Abstract
Albumin has been viewed as one of the most useful and versatile carrier proteins in pharmaceutical and clinical fields. Albumin is biocompatible and non-toxic, and so can be used as a pharmaceutical carrier more safely versus many synthetic polymers. Importantly, albumin has great ability to extend circulating half-lives of short-lived peptides and protein drugs when they are properly linked because albumin is hardly filtered in the glomerulus due to its large size (~66.4 kDa). Albumin is also an excellent material to construct nanoparticles because it has good physicochemical stability, targetability, and chemical functionality. In the first part of this review, three major methods for half-life extension of peptide/protein drugs using endogenous or exogenous albumin are described: physical non-covalent binding, covalent binding, and albumin-fusion. The second part details the most intensively utilized methods for nanoparticle preparation: desolvation, nanoparticle albumin bound (Nab™) technology, and self-assembly. The review provides in-depth understanding for albumin-based drugs and their nano-delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Albumin has expanded its pharmaceutical role as a protein carrier to include delivery systems for a wide spectrum of drugs including chemicals, peptides/proteins or genes (Bosse et al. 2005; Mendez et al. 2005; Son et al. 2013). Until the early years of this century, the clinical use of albumin was confined to the emergency maintenance of blood pressure as a blood substitute or the treatment of shock, burns, and hypoalbuminemia (Son et al. 2013), either derived from human blood or produced from yeast cells by DNA recombination (Kratz 2008; Mendez et al. 2005). However, recently, the utility of albumin as a drug carrier is surpassed its traditional pharmaceutical or medical uses. Albumin can be a carrier for short-lived drugs (including contrast agents or imaging probes) to extend their circulating life-time, such as in an albumin-drug complex, albumin-drug conjugate, or albumin fusion with a drug (usually protein). Albumin can also be a building/targeting material for nano-delivery systems comprising nanoparticles (NPs) or nano-complexes to incorporate and to carry drugs to the proper disease sites. Furthermore, albumin is very safe and well-tolerated in humans due to its endogenous origin, which has accelerated its pharmaceutical usefulness. In this review, the basic features of albumin and the utility of albumin for in vivo half-life protraction are briefly summarized, and the use of albumin as a NP carrier is discussed in depth.
Basic aspects of albumin
Human serum albumin (HSA) is an endogenous non-glycosylated protein with a molecular weight of ~66.4 kDa. It is the most abundant protein, comprising ~55 % of all plasma proteins (Kratz 2008; Sleep 2015). Albumin consists of 585 natural amino acids. It is synthesized in the liver and is normally present an average serum concentration of ~40 mg/ml (Sleep 2015). Albumin is vital physiologically. It plays a pivotal role in solubilizing hydrophobic long-chain fatty acids in the body, and binds to a variety of therapeutic drugs as well as endogenous substances including bilirubin, thyroxin, prostagladin and metal ions (Elsadek and Kratz 2012). Furthermore, it contributes to maintaining the oncotic pressure and plasma pH in the body, and can be dissimilated into its constituent amino acids as a nutrient source.
Physicochemical advantages of albumin
Pharmaceutically, albumin is very robust. While usually present at pH 7.4 in the body, albumin is quite stable over a wide pH range of 4–9, which enables various chemical modifications. Also, it is stable upon heating at 60 °C for up to 10 h and remains soluble even after exposure to denaturing solvents like 40 % ethanol with negligible formation of precipitates or aggregates; these thermal and physical properties are advantageous in the preparation of micro- or nano-systems (Kratz 2008). Owing to its endogenous natural property, human serum albumin is considered safe and well-tolerated in humans. Indeed, it is biodegradable, biocompatible, non-toxicity, and non-immunogenic (Chuang et al. 2002).
The chemical functionality of albumin is one of the significant advantages in improving its pharmaceutical usefulness. Human serum albumin has 59 amine residues (58 lysine and one N-terminus) and 99 carboxylate residues (39 aspartic acid and 60 glutamic acid). About 26 amines of albumin are surface exposed (Singh et al. 2004). Its amino and carboxylic groups can be modified using N-hydroxysuccinimide (NHS)-activated derivatives or 1-ethyl-3 3-dimethylaminopropyl carbodiimide (EDC). Furthermore, albumin has a free sulfhydryl group at Cys34, although it is less reactive because it is positioned in a 10–12 Ǻ deep pocket within the molecule (Kratz 2008; Sleep 2015), which makes possible further conjugation with a maleimide-modified group (Kim et al. 2003; Léger et al. 2004). Additionally, the artificial sulfhydryl groups can be modified to the surface amines of albumin using 2-iminthiolane (Traut’s reagent) to covalently connect with other pharmacological cores, receptor ligands, or functional moieties (Byeon et al. 2014; Kim et al. 2010, 2015).
Inherent target ability of albumin at disease sites
Albumin has prominent merits in targeting disease sites that include tumors and inflamed tissues. First, albumin accumulates in malignant tissues due to leaky capillaries (pore size 100–1200 nm) and defective lymphatic drainage system (Hobbs et al. 1998; Kratz 2008). In general, albumin (66.4 kDa) has a maximum tumor uptake that is comparable to immunoglobulin (~150 kDa), although the tumor uptake of macromolecules is proportional to their molecular size in the range of 12–150 kDa (Kratz 2008; Maeda et al. 2000). Moreover, NPs made of albumin are more efficiently trapped in tumors by passive targeting via the enhanced permeability and permeation (EPR) effect (Elsadek and Kratz 2012; Kratz 2008; Maeda et al. 2000).
