Abstract
Frequent occurrence, higher morbidity and mortality rate of atherosclerosis associated hypertension in elderly people shambles health care system worldwide. So the present study was deliberated to formulate and appraise rational combination, containing immediate release portion of atorvastatin calcium (ATR) and sustained release (SR) portion of felodipine (FEL) in bilayered multicomponent tablet formulation for elderly people. A 32 factorial design was used to prepare atorvastatin calcium and felodipine tablets separately. For each drug total nine batches (A1–A9 for ATR; F1–F9 for FEL) were prepared. The amount of sodium starch glycollate (X1) and lactose (X2) were used as independent variables for atorvastatin calcium and hydroxypropyl methyl cellulose 50 cps (X1) and talc (X2) for felodipine tablets. Talc being hydrophobic diluents has been tried as release retardant. On the basis of flowability, compressibility, compactibility, tensile strength and drug release, the both layers of formulation were optimized, and subsequently, bilayered multicomponent tablet formulation was prepared and evaluated. The optimized tablet formulations of atorvastatin calcium (Batch A3) released 76 % of drug after 30 min, while (Batch F8) of felodipine showed drug release up to 79 % after 10 h and followed peppas release pattern (r2 = 0.9862). The similar release pattern was noticed in the bilayered tablet formulation of atorvastatin calcium and felodipine meeting the USP requirements. Hence, it can be concluded that, the aforesaid bilayered formulation can meet the needs of those elderly people, who suffer from mild hypertension and hyperlipidemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In current years, a budding interest has been developed in designing drug delivery systems that take account of an immediate release (IR) component to sustained release (SR) dosages (Waterman and Fergione 2003). Multidrug therapy is very common nowadays in clinical practice to treat chronic diseases or to manage either single (Kotta et al. 2010; Indop et al. 2002) or simultaneously occurring (Raj et al. 2011) different diseases. Hypertension in the majority of patients is always associated with atherosclerosis (Alexander 1995). Framingham data analysis showed that approximately 78 % of hypertensive males and 82 % of hypertensive females found to be associated with at least one other cardiovascular risk factor (Kannel 2000). Survey showed that 19 % of males and 26 % of females with hypertension also had high total cholesterol levels (Health Survey for England 1998). So if possible, in this sort of diseases all medications that a person needs to take concurrently would be amalgamated into a single pill. Anglo-Scandinavian Cardiac Outcome Trial (ASCOT) showed that combination of antihypertensive and lipid lowering drugs reduces cardiovascular events including stroke and deaths occur in patients with moderate to high cardiovascular risk profiles (Park 2008).
Calcium channel blockers (CCBs) can best suit treatment of mild hypertension in elderly peoples (Gupta and Guptha 2010). Work supporting same has appeared recently in the scientific literature (Estacio et al. 1998). Amongst various CCBs, felodipine (FEL) has been considered safe and effective for elderly people due to its hemodynamic effects like increasing cerebral and renal blood flow (Ono et al. 1999), prevents left ventricular hypertrophy (Cacciopouli et al. 1998), increase in the ejection fraction, shortening of left ventricular end systolic length and increase in stroke volume index (Wong and Germanson 2000). If FEL can be combined with atorvastatin calcium (ATR), it can be regarded as safe and effective combination therapy. Because, ATR potent reducing total cholesterol, low density lipoproteins and triglycerides (Roger and Mangliano 2007). It improves atherosclerosis in addition to a reduction in serum lipids (Ozaki and Kubo 2006), and significantly reduces total cholesterol, low density lipoprotein-C (LDL-C), and high density lipoprotein-C (HDL-C) compared to statin—fibrate therapy. Moreover, the pharmacokinetic parameters of both drugs are suggestive of rationality of combination (Wingstrand et al. 1990; Chung et al. 2006).
Liturature reveals that, till now only single study has been carried out on bilayer tablet of FEL and ATR and restricted to liquisolid technique only (Rajesh and Rajalakshmi 2013). Caduet® is the only marketed fixed-dose combination of this type having amlodipine and atorvastatin which has the ability to improve patient adherence and the management of cardiovascular risk, improving clinical outcomes (Park 2008). Improved tolerability of FEL compared with amlodipine in elderly hypertensives has been already proved (Schaefer et al. 1998). Hence, to reap maximum benefits of FEL-ATR combination therapy, multicomponent bilayered tablet formulation having sustained release (SR) of the FEL and immediate release (IR) of ATR has been designed. An attempt has been made to incorporate talc as hydrophobic diluents to act as a release retardant in FEL tablets, which is inexpensive and safe for administration to humans (Lin and Peck 1995). A systematic studies has been performed by implementing 32 factorial experimental design to prepare immediate release ATR tablet and SR FEL tablet separately. Subsequently, the same compositions of optimized FEL and ATR tablets have been used in the fabrication of bilayered tablet formulation. And finally, the tablets were evaluated for hardness, friability, weight variation, content uniformity and drug release.
Materials and methods
Material
Felodipine and Atorvastatin Calcium was provided as a gift sample from Cipla, (Mumbai, India) and Taj Pharmaceuticals, (Mumbai, India) respetively. Lactose anhydrous, sodium starch glycollate (SSG), hydroxypropyl methyl cellulose (HPMC 50cps), microcrystalline cellulose (MCC), polyvinylpyrrolidone (PVP) K30, talc, magnesium stearate (Mg stearate), aerosil (Loba Chemie, Mumbai, India) were purchased locally and rest chemicals were of analytical grade.
