically important species and due importance has not been given to B. carinata. To our knowledge, there is no previous report in the literature on the genetic linkage mapping or development of molecular markers specific to B. carinata. Keeping this in mind, the present study was aimed at developing a genetic linkage map of B. carinata using random amplification of polymorphic DNA (RAPD) and inter-simple sequence repeat (ISSR) markers. Moreover, microsatellite or simple sequence repeat (SSR) markers already available in different Brassica species were also evaluated for their cross-transferability to B. carinata and subsequently integrated to the genetic linkage map. It is expected, the linkage map developed in the study will allow better genetic analysis of target agronomic traits through localization of major genes and QTLs and their utilization in crop improvement programmes through marker assisted selection (MAS). Besides, it will serve as a skeleton to be used for anchoring of more loci in future investigations.
Materials and Methods
Plant Material and DNA Isolation
A set of 150 randomly selected F2 individuals derived from a single F1 plant of a cross between a resynthesized B. carinata genotype, Ar29 and natural B. carinata cv. PC5, was used as a mapping population for developing the linkage map. DNAs from the parents, F1 and F2 plants, were isolated from fresh young expanded leaves following Doyle and Doyle [16].
DNA Marker Analysis
Forty-eight decamer RAPD primers obtained from Operon Technologies (Alameda, CA, USA), 100 ISSR primers chosen from the #9 ISSR primer kit (801-900) of the Biotechnology Laboratory, University of British Columbia (Vancouver, Canada) and 359 SSR primers derived from Brassica rapa, B. oleracea, B. nigra and B. napus, whose nucleotide sequences information are publicly available in the Brassica microsatellite information exchange database (www.brassica.info/resourse/markers/ssr-exchange.php), were used for detection of polymorphism between resynthesized B. carinata genotype, Ar29 and natural B. carinata cv. PC5. PCR amplifications of the genomic DNA with different primers were carried out using an MJ Research Thermo-cycler PTC 200.
RAPD and ISSR analyses were performed on the basis of protocols devised by Williams et al. [17] and Zietkiewiez et al. [18], respectively. PCR amplifications of ISSR loci were performed in a 20 μl reaction volume containing approximately 40 ng template DNA, 0.5 mM of a single primer, 200 μM each of dNTPs and 1 U of Taq DNA polymerase (MBI Fermentas, Vilnius, Lithuania) in 1× PCR buffer, and 2 mM MgCl2. For RAPD, the conditions were the same as those of ISSR except that 25 ng of template DNA was utilized. The PCR cycling conditions for ISSR analysis was 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 2 min, and a final extension of 10 min at 72 °C, while it was 94 °C for 5 min followed by 40 cycles of 94 °C for 1 min, 37 °C for 1 min, 72 °C for 2 min, and a final extension of 10 min at 72 °C for RAPD.
SSR amplifications were performed in 20 μl reactions consisting of 2.25 mM MgCl2, 250 μM of each dNTP, 10 pmol of each primer, 1× PCR buffer, 1 U Taq DNA Polymerase, and approximately 50 ng DNA. The PCR cycling conditions for SSR analysis was 94 °C for 5 min for the initial denaturation of the template DNA, followed by 35 cycles of 94 °C for 45 s, annealing temperatures of 52.5–66 °C for 30 s, and 72 °C for 2 min, and a final extension of 10 min at 72 °C.
The RAPD and ISSR amplification products were size separated by standard horizontal electrophoresis on 1.5 % agarose gels whereas, SSR-amplified products were resolved on 3.0 % Super Fine Resolution (SFR) agarose gels, at 70 V for 3 h in 1× Tris–borate-EDTA (TBE) buffer and stained with ethidium bromide (0.5 μg/ml) for 30 min. The gels were viewed and photographed in gel documentation and image analysis system (Syngene, Synoptics Group, Cambridge, UK).
Segregation Analysis and Map Construction
RAPD and ISSR banding patterns were scored from gel photographs for presence (1) and absence (0) of bands assuming that each band represents a unique genetic locus. However, the genotype of loci with SSR markers was scored as A (homozygous for allele present in resynthesized B. carinata genotype, Ar29), B (homozygous for allele present in B. carinata cv. PC5), and H (heterozygous for both the alleles). Deviation from the expected Mendelian segregation ratio of 3:1 for RAPD and ISSR, and 1:2:1 for SSR markers were tested using χ 2 analysis at 5 % level of significance with degree of freedom, 1 and 2, respectively.
Linkage analysis of polymorphic marker loci was performed using the program MapMaker v3.0 [19]. A minimum LOD threshold of 3.0 was used to group all the markers into potential linkage groups (LGs). Two-point, three-point and multi-point analyses were used in order to determine the best order of marker loci within the LGs. Three-point and multipoint analyses were used to find the most likely locus orders within each LG. The final linkage orders were determined with ‘ripple’ command [20]. In case of more than one possible arrangement of LGs; the one with smallest genetic linkage distance between adjacent marker loci of the LGs was chosen to construct the genetic map. The Kosambi mapping function was used to convert recombination frequencies into map distance [21]. Linkage map was drawn using the computer software ‘MAPCHART’ version 2.1 [22].