Interestingly, 60 kDa glycoproteins (gp60, albondin) are highly expressed in the surface of endothelial cells around tumor tissues. Albumin or an albumin-based carrier binds to gp60, forms calveolaes of albumin-gp60 complex, and undergoes transcytosis to tumor interstitium; this is termed the gp60-mediated active transcytosis pathway. The albumin-gp60 complex can be facilely disassembled by SPARC (secreted protein, acidic and rich in cysteine), a 43 kDa secreted matricellular glycoprotein that has great affinity to albumin. Consequently, the accumulation of albumin or albumin carrier can be promoted by this significant active targeting process as well (Podhajcer et al. 2008; Sage et al. 1984).
Second, albumin markedly accumulates in inflamed sites of rheumatoid arthritis (Ballantyne et al. 1971; Wilkinson et al. 1965). Albumin consumption at the inflamed tissues is significantly higher than in normal tissues because the inflamed synovial cells require more energy, which is supplied by albumin. Moreover, the permeability of albumin in inflamed joints is remarkably increased, so patients with rheumatoid arthritis show obvious enhanced hypoalbuminemia (Ballantyne et al. 1971; Kratz 2008; Levick 1981). Accordingly, albumin carriers can be efficiently targeted in inflamed tissues.
Pharmaceutical carrier use of albumin
Extension of the in vivo circulating half-lives of short-lived drugs
Human serum albumin has two significant non-covalent binding sites (binding site I and II) available for many exogenous drug substances that include benzodiazepines, antibiotics, and anti-inflammatory drugs. When bound on the surface of albumin, which has an average circulating half-life of ~19 days, the circulating life-time of these drugs is increased, prolonging their therapeutic efficacy (Kratz 2008). Albumin is very stable and hardly metabolized in blood due to its negligible immunogenicity and near absence of filtering in the glomerulus (pore size: 5–6 nm) due to its large size. This physical or chemical association with albumin provides the opportunity to improve the poor pharmacokinetic profiles of many drugs. However, this non-covalent albumin binding is only limited to hydrophobic drugs, and hydrophilic chemical drugs or peptide/protein drugs are much less available to bind circulating albumin molecules, so that they cannot retain in blood circulation in a long time. Therefore, researchers have sought to induce the artificial association with albumin; the half-life extension effect was particularly clear for small peptides.
The first approach exploits the ability of albumin to bind to several linear fatty acids including myristic, palmitic, and stearic acid. The covalent conjugation of hydrophilic peptides or proteins with such fatty acids is considered to be a good approach because the conjugated fatty acids would help tightly dock at the hydrophobic pockets of albumin molecules. A long-acting formulation of the des(B30; threonine) insulin acylated with myristic acid (C14) at the B29 lysine was developed by Novo Nordisk and approved by the United States Food and Drug Administration (FDA) in 2004; it is commercially marketed as Levemir® (NN304, insulin determer) (Kratz 2008; Kurtzhals et al. 1997; Markussen et al. 1996). Also, a glucagon-like peptide-1 (GLP-1; 7-37) analog with covalently attached palmitic acid (C16) at the lysine 26 residue via a γ-l-glutamyl spacer received FDA approval in 2009 (Victoza®: NN2211, Liraglutide; Novo Nordisk) (Kratz 2008; Juhl et al. 2002; Sleep 2015). These fatty acid-conjugated anti-diabetic peptides display greatly improved hypoglycemic efficacy (both levels of blood glucose and glycated hemoglobin, HbA1c) in rodents and humans with Type 1 or 2 diabetes (Juhl et al. 2002; Kurtzhals et al. 1997; Markussen et al. 1996). Similarly, fatty acid-derivatives or bile acid-derivatives of exendin-4 peptide were proven to have remarkable anti-diabetic efficacy in type 2 diabetic db/db mice (Chae et al. 2010; Kim et al. 2011a; Lee et al. 2012a, b).
The second approach involves the direct covalent conjugation with circulating endogenous albumin in situ or recombinantly produced exogenous albumin. ConjuChem developed a new prototype of a synthetic GLP-1(7-36) that is substituted with D-Ala8 and that is additionally attached by a 6-maleimideo derivative at the C-terminal lysine 37 residue (CJC-1131). Similarly, a synthetic exendin-4 peptide-conjugated recombinant albumin (CJC-1134-PC) using the performed conjugate-drug affinity complex (PC-DAC™) displays a significantly improved circulating life-time (~1 week) without inherent biological activity loss (Baggio et al. 2008; Sleep 2015). These novel anti-diabetic peptide-albumin conjugates have the potential to usher in a new anti-diabetes therapy, compared with conventional insulin therapy. In an especially interesting approach, artificial sulfhydryl moieties were introduced to human serum albumin by using 2-iminothiolane for further covalent conjugation to antidiabetic exendin-4 peptide and an apoptotic TRAIL (tumor necrosis factor (TNF)-related apoptosis inducing ligand) protein via a bifunctional polyethylene glycol (PEG): maleimide/NHS-activated PEG. These albumin-conjugates exhibited greatly protracted anti-diabetic or anti-rheumatic efficacy, respectively. Additionally, the albumin-TRAIL conjugate considerably accumulates in inflamed sites of an arthritis animal model.