Methods
Preparation of ATR and FEL tablets
Using 32 factorial design, 9 batches of IR ATR tablets (A1-A9) and SR FEL tablets (F1-F9) were prepared separately in triplicate. Translation code level in preparation of SR FEL and IR ATR tablets has reported in Table 1. Preparation of ATR tablets was carried out using sodium starch glycollate (SSG) and anhydrous lactose as independent variables, X1 and X2 respectively. Required quantity of ATR, lactose, PVP K30, SSG, Mg stearate, talc were weighed and mixed thoroughly in mortar for 10 min to obtain a homogeneous blend. The mixture was then passed through sieve #16 (ASTM) and directly compressed using RIMEK minipress (Karnavati engineering, Gujarat, India), using 8 mm flat faced punches.
FEL tablets (F1–F9), were prepared in triplicate by wet granulation using talc (X1) and HPMC (X2) as independent variables. All ingredients were passed through 60# mesh sieve (ASTM) separately and collected. FEL, talc, HPMC 50 cps, MCC, aerosil, Mg stearate were weighed and mixed with 1 % w/v aqueous dispersion of PVP K30. The wetted mass was forced through 20# mesh (ASTM) sieve to obtain the granules. The granules were dried in a hot air oven at 50 °C for 10 min (Mehta and Patel 2006), and subjected to tabulating as that of ATR tablets. Subsequently, ATR blend, FEL granules, ATR and FEL tablets were further evaluated separately. The detailed composition of aforesaid tablet formulation has been given in Table 2. To optimize the tablet formulations and elucidate the effect of each variable, multiple regression analysis was carried out and polynomial equation was generated. Based on the calculated slope (β) values, and their significance, the formulations were optimized.
Evaluation of ATR tablets, ATR blend, FEL granules and FEL tabelets
Particle size distribution
FEL, ATR powder, and FEL granules were subject to particle size determination by microscope and sieve analysis methods respectively. 100 particles of each drug were counted and particle size was determined and sieves were nested on each other and weighed mass was placed on top sieve, and tapped for 15 min. The mass retained on each sieve was weighed, and the resulting data were used to obtain the mean geometric diameter by plotting the graph of cumulative percentage undersize versus the average particle size (Sinko 2006).
Micromeritics
Flowability of ATR blend and FEL granules was evaluated by determining the angle of repose (AR), Hausner’s ratio (HR) and Carr’s compressibity index (CCI).
Angle of repose
AR was determined using fixed-funnel free standing cone method. The height of the funnel tip from horizontal surface was kept constant at 1.7 cm. Study was performed in triplicate for every batch of F1–F9 and A1–A9. AR was determined by using the formula (Train 1958)
where ‘θ’ is the angle of repose; h is height between the lower tip of the funnel and the base of the heap of blend or granules; and r is radius of the base of heap formed.
Carr’s compressibility Index (CCI) and Hausner’s ratio (HR)
CCI and HR were determined by measuring tapped density and bulk density (Carr 1965; Hausner 1967). Tapped density was determined by using the bulk density apparatus Lab Hosp, (Mumbai, India)
where TD is tapped density; and BD is bulk density.
Kawakita analysis
The flowability and packability of the ATR blend and FEL granules was studied by kawakita plot (Kawakita and Ludde 1971). The reduction in volume of bed with tappings was noted by using the bulk density apparatus. The plot of number of tappings (n) versus the degree of volume reduction (n/c) was plotted. And, the values of constants ‘a’ and ‘b’ was calculated by using the following equation
where ‘n’ is the number of toppings; ‘C’ is degree of volume reduction equal to
where V0 is the initial volume before tapping and V∞ is volume after tapping.
Crushing strength (CS) of FEL granules from every batch was determined by using mercury load cell method, reported by Jaroz and Parrot (1983). The mercury was poured at constant speed. Five granules were randomly sampled from every batch and subject to CS determination by using mercury as a load. The weight of mercury required to break the granules considered as CS.
Compressional properties
Tablets from each batch of F1–F9 and A1–A9 were subject to Heckel, tensile strength, Leuenberger analysis in triplicate.
Pressure–relative density relationship
ATR blend and FEL granules of all the batches were compressed separately using hydraulic
press (Techno search Instruments,Mumbai, India) having 13 mm flat faced punch and die set at the pressure of 1, 2, 3, 4, 5, 6 and 7 tons. Lubrication of punches and die was performed by 1 % w/v dispersion of magnesium stearate in acetone. Compacts were allowed to relax at ambient condition for 2 h. Heckel study was performed in triplicate for batch F1–F9 and A1–A9 using the Heckel Equation (Heckel 1961; Jadhav et al. 2013)
where Pf is packing fraction of the tablet; P is the applied pressure in tons; and K is Heckel constant; K = 1/3 σ0, where σ0 is yield strength; and mean yield pressure (MyP) is equal to 3σ0. The constant A expresses densification at low pressure.
Pressure–tensile strength (σt) relationship
After determination of the diameter (D) and thickness (t), the compacts used for compression study were subjected to determination of the force (F) required to break the compacts (hardness) by a Monsanto-type hardness tester (Kshitij Innovations,Mumbai India) and the data were subjected to tensile strength (σt) determination by using following equation (Fell and Newton 1971)
where ‘D’ is diameter, and ‘t’ is thickness of compacts; and ‘F’ is the force required to break the compacts.
Leuenberger analysis
Pressure-tensile strength data were subject to Leuenberger analysis to calculate compression susceptibility (γ) and compactibility (σtmax) (Jetzer et al. 1983). The study was performed in triplicate for FEL granule (F1–F9) and ATR blend (A1–A9)
where σt is tensile strength at time t, σtmax; maximum tensile strength at infinite pressure, compactibility (σtmax), and compression susceptibility (γ), pressure (P) and the relative density (rd) was calculated from tablet dimensions.