Results and Discussion
Primer Selection
Parental polymorphism survey was performed using 507 different markers comprising of 48 RAPD, 100 ISSR and 359 SSRs. Poor level of polymorphism (13.3 %) was apparent for SSR and 45.0 % for ISSR markers. However, RAPD showed relatively higher polymorphism (62.5 %) as reported earlier [23]. Finally, only 140 markers comprising of 35 RAPD, 57 ISSR and 48 SSR markers were found to be polymorphic and utilized for genotyping the mapping population. Apparent lack of polymorphism for SSR markers have been reported in the past for related Brassica species [24]. However, there is no previous reference indicating the level of polymorphism with ISSR markers.
Extensive distortion of allele transmission ratios was observed for all marker types. A total of 7 (20 %) RAPD and 11 (19.3) ISSR markers showed significant deviations from expected Mendelian segregation ratio of 3:1, and out of them 10 markers showed segregation biasness towards B. carinata cv. PC5. Furthermore, 11 (22.9 %) SSR markers also showed segregation distortion from expected Mendelian segregation ratio of 1:2:1 and all the 11 loci showed segregation biasness towards B. carinata cv. PC5 (Table 1). Such a high frequency of segregation distortion could be attributed to the fact that one of the parents, B. carinata Ar29, was resynthesized and may still be suffering from a degree of genomic instability. Distortions in segregation ratios are known to increase with the level of divergence of the parents and have been attributed to differentiation of parental chromosomes [25]. Moreover, high level of segregation distortion could also be indicative of chromosome rearrangements such as translocations, as observed earlier [26]. Relatively higher polymorphism observed for RAPD markers and lower segregation distortion associated with them underlined the usefulness of RAPD markers for B. carinata map construction. In the present study, RAPDs constitute an abundant source of markers loci dispersed randomly across BC genomes of B. carinata.
Construction of Linkage Map
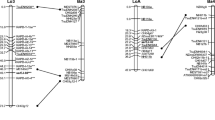
A total of 140 RAPD, ISSR and SSR markers, which were found to be polymorphic among the resynthesized B. carinata genotype, Ar29 and natural B. carinata cv. PC5, were used for the construction of the linkage map. However, MapMaker allowed the assembly of only 69 marker loci amplified using 16 RAPD, 24 ISSR and 17 SSR primers (Table 2) into 17 major LGs and one subgroup of LG5 (Fig. 1). A very large number of markers (71) were left stranded out of the 17 LGs constructed. The number of major LGs obtained in the study is in agreement with the haploid chromosome number (n = 17) of B. carinata. The total map distance was 2,166 cM with an average distance of 31.39 cM between marker loci. Although, it compared poorly with the map lengths recorded in B. napus (2,429 cM) and B. juncea (1,629 cM) by more elaborate studies of Lombart and Delourme [26] and Pradhan et al. [27] with average marker interval of 3–4 cM, the map present in the present study can serve as a framework map and will be highly useful for anchoring of more loci in future investigations.
Linkage map of B. carinata. Map distances in cM are indicated in left side and locus names are on the right side of LGs. Loci marked in asterisk deviated significantly from Mendelian ratio (1:3 and 1:2:1) at 5 % level of significance. A and P indicate that Ar29 or PC5 genotypes predominated, respectively
The present study reveals a non-random distribution of the assigned markers for some of the LGs. It is an indication of non-random sampling of the genome, by an uneven recombination rate along the LGs [28] or by a clustering tendency of some markers due to their preferential targeting of specific genome regions. The size and number of markers, density of markers of each LGs are summarized in Table 3.
The size of LGs varied substantially between 19.0 cM (LG10) to 512.9 cM (LG5). It was possible to align 21 RAPD, 27 ISSR 14 SSR markers to 17 different LGs. Besides, some markers clustered to develop another subgroup and appeared separately as LG5A within LG5 due to unavailability of linked markers that could fill the large gap. The length of the subgroup LG5A was 540.3 cM having 7 markers comprised of 2 RAPD, 2 ISSR and 3 SSR markers. The map reported in the study represents the first skeleton map of B. carinata for anchoring of more loci in further investigations. The F2 mapping population generated in the study and the genetic linkage map obtained will be highly useful to locate genes controlling several traits of agronomic interest and enrich the genetic map of B. carinata with more markers.