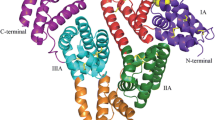
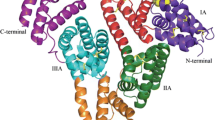
The third strategy is associated with an albumin fusion with therapeutic peptides or proteins using yeast or mammalian cells based on genetic engineering techniques (Chuang et al. 2002; Sleep 2015). This technique has been successfully applied to various peptides or proteins, such as glucagon-like peptode-1 (GLP-1), granulocyte colony stimulating factor (G-CSF), interferon α-2b (albinterferon alpha-2b) (Subramanian et al. 2007), and human growth hormone (Albutropin™), by Human Genome Sciences, Inc. Similarly, these albumin fusion proteins overcome the pharmacokinetic shortcomings of a short life and low therapeutically active peptide or protein. Albiglutide is a novel drug comprising GLP-1 dimer fused to the N-terminus of HSA. The drug was recently developed by GlaxoSmithKline and was approved by the US FDA in 2013 (Tanzeum®). The drug has a circulating half-life of 138–163 h (Fig. 1 and Table 1).
Albumin as a key building material for nanoparticle preparation
Nanoparticulate systems made of HSA have gained great interest in clinical targeted therapy. Thus far, natural or synthetic linear polymers, such as chitosan, hyaluronic acid, polyethylene glycol, poly(d,l-lactic-co-glycolic) acid, and pluronic acid have been restrictively used in the preparation of NPs for human use. Safety concerns remains and extra receptor ligands should be incorporated into these polymers for active targeting. In contrast, the use of albumin has all possible practical advantages of safety, chemical functionality, and targetablity. Hence the use of albumin as a building material is considered to be one of the most effective way of making NPs. On the basis of these benefits, unique specialized methods using desolvation, NP albumin-bound (Nab™) technology, self-assembly, thermal gelation, and nano spray-drying have been developed. In this review, the first three methods will be discussed in depth; they are the most refined methods and they are practical clinically.
Desolvation (coacervation)
Desolvation was the first established preparation of albumin NPs (Weber et al. 2000). In this method, an amphiphilc solvent (most commonly ethanol) is added drop-wise to a very concentrated albumin solution. The ethanol is approximately 4–5 times greater than the volume of the aqueous phase. Drop-wise addition of ethanol is important; the addition of the entire volume at once can result in the formation of insoluble albumin aggregates, or damage to the surface of the albumin, or irregular albumin associations. Adding ethanol drop-by-drop induces slow absorption of water from the aqueous albumin phase, which traps albumin molecules in a very small amount of water phase (coacervate). In a coacervate state, the interparticulate and intermolecular distances are extremely close, facilitating formation of albumin nano-clusters. Slow addition of ethanol also promotes the stable and homogenous formation of albumin coacervate, which results in regular spherical colloid particles. Specifically, considering albumin’s pI is 4.0, the surface charge will be significantly negative at neutral pH. Therefore, electron repulsion between albumin molecules is markedly increased, which prevents NP formation, even in coacervate. Therefore, the preferred pH of albumin solution should be ≤ 5.5 to facilitate the association (Fig. 2 and Table 2).
However, the use of cross-linking agents is a clear disadvantage in desolvation process. The albumin coacervate (nanoparticles) formed can be easily restored to individual albumin molecules after centrifugation and reconstitution because they are only physically and temporarily associated. Thus, cross-linkers are inevitably used to ‘harden’ the albumin NPs for maintaining its basic structure or morphology. Glutaraldehyde is most frequently used to cross-link albumin surface amines (including lysine, arginine and or N-terminus residues). Subsequently, the solidified albumin NPs maintain their shape and physical state even after many cycles of centrifugation and reconstitution. After several cycles of washing or evaporation steps (if necessary) to remove ethanol and glutaraldehyde, the albumin NPs are highly purified and lyophilized using proper cryopectants like 5 % mannitol. In general, drug loading can be performed in three ways: covalent modification of drugs to the particle surface (Bae et al. 2012) or to albumin itself (Choi et al. 2015), adsorption of drugs onto the surface or inner network of NPs formed (Dreis et al. 2007; Kim et al. 2011b), and thirdly by incorporation of drugs during particle preparation (Dreis et al. 2007). The overall incorporation efficiency of drugs into NPs can exceed 80 % (Dreis et al. 2007).
Weber and Langer et al. first established the desolvation method for albumin NP preparation and optimized all details including the amount of glutaraldehyde needed for cross-linking, volume ratio of ethanol versus aqueous phase, albumin drug loading, control of particle size, and zeta potential (Dreis et al. 2007; Langer et al. 2003; Weber et al. 2000). During cross-linking, the number of amino groups of albumin depending on glutaraldehyde increase was carefully determined using 2,4,6,-trinitrobenzensulfonic acid (TNBS) (Weber et al. 2000). Dreis and Langer et al. compared the loading efficiency of doxorubicin into albumin NPs according to adsorption (i.e., drug loading after NP preparation) and incorporation (i.e., drug loading during NP formation) (Dreis et al. 2007).