Hardness and friability
Three tablets from each batch of ATR (A1–A9) and FEL tablets (F1–F9) were subjected to hardness and friability test. The hardness of tablets was determined using Monsanto hardness tester (Kshitij Innovations Mumbai,India). The friability study of tablets was performed using Roche friabilator. Three tablet samples from each batch (A1–A9) and (F1–F9) were placed in friabilator which is then operated at 100 rpm. Percent weight loss was calculated at fixed time interval by using the following equation:
where Wo and Wt are initial weight and weight after time t, respectively.
Weight variation and content uniformity
Twenty tablets form each batches of FEL (F1–F9) and ATR (A1–A9) tablets were subjected to weight variation and content uniformity test. Each tablet was weighed individually to calculate the average mass and then percent variation in each tablet was calculated. Content uniformity test was performed by UV- visible spectrophotometry. Standard stock solution of ATR and FEL pure drug was prepared by dissolving 10 mg of ATR and 10 mg of FEL separately in 100 ml of 0.1 N HCl and phosphate buffer (pH 6.8) separately in two different 100 ml volumetric flask. Dilution of different concentrations were prepared and there absorbance was recorded. Wavelength of maximum absorption for ATR and FEL was found to be 247 and 268 nm. Calibration curve was plotted for absorbance versus concentration. Twenty tablets form each batches of F1–F9 and A1–A9 were used for assay.
Tablets were triturated initially and amount of powder equivalent to 10 mg of ATR and FEL was weighed. Standard stock solutions of ATR and FEL were prepared by dissolving powder equivalent to 10 mg of ATR and 10 mg of FEL separately in 60 ml of 0.1 N HCl and phosphate buffer (pH 6.8) separately and final volume of both the solutions was made up to 100 ml with 0.1 N HCl and phosphate buffer (pH 6.8), to get stock solutions containing 100 μg/ml each of ATR and FEL in two different 10 ml volumetric flasks. From standard drug solutions six working test solutions were prepared and concentration of ATR and FEL in tablet was estimated and reported.
FTIR, PXRD and DSC analysis
ATR and FEL drug were subjected to Fourier transform Infrared (FTIR), Differential scanning calorimetry (DSC) and powder X-ray diffractometry. FTIR spectra of purified drugs were recorded using an infrared spectrophotometer (Jasco-V-730 model). About 2 mg of sample was ground thoroughly with KBr; uniformly mixed sample was kept in sample holder and a spectrum was recorded over the wave number 400–4,000 cm−1. Crystallinity of the drug and excipients was analyzed by PXRD study. Dry powder of drug & excipients was used separately. The sample was irradiated with monochromatic Cu Kα radiation (1.742 Å) between 7° and 77° (using 2θ) on an X-ray diffractometer (Philips analytical XRD, PW 3710). The voltage and current applied were 40 kV and 30 mA respectively. Thermal behavior of the drug and excipients was analyzed by DSC. Shimadzu differential scanning calorimeter (TA instruments, model SDT 2960, USA) equipped with intracooler and refrigerated cooling system was used to analyse the sample. Samples were kept in aluminum crucibles, prior to heating under nitrogen flow (50 ml/min) at a scanning rate of 10 °C/min. Aluminum crucible devoid of sample was used as reference.
Disintegration time and dissolution studies
Three intact tablets from each batch of F1–F9 and A1–A9 were subjected to in vitro disintegration and dissolution test using disintegration test apparatus Type-II (TDT 08L, Electrolab, Mumbai, India) at 37 ± 0.5 °C in 900 ml 0.1 N HCl and the dissolution study was performed in USP Type-II dissolution test apparatus (TDT 08L, Electrolab, Mumbai, India). The dissolution medium used was 900 ml of 0.1 N HCl for first 2 h and phosphate buffer (pH 6.8) for next 10 h at 37 ± 0.5 °C. The paddle speed was kept constant at 50 rpm. Each time, 5 ml of samples were withdrawn at the interval of 30 min for FEL and at the interval of 5 min for ATR, for first 1 h and thereafter at interval of 1 h. The withdrawn samples were analyzed spectrophotometrically at 268 nm for FEL and 247 nm for ATR. The same amount of fresh 0.1 N HCl and phosphate buffer (pH 6.8) was used to replace the amount withdrawn for respective dissolution media. Percent cumulative release of both drugs from the tablet was calculated.
Preparation of bilayered tablets
Out of the nine batches of both FEL (F1–F9) and ATR (A1–A9) tablets, optimization was done on the basis of flowability, friability, compressibility, tensile strength, and release profile. Batch having angle of repose (θ) <25°, CCI in between 12 and 16 % friability <0.4 and tensile strength value >1 kg/cm2 were considered optimized and bilayered tablet was prepared from that optimized batch of ATR (A3) and FEL (F8) tablets (Table 2). The six formulations of bilayered tablets, each weighing 300 mg containing ATR layer of 150 mg and FEL layer of 150 mg (each layer contained 10 mg of drug) were prepared from optimized batches and further subjected to evaluation as per USP specifications.
Evaluation bilayered of tablets
Hardness and weight variation
Six tablets of bilayered tablets were subjected to hardness and friability test. The hardness of tablets was determined using Monsanto hardness tester. Friability like hardness is another measure of tablet strength. The friability study of tablets was performed using Roche friabilator. Six tablets were placed in friabilator which is then operated at 100 rpm. Percent weight loss was calculated at fixed time interval by using the equation no. 10.
Weight variation and content uniformity
Twenty bilayered tablets were subjected to weight variation and content uniformity test. Twenty tablets were weighed individually and calculated the average weight. Percent variation between tablets was calculated. Tablets form each batches were assayed individually for ATR and FEL content by UV–Vis spectrophotometry using simultaneous equation method.