References
Prakash S, Hinata K (1980) Taxonomy, cytogenetics and origin of crop Brassicas, a review. Opera Bot 55:1–57
Knowles PF, Kearney TE, Cohen DB (1981) Species of rapeseed and mustard as oil crops in California. In: Pryde EH (ed) New sources of fats and oils. American Oil Chemical Society, Champaign, pp 255–268
Fereres E, Fernández-Martínez J, Minguez I, Domínguez J (1983) Productivity of B. juncea and B. carinata in relation to rapeseed. In: Proceedings of the 6th International Rapeseed Conference, 17–19 May 1983, GCIRC, Paris, pp 293–298
Warwick SI, Francis A, Al-Shehbaz IA (2006) Brassicaceae: species checklist and database on CD-Rom. Plant Syst Evol 259:249–258
Velasco L, Fernandez-Martinez JM, Haro-A de, De-Haro A (1997) Selection for high sum of oil and protein in Ethiopian mustard (Brassica carinata). Crucif Newsl 19:97–98
Sheikh FA, Banga S, Banga SS, Najeeb S (2011) Development of Ethiopian mustard (Brassica carinata) with broad genetic base through interspecific hybridization with elite lines of Brassica napus and Brassica juncea. JABSD 3:77–84
UN (1935) Genomic analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot 7:389–452
Snowdon RJ, Friedt W (2004) Molecular markers in Brassica oilseed breeding: current status and future possibilities. Plant Breed 123:1–8
Mahmood T, Rahman MH, Stringam GR, Yeh F, Good AG (2006) Identification of quantitative trait loci (QTL) for oil and protein contents and their relationships with other seed quality traits in Brassica juncea. Theor Appl Genet 113:1211–1220
Qiu D, Morgan C, Shi J, Long Y, Liu J, Li R, Zhuang X, Wang Y, Tan X, Dietrich E, Weihmann T, Everett C, Vanstraelen S, Beckett P, Fraser F, Trick M, Barnes S, Wilmer J, Schmidt R, Li J, Li D, Meng J, Bancroft I (2006) A comparative linkage map of oilseed rape and its use for QTL analysis of seed oil and erucic acid content. Theor Appl Genet 114:67–80
Chen W, Zhang Y, Liu X, Chen B, Tu J, Tingdong F (2007) Detection of QTL for six yield-related traits in oilseed rape (Brassica napus) using DH and immortalized F(2) populations. Theor Appl Genet 115:849–858
Fu FY, Liu LZ, Chai YR, Chen L, Yang T, Jin MY, Ma AF, Yan XY, Zhang ZS, Li JN (2007) Localization of QTLs for seed colour using recombinant inbred lines of Brassica napus in different environments. Genome 50:840–854
Okazaki K, Sakamoto K, Kikuchi R, Saito A, Togashi E, Kuginuki Y, Matsumoto S, Hirai M (2007) Mapping and characterization of FLC homologs and QTL analysis of flowering time in Brassica oleracea. Theor Appl Genet 114:595–608
Ramchiary N, Padmaja KL, Sharma S, Gupta V, Sodhi YS, Mukhopadhyay A, Arumugam N, Pental D, Pradhan AK (2007) Mapping of yield influencing QTL in Brassica juncea: implications for breeding of a major oilseed crop of dryland areas. Theor Appl Genet 115:807–817
Soengas P, Hand P, Vicente JG, Pole JM, Pink DA (2007) Identification of quantitative trait loci for resistance to Xanthomonas campestris pv. campestris in Brassica rapa. Theor Appl Genet 114:637–645
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissues. Focus 12:13–15
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Zietkiewiez E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Lander E, Lincoln S, Daly M (1993) Constructing genetic linkage maps with MAPMAKER/EXP version 3.0, Whitehead Institute for Biomedical Research; technical report, 3rd edn. Whitehead, Cambridge
Lincoln P, Mitchell J, Scedrov A, Shankar N (1992) Decision problems for propositional linear logic. Ann Pure Appl Logic 56:239–311
Kosambi DD (1944) The estimation of map distance from recombination value. Ann Eugen 12:172–175
Voorrips RE (2002) Mapchart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Jain A, Bhatia S, Banga SS, Prakash S, Lakshmikumaran M (1994) Potential use of random amplified polymorphic DNA (RAPD) technique to study the genetic diversity in Indian mustard (Brassica juncea) and its relationship to heterosis. Theor Appl Genet 88:116–122
Snowdon RJ (2007) Cytogenetics and genome analysis in Brassica crops. Chromosome Res 15:85–95
Kianian SF, Quiros CF (1992) Generation of a Brassica oleracea composite RFLP map: linkage arrangements among various populations and evolutionary implications. Theor Appl Genet 84:544–554
Lombard V, Delourme R (2001) A consensus linkage map for rapeseed (Brassica napus L.) construction and integration of three individual maps from DH populations. Theor Appl Genet 103:491–507
Pradhan AK, Gupta V, Mukhopadhyay A, Arumugam N, Sodhi YS, Pental D (2003) A high-density linkage map in Brassica juncea (Indian mustard) using AFLP and RFLP markers. Theor Appl Genet 106:607–614
Tanksley SD, Ganal MW, Prince JP, deVicente MC, Bonierbale MW, Broun P, Fulton TM, Giovannoni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Paterson AH, Pineda O, Roder MS, Wing RA, Wu W, Young ND (1992) High density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
Acknowledgments
Priyamedha sincerely acknowledges the fellowship support (JRF) from the Indian Council of agricultural Research (ICAR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Priyamedha, Singh, B.K., Kaur, G. et al. RAPD, ISSR and SSR Based Integrated Linkage Map From an F2 Hybrid Population of Resynthesized and Natural Brassica carinata . Natl. Acad. Sci. Lett. 35, 303–308 (2012). https://doi.org/10.1007/s40009-012-0057-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40009-012-0057-3