A new approach for attaching receptor ligands to the surface of albumin NPs prepared by a desolvation method was first tried by Michaelis et al. (Michaelis et al. 2006). Apolipoprotein E was covalently modified with the surface of loperamide-loaded albumin NPs using a bifunctional PEG3400 spacer (N-hydroxysuccinimide-activated PEG-maleimide); these NPs displayed greatly enhanced delivery to the brain via facilitated transport across the blood–brain barrier.
Kim et al. tried to load an apoptotic hydrophilic TRAIL protein into albumin NPs by adsorption. TRAIL is a type-II transmembrane homotrimeric protein that selectively induces apoptosis in many human cancer cells, and little or none in normal cells, since this protein only binds to the death receptors (DR4, DR5) overexpressed on tumor cells. Despite its potential as an antitumor agent, TRAIL has a very short in vivo half-life (~20 min: terminal). Kim et al. suggested an optimal formulation in terms of the TRAIL loading efficiency and NP size depending on factors including pH, and the ratio of TRAIL to albumin. Consequently, the TRAIL-load albumin NPs displayed greatly improved pharmacokinetic disposition vs. naïve TRAIL (half-lives: 90.2 vs. 9.8 min, respectively) (Kim et al. 2011b).
A unique approach for making albumin NPs surface-modified with TRAIL and transferrin, and containing doxorubicin was reported (Bae et al. 2012). As mentioned above, one of the obvious disadvantages of the desolvation method is the use of cross-linking agents. Apart from toxicity of cross-linkers, a cross-linker like glutaraldehyde non-specifically and irreversibly binds to the surface amino groups of albumin NPs. Therefore, the use of excess glutaraldehyde abolishes availability of surface amines for further functionalization of drugs or targeting ligands. To overcome this, surface amines of HSA were reversibly protected with dimethylmaleic anhydride (DMMA), and the resulting DMMA-protected HSA NPs were prepared using a desolvation/cross-linking technique. The protected DMMA moiety was easily detached from the prepared HSA NPs by decreasing the pH to 5.5, and the thiolated TRAIL or transferrin was modified with the protected surface amines on HSA NPs using sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Sulfo-SMCC). The prepared albumin HSA NPs in which the surface was modified by TRAIL and transferrin, and which contained doxorubicin, displayed great cytotoxic potential to multiple cancer cells including regular, resistant cancer cell lines, and necrosis/apoptosis less-sensitive cells (HCT116, MCF-7/ADR, Capan-1 cells, respectively), and markedly accumulated in the tumor site of HCT116 cell-xenografted mice.
Nanoparticle albumin bound technology
An innovative new way of fabricating albumin NPs was developed by Abraxis Bioscience, Inc., and was termed NP albumin-bound technology (nab™-technology). The innovation exploited the characteristic feature of albumin of having numerous binding sites for hydrophobic drugs in its molecular structure. In this technology, hydrophobic drugs are bound on the albumin surface. They participate in physically linking albumin molecules, which in part helps the association of albumin molecules and generates NPs under high-pressure conditions; high-pressure promotes physical exposure by decreasing the molecular distance between albumin molecules.
In general, albumin is dissolved in an aqueous solution (e.g., deionized water or buffers of low concentrations; albumin concentration usually >20 mg/ml). Hydrophobic drugs should be dissolved in non-polar solvents, typically chloroform or methylene chloride, and sometimes acetone, prior to mixing with ethanol (usually >20–50 mg/ml). The resulting emulsion mixture is subjected to high-pressure homogenization with ~9 cycles at ~20,000 psi: the primary emulsion is squeezed through a narrow slit at high shear stress to produce a nano-sized dispersion. This resulting dispersion is then rotary evaporated to remove non-polar solvents under reduced pressure. The generated NPs are gently centrifuged and the supernatant is lyophilized for further use. For some cases, a cryoprotectant, such as mannitol or trehalose, can be added before lyophilization to avoid particle aggregation after reconstitution (Fig. 2).
Using the nab™ technology, an albumin-based NP formulation of paclitaxel (Abraxane® ABI-007: nab-paclitaxel; Calgene Corporation, Davis, CA) with a diameter of ~130 nm was approved by the US FDA in 2005. The formulation has been clinically used for treating metastatic breast cancer and is being investigated in clinical phase II/III trials concerning melanoma, lung, and pancreas therapy (Desai et al. 2006; Elsadek and Kratz 2012; Moreno-Aspitia and Perez 2005). Nab™-docetaxel and nab™-rapamycin or other candidates are in preclinical phase development for various therapeutic indications (Table 3).
The pharmaceutical usefulness and clinical efficacy of cremophor-free ABI-007 (Abraxane®) has been reported and compared with cremophor-based paclitaxel formulations (Desai et al. 2006). Paclitaxel-bound non-crosslinked albumin NPs significantly accumulated in tumors as compared to crosslinked albumin NPs (p < 0.05) (Li et al. 2014). The curcumin-bound albumin NPs prepared by Kim et al. displayed remarkable cytotoxicity and antitumor efficacy in HCT116 and Mia Paca-2 cells or their xenografted mice models (Kim et al. 2011c). Ibrahim et al. used the nab™ technology to prepare indolone-N-oxides derivative-loaded albumin NPs for chloroquine-resistant antimalarial efficacy; this lipophilic drug was solubilized in acetone but not chloroform. The resulting albumin NPs had a rectangular (not spherical) shape with a mean diameter of 368 nm after lyophilization (Ibrahim et al. 2014).