Six bilayered tablet formulation containing ATR 10 mg and FEL 10 mg were triturated initially. From the triturated mixture, an amount equivalent to 10 mg of ATR and 10 mg of FEL drug was weighed and dissolved in ACN: Water (70:30 % v/v) separately in two different 100 ml volumetric flask. Solution was filtered through Whatman filter paper no. 41 and final volume of the solution was made up to ACN: Water (70:30 % v/v) to get a stock solution. Dilutions from stock were prepared and the concentration of ATR and FEL present in the mixture solution was calculated by using the simultaneous equation.
where Cx is the concentration of FEL in gm/lit, Cy is the concentration of ATR in gm/lit, A1 is the absorbance of sample solution at 268 nm, A2 is the absorbance of sample solution at 245 nm, ax1 is the absorptivity of FEL at 268 nm, ay1 is the absorptivity of ATR at 286 nm, ax2 is the absorptivity of FEL at 245 nm and ay2 is the absorptivity of ATR at 245 nm.
In vitro disintegration and dissolution studies
Three bilayered tablets were subjected to in vitro disintegration and dissolution test using disintegration test apparatus Type-II, (TDT 08L, Electrolab Mumbai, India) at 37 ± 0.7 °C in 900 ml 0.1 N HCl and dissolution study was performed in USP Type-II dissolution test apparatus (TDT 08L,Electrolab, Mumbai, India). The dissolution medium was 900 ml of 0.1 N HCl for first 2 h and phosphate buffer (pH 6.8) for next 10 h at 37 ± 0.7 °C. The paddle speed was kept constant at 50 rpm. Each time, 5 ml of samples was withdrawn at the interval of 5 min for first 1 h and thereafter at intervals of 1 h. The withdrawn samples were analyzed spectrophotometrically at 268 nm for FEL and 247 nm for ATR. The same amount of fresh 0.1 N HCl and phosphate buffer (pH 6.8) was used to replace the amount withdrawn for respective dissolution media. Percent cumulative release of both drugs from the tablet was calculated.
Stability studies (ICH 2003)
Six bilayered tablets were subjected to accelerated stability studies. Accelerated stability studies were conducted for a period of 3 months at temperature 40 ± 2 °C, and humidity 75 ± 5 % RH. Three tablets from each batch of FEL and ATR was subject to accelerated stability studies. Drug release studies were performed on the tablet at different time points.
RP-HPLC method development for simultaneous estimation of ATR and FEL from plasma
Selection of animals
The experiments were carried out as per the guidelines and prior approval of Animal Ethics Committee (BVCPK/CPCSEA/IAEC/01/12). The albino rabbits of either sex having weight 2–2.7 kg were used for estimation of plasma concentration of pure drugs and their comparison with prepared tablet was done. The albino rats were kept under standerd conditions in animal house of Bharati Vidyapeeth College of Pharmacy, Kolhapur, as per CPCSEA guidelines.
Treatment of plasma for extraction of drug
Blood (1–2 ml) was withdrawn at the interval of 3, 6, 9 and 12 h. The blood samples were directly collected in micro cups containing 200 μl trichloroacetic acid and 70 μl of glacial acetic acid was added to decrease hydrogen bonding between nucleotide and proteins. Diethyl ether: Water (80:20 % v/v) (4 ml) was added to blood samples; the mixture was vortexed and centrifuged for 15 min to get supernatant plasma. Plasma sample was filtered through 0.22 μm syringe filter to filter the plasma proteins and the filtrate was heated to obtain dry residue and the residue obtained was dissolved on mobile phase of acetonitrile: water (70:30 % v/v) for prepration of stock solution.
Calibration curve in plasma
Preparation of standerd stock solution
Standerd stock solution was prepared by adding 10 mg of ATR and 10 mg of FEL in 10 ml mobile phase, ACN: double distilled water (70:30 % v/v) taken into a 100 ml volumetric flask to get the stock solution of 100 μg/ml, ultrasonicated for about 10 min. The solution was filered through whatmann’s filter paper No. 40; previously separated filtrate of plasma solution was added to the stock solution. Final volume was made up to 100 ml with mobile phase. Aliquots in suitable concentration were prepared with addition of internal standerd Ezetimibe (EZT) and analysed using proposed method.
Preparation of internal standerd solution
Standerd stock solution containing EZT was prepared by dissolving 10 mg of EZT in 20 ml of mobile phase. It was then sonicated for 10 min and the final volume of solution was made up to 100 ml with mobile phase to get 100 μg/ml of EZT in 100 ml volumetric flask.
Linearity study of ATR and FEL in plasma
0.5 ml of treated plasma was taken in 10 ml volumetric flask and volume was made up to 10 ml with mobile phase (stock solution). Dilutions of different concentration (5–25 μg/ml) of ATR and FEL were prepared from stock solution. Fixed concentration of EZT (2 ml) was added in each dilution as internal standerd. Prepared solutions were injected in HPLC. Peaks were observed at their retention time. Calibration curve was plotted for concentration versus response factor.
In vivo release study
Initially nine albino rabbits were selected for experiment and divided into three groups (3 animal in each). To the control group plain 4 mm tablet (without drug) was administered through oral route. Test group was administered with prepared tablet formulation and pure drug was given to standerd group. Blood (1–1.5 ml) was withdrawn from the juglar vein of ear at the interval of 3, 6, 9 and 12 h. The blood samples were directly collected in micro cups containing 200 μl trichloroacetic acid and 70 μl of glacial acetic acid was added to decrease hydrogen bonding between nucleotide and proteins. Diethyl ether: Water (80:20 % v/v) (4 ml) was added to blood samples; the mixture was vortexed and centrifuged for 15 min to get supernatant plasma. Plasma sample was filterd through 0.22 μm syringe filter to filter the plasma proteins and the filtrate was heated to obtain dry residue and the residue obtained was dissolved on mobile phase of acetonitrile: water (70:30 % v/v), diluted to different concentrations with mobile phase and internal standerd solution of EZT (10 μg/ml) was added to it and injected into HPLC. The flow rate was 1 ml/min and UV detection was performed at 238 nm. The concentrations of pure ATR, pure FEL, ATR and FEL from tablet was estimated after interval of 3, 6, 9 and 12 h.
Results and discussion
Evaluation of ATR tablets, ATR blend, FEL granules and FEL tabelets
Particle size distribution
Particle size analysis of FEL blend and ATR granules were performed by microscope and sieve analysis respectively. Observations showed that the major fraction of particles of the FEL and ATR powder was found to be in the range of 143–150 µm and 110–130 µm. The average FEL granule size was found to be in the range of 746.25–834.68 μm for all batches.
Micromeritics
Angle of Repose (θ)
For ATR blend (Batch A1-A9) and EFL granules (Batch F1–F9), angle of repose (θ) was found to be in the range of 24.85 ± 0.57–32.11 ± 0.34 and 17.85 ± 0.24–27.42 ± 0.4 respectively, which indicates good flow properties (<25 excellent, 25–30 good, 30–40 passable, >40 poor). For FEL granules angle of repose was found to be maximum for the batch F9 and minimum for batch F5. While, for ATR it was maximum for batch A5 and minimum for batch A3 (Singh and Kumar 2012).
Carr’s compressibility index (CCI) and Hausner’s ratio (HR)
Carr’s compressibility index for FEL granules and ATR blend was found to be in the range of 13.46 ± 0.47–22.24 ± 0.55, which is good to fair (11–15 good, 16–20 fair, 21–25 passeble, 26–31 poor) and 14 ± 0.54–29 ± 0.35 % respectively. Carr’s compressibility index for FEL granules was found to be maximum for batch F9 and minimum for batch F5 and for ATR blend; maximum for batch A1 and minimum for batch A3.
For ATR blend Hausners ratio was found to be in the range of 1.14 ± 0.87–1.40 ± 0.67. Whilst, for FEL granules it was found to be 1.09 ± 0.86–1.34 ± 0.49. Both ranges indicates good flowability (1.00–1.11 excellent, 1.12–1.18 good, 1.19–1.25 fair, 1.26–1.34 passeble, 1.35–1.45 poor). Haussners ratio for FEL granules was found to be maximum for the batch F2 and minimum for batch F1 and for ATR; maximum for batch A1 and minimum for batch A8 (Singh and Kumar 2012).
Kawakita analysis
It was observed that for ATR blend and FEL granules the value of ‘a’ was lower as compared to the value of ‘b’ which indicates a good flowability. In case of FEL ‘a’ value was found to be maximum for the batch F3 and minimum for batch F6 and for ATR; maximum for the batch A8 and minimum for batch A4. Whereas ‘b’ value of FEL was found to be maximum for the batch F7 and was minimum for batch F5 and for ATR, maximum for batch A6 and was minimum for batch A8. The compression strength of every batch of FEL granules was found to be within 48–53 gm/cm2. Compression strength of FEL granules was found to be maximum for batch F2 and minimum for batch F9 (Table 3).
Compressional properties
Pressure–relative density relationship
ATR tablets were prepared by direct compression method for that ATR blend was prepared and from blend tablets were prepared. From the full Factorial design the binomial equation was set and values regarding with the equation are found out from the data. Regression coefficients (β) indicated the predominant effect of SSG (β1 = 0.4489), Lactose (β2 = 0.3222) and a combination of SSG and Lactose (β12 = 0.2748) for ATR (Fig. 1b) and for FEL, regression coefficient indicated the predominant effect of HPMC (β1 = 1.1580) and Talc (β2 = 0.8995) and a combination of Talc and HPMC (β12 = 0.6750) (Fig. 1a). MyP for FEL was found to be maximum for batch F9 and minimum for batch F2 indicating good compressibility and for ATR it was found to be maximum for batch A9 and minimum for batch A1(Table 3).
Pressure–tensile strength (σt) relationship
Tensile strength for FEL and ATR compact was found to be in the range of 1.876 ± 0.12–2.81 ± 0.19 kg/cm2 and 0.8981 ± 0.04–1.1951 ± 0.003 kg/cm2. Tensile strength for FEL compact was found to be maximum for batch F9 and was found to be minimum for batch F5 and for ATR compact; maximum for batch A1 and minimum for batch A3 (Table 4).
Leuenberger analysis
Compactibility of FEL granule was found to be maximum for the batch F3 and was minimum for batch F9 and for ATR blend; maximum for A7 and was minimum for batch A9. Compression susceptibility of FEL was found to be maximum for the batch F4 and minimum for batch F3 similarly for ATR it was found to be maximum for batch A7 and was minimum for batch A2 (Table 3).
Hardness and friability
Hardness of FEL tablet was found to be in the range of 4–6.5 kg/cm2 and 4.8–6.8 kg/cm2 for ATR tablet. Friability of FEL tablets and ATR tablets was within 0.2–0.6. Hardness of FEL tablet was found to be maximum for batch F9 and minimum for batch F2 and for ATR tablets, maximum for batch A6 and minimum for batch A4. Highest percent friability was found with batch F1, while F7 showed least percent weight loss and for ATR, maximum percent weight loss was found in batch A1 and minimum in batch A7 (Table 4).
Weight variation and content uniformity
Weight of ATR and FEL tablets was found to be in the range of 194.4 ± 0.34–203.8 ± 0.46 mg and 186 ± 0.92–246 ± 8 mg respectively. ATR dilutions were found to be linear within the concentration range of 2–12 µg/ml and FEL within 20–120 µg/ml. The regression coefficient for calibration curve of ATR and FEL was found to be 0.9992 and 0.9997 respectively. Content recovery of ATR and FEL tablets was found to be in the range of 94.31 ± 0.65–101.6 ± 0.79 % and 95.66 ± 0.65–102.5 ± 0.48 % (Table 4).