Unique forms of apoptotic protein (TRAIL)-embedded and chemotherapeutics (paclitaxel or doxorubicin)-bound albumin NPs were produced (Min et al. 2015; Thao et al. 2016a). The authors sought to achieve synergistic antitumor efficacy by co-delivery of these two different categories of antitumor agents using albumin NPs. The hydrophobic drugs (paclitaxel or doxorubicin) were tightly bound inside albumin NPs, but a hydrophilic TRAIL protein seemed to be embedded between albumin molecules due to their opposite charges at neutral pH (TRAIL: +charge; albumin: −charge). The TRAIL-embedded and paclitaxel/doxorubicin-bound albumin NPs displayed remarkably greater antitumor efficacy than NPs containing paclitaxel/doxorubicin alone in HCT116 or Mia Paca-2 cell-xenografted mice, respectively, in terms of tumor size and weight.
The nab™ technology has been used to fabricate tacrolimus-bound albumin NPs for the treatment of rheumatoid arthritis or pulmonary fibrosis (Thao et al. 2016b). Tacrolimus is specifically effective in a rodent collagen-induced arthritis model and a rodent bleomycin-induced pulmonary fibrosis model. Thao et al. and Seo et al. tried to make tacrolimus-bound albumin NPs using a high-pressure homogenizer (Thao et al. 2016b; Seo et al. 2016). However, tacrolimus did not sufficiently promote the formation of albumin NPs despite its obvious hydrophobicity, so cholesterol was co-incorporated to boost the binding force during the preparation process (Thao et al. 2016b; Seo et al. 2016; Zhao et al. 2015). Tacrolimus-bound albumin NPs co-loaded with cholesterol showed good morphology, acceptable stability, excellent targetability because it significantly accumulated at inflamed arthritis sites and greatly enhanced the incidence of arthritis and clinical scores of mice with collagen-induced arthritis (Thao et al. 2016b). Furthermore, intratracheally aerosolized tacrolimus-bound albumin NPs clearly ameliorated the histopathological results of the lungs of mice with bleomycin-induced pulmonary fibrosis, on the basis of hematoxylin and eosin (H&E) and Masson’s trichrome staining (Seo et al. 2016).
Unique albumin NPs formulated using the nab™ technology were designed for brain targeting via blood–brain barrier transport (BBB). The mannose and cationic moieties tend to traverse the BBB efficiently through absorptive-mediated transcytosis and glucose transport, respectively. Using this property, Byeon et al. sought to fabricate a prototype of doxorubicin-loaded albumin NPs co-embedded by ethylenediamine- or mannose-modified albumin in a one-pot reaction using high-pressure homogenization (Byeon et al. 2016). In this study, doxorubicin (no salt form) was dissolved in a 9:1 mixed solution of chloroform and ethanol and then used for making albumin nanoparticles because doxorubicin hydrochloride is soluble in water or buffers of low pH, not non-polar solvents. Authors demonstrated that the 85: 5: 10 composition of HSA, c-HSA (ethylenediamine modified), and m-HSA (mannose modified) was optimal for albumin NPs in the context of physicochemical stability, zeta potential, BBB permeation, and brain targeting. Subsequently, doxorubicin-loaded NPs consisted of naïve HSA plus cationic- and mannose-modified-albumin displayed much better antitumor efficacy than doxorubicin-loaded plain albumin NPs in the U87MG glioma mouse model.
Self-assembly
Self-assembly is considered to be a convenient method to prepare albumin NPs. In this method, hydrophobic moieties are introduced to albumin in a covalent or non-covalent manner to assemble them because albumin is hydrophilic and hence water-soluble. Hydrophobically modified albumin molecules self-assemble and form a nano-scale micelle-like structure (core–shell nano-micelles) consisting of a hydrophobic inner core and a hydrophilic outer layer. During NP formation, hydrophobic drugs incorporate into the core and help incrementally collect the hydrophobically modified albumin.
The first trial for this approach involved the introduction of octyl groups to bovine serum albumin (BSA) (Gong et al. 2009). Octyl aldehyde was modified with the primary amino groups of albumin to increase hydrophobicity that promotes self-assembly. The drug loading and entrapment efficiency for paclitaxel in these albumin NPs was as much as 33.1(w/w) % and 90.5 %, respectively (Elsadek and Kratz 2012; Gong et al. 2009). The authors carefully examined the representative features of particle size, critical micelle concentration, and zeta potential of prepared albumin NPs according to degree of octyl group substitution.
Similarly, Battogtokh et al. tried to prepare self-assembled NPs made of cholesterol-modified BSA (Battogtokh et al. 2015). Cholesterol was conjugated with BSA using N,N-disuccinimidyl carbonate; cholesterol-modified BSA molecules associated together in the presence of paclitaxel and formed spherical NPs during sonication with a particle size of ~148 nm.