FTIR, PXRD and DSC analysis
FTIR spectrum analysis was performed in order to recognize drug (ATR and FEL) and excipients characteristic functional groups and to evaluate the possible interactions between them. FT-IR spectra of pure FEL physical mixture and optimized formulation are shown in Fig. 2. IR spectra of FEL showed characteristic peaks at 3,069.21, 3,362.59, 1,224.14, 755.42 and 1,732.36 cm−1 assigned to C–H stretch (aromatic), N–H stretch (secondary), C–N stretch, C–Cl stretch and C=O (aromatic) respectively (Tapas et al. 2011). Charactristic IR peaks were retained in physical mixture (PM) and optimized formulation (Batch F8) when compared with pure FEL, which indicated the absence of well identified chemical interactions between FEL and other excipients. ATR spectrum (Fig. 2) showed characteristics peaks ascribed to C–H stretching, C–N stretching, C=O stretching, C=C bending and C–F stretching at 2,962.13, 1,315.21, 1,558.2, 1,650.77 and 701.96 cm−1 (Narasaiah et al. 2010). All peaks of ATR were retained in their physical mixture with excipients and in final formulation (batch A3). Means ATR and FEL structure did not change and there is no incompatabilities between ATR, FEL and excipients.
To determine the crystalline nature of active molecules, PXRD studies were performed. PXRD spectra of pure FEL and ATR, PM and optimized formulation are shown in Fig. 2. PXRD spectra of pure FEL showed intense and long peaks attributed to its crystalline nature (Tapas et al. 2011). While, PM showed reduction in peak intensity up to greater extent which indicate reduction in crystallinity or amorphization of FEL. However, optimized formulation (F8) showed crystalline nature might be due to moisture absorption (Konno and Taylor 2008). Like FEL Pure ATR also showed intense and long peaks attributed to its crystalline nature whereas PM and optimized formulation (A3) showed less intense peaks attributed to amorpization of drug in formulation (Skorda and Kontoyannis 2008).
The DSC thermogram of pure FEL, Pure ATR and formulations (Batch F8 and A3) are shown in Fig. 4. DSC thermogram of FEL and ATR showed single endotherm at 147.19 and 159.35 °C respectively attributed their melting point (Tapas et al. 2011; Skorda and Kontoyannis 2008). However, endotherm was found to be slightly shifted in DSC thermogram of optimized batch F8 and batch A3 (Fig. 4).
In vitro disintegration and dissolution studies
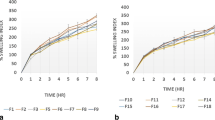
Disintegration time of ATR and FEL tablets was found to be in the range of 10–16 s and 9.3 ± 0.21–16.5 ± 0.33 min respectively. ATR blend and FEL granules of all batches were compacted at a pressure of 2 tons and the dissolution study was performed. It was observed that batch F7 (100.36 ± 1.4) showed the maximum % release for 12 h and batch F3 (96.38 ± 0.5) showed minimum % cumulative release. Batch F2, F6, F8 and F9 follows Korsmeyer Peppas release kinetic model, while other follows Hixson Crowell model.
For ATR tablets; maximum dissolution was shown by A7 (102.14 ± 0.05) and minimum by A1 (96.24 ± 0.02). As per USP specification only batch F8 and batch A3 had the appropriate % cumulative release results as shown in Table 4. Plot of % cumulative drug release versus time has shown in Fig. 5.
Preparation of bilayered tablet
ATR and FEL tablets were optimized on the basis of flowability, friability, compressibility, tensile strength, and release profile. It was found that batch A3 had good flowability, high tensile strength, high compressibility less friability, drug release meet the specifications of USP. Batch A3 contained SSG 8 mg and lactose 169.2 mg. FEL batches were optimized on the basis of flowability, compressibility, tensile strength, and release profile and it was found that batch F8 was optimum as batch F8 had good flowability, high tensile strength, and good compressibility. Batch F8 released 18 % of drug after 2 h, 59 % after 6 h and 96 % after 10 h which is as per USP specification. It contains talc 80 mg and HPMC 50 cps 40 mg. FEL tablet follows peppas release model (n = 0.45) for drug release. FEL tablet followed peppas model for drug release. Hence six batches of bilayered tablets were prepared from optimized batch F8 of FEL and A3 of ATR and were further subjected to evaluation as per USP.
Hardness and friability
Six tablets of bilayered tablet were subjected to friability and hardness test. Percent weight loss of each batch was determined by calculating the initial weight and weight after time ‘t’, thus giving its friability. It was observed that all tablets were within the specifications hence passes the tests (Table 5). For ATR blend as the content of SSG and lactose increases compressibility was found to increase, but tensile strength decreases. As a general trend as lactose content increases hardness of tablet increase but in ATR hardness of the tablet was found decreased and drug release was found to be increased up to certain extent and then decreased. For FEL as a general trend as the HPMC increases compressibility decreases, but in FEL tablet it was found that as the content of talc and HPMC increases compressibility increases and tensile strength increases up to certain extend then, it decreases and hardness increases.
Weight variation and content uniformity
Six tablets of bilayered tablet were subjected to weight variation and content uniformity test. It was observed that all tablets were within the specifications hence passes the tests. The content of bilayered tablets was found to be 100.12 ± 1.03 % for ATR and 99.98 ± 0.98 % for FEL (Table 5).