For the purpose of self-assembly, Xu et al. sought to conjugate doxorubicin with albumin using a sulfo-LC-SPDP linker (Xu et al. 2011). The doxorubicin-conjugated albumin molecules self-associated in the presence of free doxorubicin (non-conjugated) due to the hydrophobic interaction between free or conjugated doxorubicin. Before self-assembly, the cyclic arginylglycylaspartic acid peptide was modified with the albumin derivatives for further binding to the αvβ3 integrin, a membrane protein highly expressed in angiogenic endothelia and in some cancer cells, for the purpose of tumor targeting. These NPs displayed greatly improved cellular uptake and cytotoxicity in M21+ cells.
Another unique example of self-assembled HSA nanoparticles was shown by Choi and Youn et al., using a strategy of conjugation of both octyl aldehyde and doxorubicin to HSA molecules (Choi et al. 2015). Especially, doxorubicin was modified with 2-iminothiolane to obtain sulfhydryl group and then further conjugated with HSA using sulfo-SMCC. The modified albumin exhibited greatly increased hydrophobicity in reverse phase-high performance liquid chromatography. Nevertheless, this modified HSA was considered acceptable in terms of molecular integrity that was identified by circular dichroism versus naïve albumin. After forming such self-assembled albumin NPs, extra doxorubicin (as an unconjugated free form) and TRAIL tightly adsorbed onto the surface of the NPs. The resulting TRAIL/Dox HSA NPs displayed synergistic cytotoxicity and apoptotic activity in H226 lung cancer cells versus HSA NPs containing TRAIL or doxorubicin alone. Furthermore, these NPs showed remarkable antitumor efficacy in H226 cell-implanted mice in terms of tumor size and weight, when compared with the mice treated with TRAIL or doxorubicin HSA–NPs alone (Table 4).
Conclusions
Albumin is considered to be a key natural material of great safety due to a number of advantages in pharmaceutical and clinical field. Its pharmaceutical utility has been gradually increasing since the approval of Levemir® and Abraxane® in 2005 and 2012, respectively, because many potential albumin-based drug candidates are under clinical phase or in the pipeline. In the view of in vivo half-life extension of drugs with poor pharmacokinetics, albumin association seems to be one of the best strategies that can be achieved recently since the development of PEGylation technology. Many successful examples have already been achieved, bolstering the attractiveness of the technology for attaining therapeutic efficacy extension if proper drugs are chosen. Albumin is viewed as a superior material carrier in NPs. Albumin NPs have an inherent advantage of the lack of Cremophor® and polysorbates that are present in Taxol® and Taxotere®, respectively. Furthermore, these NPs have intrinsic targetability to tumors via a gp60-mediated pathway, which comes from the biological or physiology-related features of albumin. We believe that albumin will be an increasingly influencing natural material carrier in the pharmaceutical field.
References
Bae S, Ma K, Kim TH, Lee ES, Oh KT, Park ES, Lee KC, Youn YS (2012) Doxorubicin–loaded human serum albumin nanoparticles surface–modified with TNF–related apoptosis–inducing ligand and transferrin for targeting multiple tumor types. Biomaterials 33(5):1536–1546
Baggio LL, Huang Q, Cao X, Drucker DJ (2008) An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose homeostasis. Gastroenterology 134(4):1137–1147
Ballantyne FC, Fleck A, Dick WC (1971) Albumin metabolism in rheumatoid arthritis. Ann Rheum Dis 30(3):265–270
Battogtokh G, Kang JH, Ko YT (2015) Long-circulating self-assembled cholesteryl albumin nanoparticles enhance tumor accumulation of hydrophobic anticancer drug. Eur J Pharm Biopharm 96:96–105
Bosse D, Praus M, Kiessling P, Nyman L, Andresen C, Waters J, Schindel F (2005) Phase I comparability of recombinant human albumin and human serum albumin. J Clin Pharmacol 45(1):57–67
Byeon HJ, Min SY, Kim I, Lee ES, Oh KT, Shin BS, Lee KC, Youn YS (2014) Human serum albumin-TRAIL conjugate for the treatment of rheumatoid arthritis. Bioconjug Chem 25(12):2212–2221
Byeon HJ, le Thao Q, Lee S, Min SY, Lee ES, Shin BS, Choi HG, Youn YS (2016) Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J Control Release 225:301–313
Chae SY, Choi YG, Son S, Jung SY, Lee DS, Lee KC (2010) The fatty acid conjugated exendin-4 analogs for type 2 antidiabetic therapeutics. J Control Release 144(1):10–16
Chen W, Gu B, Wang H, Pan J, Hou H (2008) Development and evaluation of novel itraconazole-loaded intravenous nanoparticles. Int J Pharm 362(1–2):133–140
Choi SH, Byeon HJ, Choi JS, Thao L, Kim I, Lee ES, Shin BS, Lee KC, Youn YS (2015) Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J Control Release 197:199–207
Chuang VT, Kragh-Hansen U, Otagiri M (2002) Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm Res 19(5):569–577
Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P (2006) Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI–007, compared with cremophor–based paclitaxel. Clin Cancer Res 12(4):1317–1324
Dreis S, Rothweiler F, Michaelis M, Cinatl J Jr, Kreuter J, Langer K (2007) Preparation, characterisation and maintenance of drug efficacy of doxorubicin-loaded human serum albumin (HSA) nanoparticles. Int J Pharm 341(1–2):207–214
Elsadek B, Kratz F (2012) Impact of albumin on drug delivery—new applications on the horizon. J Control Release 157(1):4–28
Gong J, Huo M, Zhou J, Zhang Y, Peng X, Yu D, Zhang H, Li J (2009) Synthesis, characterization, drug-loading capacity and safety of novel octyl modified serum albumin micelles. Int J Pharm 376(1–2):161–168
He X, Xiang N, Zhang J, Zhou J, Fu Y, Gong T, Zhang Z (2015) Encapsulation of teniposide into albumin nanoparticles with greatly lowered toxicity and enhanced antitumor activity. Int J Pharm 487(1–2):250–259
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 95(8):4607–4612
Ibrahim N, Ibrahim H, Dormoi J, Briolant S, Pradines B, Moreno A, Mazier D, Legrand P, Nepveu F (2014) Albumin-bound nanoparticles of practically water–insoluble antimalarial lead greatly enhance its efficacy. Int J Pharm 464(1–2):214–224
Ibrahim N, Ibrahim H, Sabater AM, Mazier D, Valentin A, Nepveu F (2015) Artemisinin nanoformulation suitable for intravenous injection: preparation, characterization and antimalarial activities. Int J Pharm 495(2):671–679
Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agersø H, Veldhuis J, Pørksen N, Schmitz O (2002) Bedtime administration of NN2211, a long–acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 51(2):424–429
Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jetté L, Benquet C, Drucker DJ (2003) Development and characterization of a glucagon–like peptide 1-albumin conjugate: the ability to activate the glucagon–like peptide 1 receptor in vivo. Diabetes 52(3):751–759
Kim I, Kim TH, Ma K, Lee ES, Kim D, Oh KT, Lee DH, Lee KC, Youn YS (2010) Synthesis and evaluation of human serum albumin-modified exendin-4 conjugate via heterobifunctional polyethylene glycol linkage with protracted hypoglycemic efficacy. Bioconjug Chem 21(8):1513–1519
Kim H, Park H, Lee J, Kim TH, Lee ES, Oh KT, Lee KC, Youn YS (2011a) Highly porous large poly(lactic-co-glycolic acid) microspheres adsorbed with palmityl-acylated exendin-4 as a long–acting inhalation system for treating diabetes. Biomaterials 32(6):1685–1693
Kim TH, Jiang HH, Youn YS, Park CW, Lim SM, Jin CH, Tak KK, Lee HS, Lee KC (2011b) Preparation and characterization of Apo2L/TNF-related apoptosis-inducing ligand-loaded human serum albumin nanoparticles with improved stability and tumor distribution. J Pharm Sci 100(2):482–491
Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, Lee S, Kim H, Jon S, Chen X, Lee KC (2011c) Preparation and characterization of water-soluble albumin–bound curcumin nanoparticles with improved antitumor activity. Int J Pharm 403(1–2):285–291
Kim I, Choi JS, Lee S, Byeon HJ, Lee ES, Shin BS, Choi HG, Lee KC, Youn YS (2015) In situ facile-forming PEG cross-linked albumin hydrogels loaded with an apoptotic TRAIL protein. J Control Release 214:30–39
Kouchakzadeha O, Shojaosadatia SA, Shokrib F (2014) Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique. Chem Eng Res Des 92:1681–1692
Kratz F (2008) Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release 132(3):171–183
Kurtzhals P, Havelund S, Jonassen I, Markussen J (1997) Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J Pharm Sci 86(12):1365–1368
Langer K, Balthasar S, Vogel V, Dinauer N, von Briesen H, Schubert D (2003) Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm 257(1–2):169–180
le Thao Q, Byeon HJ, Lee C, Lee S, Lee ES, Choi HG, Park ES, Youn YS (2016a) Pharmaceutical potential of tacrolimus–loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int J Pharm 497(1–2):268–276
le Thao Q, Byeon HJ, Lee C, Lee S, Lee ES, Choi YW, Choi HG, Park ES, Lee KC, Youn YS (2016b) Doxorubicin-bound albumin nanoparticles containing a TRAIL protein for targeted treatment of colon cancer. Pharm Res 33(3):615–626
Lee J, Lee C, Kim I, Moon HR, Kim TH, Oh KT, Lee ES, Lee KC, Youn YS (2012a) Preparation and evaluation of palmitic acid-conjugated exendin-4 with delayed absorption and prolonged circulation for longer hypoglycemia. Int J Pharm 424(1–2):50–57
Lee J, Lee C, Kim TH, Chi SC, Moon HR, Oh KT, Lee ES, Lee KC, Youn YS (2012b) Pulmonary administered palmitic–acid modified exendin-4 peptide prolongs hypoglycemia in type 2 diabetic db/db mice. Regul Pept 177(1–3):68–72
Léger R, Thibaudeau K, Robitaille M, Quraishi O, van Wyk P, Bousquet-Gagnon N, Carette J, Castaigne JP, Bridon DP (2004) Identification of CJC-1131-albumin bioconjugate as a stable and bioactive GLP-1(7-36) analog. Bioorg Med Chem Lett 14(17):4395–4398