In vitro disintegration and dissolution studies
Disintegration time for ATR layer was found to be 12 s and FEL layer was 13 min. Tablets passes in vitro disintegration and dissolution test as per USP specifications. Release of FEL was extended up to 12 h and followed peppas release model. It was observed that batch B6 (101.24 ± 0.56) showed the maximum % release for 12 h and batch B4 (98.74 ± 0.94) showed minimum % cumulative release. This drug release retardation was attributed to a high viscosity rigid gel structure around the tablet, due to swelling of HPMC in contact with water (Mughal et al. 2011). Higher the viscosity more the retardation of drug release from formulation. While for ATR, Maximum release was shown by B6 (101.12 ± 0.09) and minimum by Batch B3 (98.32 ± 0.12). Values are reported in Table 5. For all batches of ATR, swelling of SSG in presence of water, overcomes the tackiness of all added ingredients which causes tablet to fall apart (Mangal et al. 2012). Disintegration time for liquisolid tablet of FEL prepared by Ramesh and Rajalakshmi was found to be in the range of 12.6–14.3. Also in their study at the end of 30 min more than 90 % of ATR get released from Liquisolid tablet and FEL release was extended up to 12 h which is equivalent to our study (Rajesh and Rajalakshmi 2013).
Stability studies
During stability studies, it was observed that with time, drug release was not affected and in acceptable limits. So these matrices were found to be suitable in 3 months period of stability study without causing any incompatible change in formulation (Table 6). Plot of % cumulative drug release versus time has shown in Fig. 6.
In vivo study
Overlain chromatogram of physical mixture in plasma has shown in Fig. 7. Plasma analysis of blood samples of standard and test group were carried out and chromatogram was recorded at 3, 6, 9 and 12 h as shown in Fig. 8. Moreover, Plasma concentration of ATR and FEL both in pure and tablet dosage form has been determined at 3, 6, 9 and 12 h. Initially i.e. at 3 and 6 h, concentration of FEL in plasma of Test group (after tablet administration) was found to be 0.94 and 1.34 ng/ml respectively, which was extremely less as compare to standard group (1.83 and 3.68 ng/ml at 3 and 6 h respectively). However, at 9 h (4.38 ng/ml) and 12 h (3.19 ng/ml) concentration of FEL in test group was found to be enormously higher as compare to standard group (2.67 and 0.66 ng/ml respectively). Cmax and Tmax for FEL pure (Standard group) was found to be 3.68 ng/ml and 6 h respectively. Whereas, Cmax and Tmax for FEL tablet (Test group) was 4.38 ng/ml and 9 h respectively. Area under curve (AUC) was found to be 18,325.11 μg/ml h for FEL at Tmax. This indicate the increased bioavailability and extended release of FEL up to 12 h from the tablet formulation, while in standerd group near about zero percent drug was available at the end of 12 h. In case of ATR at initial phase i.e. At 3 h, concentration of ATR in test group plasma sample was found to be less (0.52 ng/ml) compared to standard group (0.68 ng/ml). Which ultimately supports higher onset of action of tablet formulation i.e. IR ATR layer. Cmax and Tmax for ATR pure (standard group) was found to be 2.78 ng/ml and 6 h. While, Cmax and Tmax for ATR tablet (test group) was found to be 2.46 ng/ml and 6 h and AUC was found to 3,218.29 μg/ml h at 6 h. the Result showed zero concentration of ATR in both form at end of 12 h (Fig. 9).
In this study there were no interfering peak of biological samples found at the time of analysis. Good separation and baselines with low noise were observed. Linearity range was observed in the concentration range of 5–10 µg/ml for both ATR and FEL having correlation coefficient value 0.9956 and 0.9998 respectively.
Conclusions
Bilayered multicomponent tablet formulation of immediate release atorvastatin calcium and sustained release felodipine was successfully developed. Felodipine showed sustained release up to 12 h. The release profiles of both drugs comply with the pharmacopoeial specifications, hence meeting the requirements of immediate release and sustained release. Talc had been successfully used as a drug release retardant; being cheaper and hence can be more economic. Hence, finally it can be concluded that, ATR-FEL bilayered tablet formulation, having sound rational, can be used in elderly patients having mild hypertension associated with atherosclerosis. Biphasic drug release pattern was successfully achieved through the formulation of bilayer tablets in this study. In future, studies in human are needed to establish IVIVC for the developed formulation.