Levick JR (1981) Permeability of rheumatoid and normal human synovium to specific plasma proteins. Arthritis Rheum 24(12):1550–1560
Li C, Li Y, Gao Y, Wei N, Zhao X, Wang C, Li Y, Xiu X, Cui J (2014) Direct comparison of two albumin–based paclitaxel–loaded nanoparticle formulations: is the crosslinked version more advantageous? Int J Pharm 468(1–2):15–25
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65(1–2):271–284
Markussen J, Havelund S, Kurtzhals P, Andersen AS, Halstrøm J, Hasselager E, Larsen UD, Ribel U, Schäffer L, Vad K, Jonassen I (1996) Soluble, fatty acid acylated insulins bind to albumin and show protracted action in pigs. Diabetologia 39(3):281–288
Mendez CM, McClain CJ, Marsano LS (2005) Albumin therapy in clinical practice. Nutr Clin Pract 20(3):314–320
Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K (2006) Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther 317(3):1246–1253
Min SY, Byeon HJ, Lee C, Seo J, Lee ES, Shin BS, Choi HG, Lee KC, Youn YS (2015) Facile one-pot formulation of TRAIL-embedded paclitaxel-bound albumin nanoparticles for the treatment of pancreatic cancer. Int J Pharm 494(1):506–515
Moreno-Aspitia A, Perez EA (2005) Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncol 1(6):755–762
Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E, Llera AS (2008) The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev 27(4):691–705
Sage H, Johnson C, Bornstein P (1984) Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem 259(6):3993–4007
Seo J, Lee C, Hwang HS, Kim B, le Thao Q, Lee ES, Oh KT, Lim JL, Choi HG, Youn YS (2016) Therapeutic advantage of inhaled tacrolimus-bound albumin nanoparticles in a bleomycin-induced pulmonary fibrosis mouse model. Pulm Pharmacol Ther 36:53–61
Singh KV, Kaur J, Varshney GC, Raje M, Suri CR (2004) Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconjug Chem 15(1):168–173
Sleep D (2015) Albumin and its application in drug delivery. Expert Opin Drug Deliv 12(5):793–812
Son S, Song S, Lee SJ, Min S, Kim SA, Yhee JY, Huh MS, Chan Kwon I, Jeong SY, Byun Y, Kim SH, Kim K (2013) Self-crosslinked human serum albumin nanocarriers for systemic delivery of polymerized siRNA to tumors. Biomaterials 34(37):9475–9485
Subramanian GM, Fiscella M, Lamousé-Smith A, Zeuzem S, McHutchison JG (2007) Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nat Biotechnol 25(12):1411–1419
Wagner S, Rothweiler F, Anhorn MG, Sauer D, Riemann I, Weiss EC, Katsen-Globa A, Michaelis M, Cinatl J Jr, Schwartz D, Kreuter J, von Briesen H, Langer K (2010) Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 31(8):2388–2398
Weber C, Coester C, Kreuter J, Langer K (2000) Desolvation process and surface characterisation of protein nanoparticles. Int J Pharm 194(1):91–102
Wilkinson P, Jeremy R, Brooks FP, Hollander JL (1965) The mechanism of hypoalbuminemia in rheumatoid arthritis. Ann Intern Med 63:109–114
Wilson B, Lavanya Y, Priyadarshini SR, Ramasamy M, Jenita JL (2014) Albumin nanoparticles for the delivery of gabapentin: preparation, characterization and pharmacodynamic studies. Int J Pharm 473(1–2):73–79
Xu R, Fisher M, Juliano RL (2011) Targeted albumin-based nanoparticles for delivery of amphipathic drugs. Bioconjug Chem. 22(5):870–878
Yang Z, Gong W, Wang Z, Li B, Li M, Xie X, Zhang H, Yang Y, Li Z, Li Y, Yu F, Mei X (2015) A novel drug-polyethylene glycol liquid compound method to prepare 10-hydroxycamptothecin loaded human serum albumin nanoparticle. Int J Pharm 490(1–2):412–428
Yuan A, Wu J, Song C, Tang X, Qiao Q, Zhao L, Gong G, Hu Y (2013) A novel self-assembly albumin nanocarrier for reducing doxorubicin-mediated cardiotoxicity. J Pharm Sci 102(5):1626–1635
Zhang S, Kucharski C, Doschak MR, Sebald W, Uludağ H (2010) Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials 31(5):952–963
Zhao L, Zhou Y, Gao Y, Ma S, Zhang C, Li J, Wang D, Li X, Li C, Liu Y, Li X (2015) Bovine serum albumin nanoparticles for delivery of tacrolimus to reduce its kidney uptake and functional nephrotoxicity. Int J Pharm 483(1–2):180–187
Zimmera AK, Zerbe H, Kreuter J (1994) Evaluation of pilocarpine-loaded albumin particles as drug delivery systems for controlled delivery in the eye I. In vitro and in vivo characterization. J Control Release 32(1):57–70
Acknowledgments
All authors (E. S. Lee and Y. S. Youn) declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors. The corresponding author Y. S. Youn would like to express special thanks to the past Dr. Tae Hyung Kim for this review work. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2014R1A2A2A05002133).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, E.S., Youn, Y.S. Albumin-based potential drugs: focus on half-life extension and nanoparticle preparation. Journal of Pharmaceutical Investigation 46, 305–315 (2016). https://doi.org/10.1007/s40005-016-0250-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0250-3