References
Alexander RW (1995) Hypertension and the pathogenesis of atherosclerosis oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension 25:155–161
Cacciopouli F, Capasso A, Mirra G (1998) Prevention of left ventricular hypertrophy by ACE inhibitors ramipril in comparison with calcium channel antagonist; felodipine. Inter J Cardiol 63:175–178
Carr RL (1965) Evaluating flow properties of solids. Chem Eng 72:163–167
Chung M, Calcagne A, Glue P (2006) Bioavailability of amlodipine besylate/atorvastatin calcium combination tablet. J Clinical Pharmacol 46:1036–1037
Estacio RO, Jeffers BW, Hiatt WR (1998) Effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non insulin dependent diabetes and hypertension. N Engl J Res 342:145–153
Fell T, Newton JM (1971) Effect of particle size and speed of compaction on density changes in tablets of crystalline and spray dried lactose. J Pharm Sci 60:1866–1869
Gupta R, Guptha S (2010) Strategies for initial management of hypertension. Indian J Med Res 132:531–542
Hausner HH (1967) Friction conditions in a mass of metal powder. Int J Powder Metal 3:7–13
Health Survey for England (1998) http://www.archive.official-documents.co.uk/document/doh/survey98/hse-00.htm. Accessed 20 Dec 2013
Heckel RW (1961) Density-Pressure relationship in powder compaction. Trans Metal Soc AIME 221:671–675
Indop MA, Bhosle SC, Tayade PT, Vavia PR (2002) Development and Evaluation of two layered tablet of Glimepiride and Metformin hydrochloride for the treatment of hyperglycemia. Ind J of Pharm Sci 64(4):349-343
International Conference on Harmonization (ICH) (2003) Harmonized tripartite guideline for stability testing of new drugs substances and products Q1A (R2). ICH, New York
Jadhav NR, Souche P, Patil S, Nadaf SJ (2013) Strength enhancement of talc pellets by incorporation of high percentage of hydroxypropyl methyl cellulose. Der Pharmacia Lettre 5(2):17–26
Jaroz PJ, Parrot EJ (1983) Comparison of granule strength and tablet strength. J Pharm Sci 72:530–535
Jetzer W, Leuenberger H, Sucker H (1983) The compressibility and compactibility of pharmaceutical powders. Pharm Technol 7:33–39
Kannel WB (2000) Fifty years of Framingham Study contributions to understanding hypertension. J Hum Hypertens 14:83–90
Kawakita K, Ludde KH (1971) Some considerations on powder compression equations. Powder Technol 4:61–68
Konno H, Taylor LS (2008) Ability of different polymers to inhibit the crystallization of amorphous felodipine in the presence of moisture. Pharm Res 25(4):969–978
Kotta KK, Mahesh M, Sasikanth K (2010) Design, development and characterization of sustained release of metformin and gliclazide bi-layered Tablets. Inter J Biopharm 1(2):67–71
Lin K, Peck GE (1995) Development of agglomerated talc I. Evaluation of fluidized bed granulation parameters on the physical properties of agglomerated talc. Drug Dev Ind Pharm 21:159–173
Mangal M, Thakral S, Goswami M, Ghai P (2012) Superdisintegrants: an Updated Review. Int J Pharm Pharm Sci Res 2(2):26–35
Mehta KK, Patel NK (2006) Formulation and process optimization of gastro retentive floating tablet of ondansetron HCl tablet. J Pharm Res Health Care 3:253–257
Mughal MA, Iqbal Z, Neau SH (2011) Guar gum, xanthan gum, and HPMC can define release mechanisms and sustain release of propranolol hydrochloride. AAPS Pharm Sci Technol 12(1):77–87
Narasaiah LV, Reddy VB, Kumar RM, Kumar KA, Raju C, Kumar SA, Reddy VB (2010) Improved dissolution rate of Atorvastatin calcium using solid dispersions with PEG-4000. J Chem Pharm Res 2(3):304–311
Ono Y, Mijono K, Goto M (1999) Hemodynamic and antihypertensive effect of felodipine in patients with essential hypertension: a pilot study. Curr Ther Res 60:392–401
Ozaki K, Kubo T (2006) The antiatherosclerotic effect of lipid lowering with atorvastatin in patients with hypercholesterolemia. J Atheroscler Thromb 13:216–219
Park CG (2008) Recent Update on Fixed Combinations of Antihypertensive Agents. Korean Circ J 38:237–243
Raj SB, Sravani G, Bhanupriya N, Veerupakshi M, AnilKumar B, Wasim RS (2011) Design and evaluation of sustained release bilayer tablet of Metformin hydrochloride with Metoprolol tartrate. Int J Novel Trends Pharm Sci 1(1):10–17
Rajesh K, Rajalakshmi R (2013) Formulation and evaluation of bilayer liquisolid tablets of Atorvastatin calcium and Felodipine. Int Res J Pharm 4(1):138–145
Roger SL, Mangliano D (2007) A dose specific meta analysis of lipid changes in randomized controlled trials of atorvastatin and simvastatin. Clin Ther 29:242–252
Schaefer RM, Aldons PM, Burgess ED, Tilvis R, Girerd X, Singh GP et al (1998) Improved tolerability of felodipine compared with amlodipine in elderly hypertensives: a randomised, double-blind study in 535 patients, focusing on vasodilatory adverse events. The International Study Group. Int J Clin Pract 52(6):381–386
Singh I, Kumar P (2012) Preformulation studies for direct compression suitability of Cefuroxime Axetil and Paracetamol: a graphical representation using sedem diagram. Acta Poloniae Pharm 69(1):87–93
Sinko PJ (2006) Martins Physical Pharmacy and Pharmaceutical sciences. Lippincott Williams& Wilkins
Skorda D, Kontoyannis CG (2008) Identification and quantitative determination of atorvastatin calcium polymorph in tablets using FT-Raman spectroscopy. Talanta 74(4):1066–1070
Tapas AR, Kawtikwar PS, Sakarkar DM (2011) Modified of Felodipine properties using spherically agglomerated solid dispersions. American j drug discov dev 1(3):160–173
Train D (1958) Some aspect of property of angle of repose of powder. J Pharm Pharmacol 10:127T–134T
Waterman KC, Fergione MB (2003) Press-coating of immediate release powders onto coated controlled release tablets with adhesives. J Control Release 89:387–395
Wingstrand K, Abrahamsson B, Edger B (1990) Bioavailability from felodipine extended release tablet with different dissolution profile. Int J Pharm 60:151–156
Wong M, Germanson T (2000) Felodipine improves left ventricular emptying in patients with chronic heart failure; V-HeFT III Echocardiographic substudy of multicentre reproducibility and detecting functional changes. J Cardiac Fail 6:19–27
Acknowledgements
This article does not contain any studies with human and animal subjects performed by any of the authors. All authors (N.R. Jadhav, R.S. Kambar, S.J. Nadaf, and P.D. Phadatare) declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jadhav, N.R., Kambar, R.S., Nadaf, S.J. et al. Design, development, in vitro and in vivo evaluation of multicomponent tablet formulation for enteral delivery of atorvastatin calcium and felodipine. Journal of Pharmaceutical Investigation 45, 115–130 (2015). https://doi.org/10.1007/s40005-014-0148-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0148-x