Abstract
In the present investigation simvastatin electrospun fibers were developed using electrospinning apparatus with drug–polymer w/w ratios of 1:1, 1:2, 1:3 and 1:4. Also solid mixtures were prepared with same ratios by employing kneading technique as conventional approach for comparison in drug release rate. Polyethylene oxide WSR coagulant 301, a hydrophilic matrix forming polymer, was used as carrier for sustained release of simvastatin. The ability of polyethylene oxide to control the drug release rate in both the formulations was also investigated. Studies were performed to characterize the optimized dosage form. Drug was crystalline in pure form. SEM surface morphology studies as well as powder X-ray diffractometry studies to developed fibers reveals that the crystalline drug was converted into amorphous form after fiber development. No physical incompatibility was found in FTIR and DSC studies of pure drug and physical mixture of drug, polymer. In vitro studies were performed in sodium phosphate buffer (pH 7.0) containing 0.5 % SLS. Simvastatin release was sustained over a period of 12 h in electrospinning fibers developed with drug to polymer w/w ratio 1:4 and 98.86 ± 0.42 % drug release was observed, interestingly with the same ratio there was a burst release of drug was obtained in case of solid mixtures “within span of 1 h”. Polyethylene oxide showed vast difference in drug release rate due to the techniques chosen to prepare formulations. The stability studies were also performed to the optimized product and no significant variance was observed in all the evaluation parameters. From the various mathematical models the drug release kinetics was estimated and found that the drug release followed zero order release rate kinetics with non fickian process as drug release mechanism.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
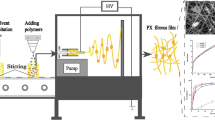
Apart from the preparation of matrix systems by compression, solvent casting techniques are also used to produce monolithic matrix systems. In these techniques the carrierr is liquefied, drug is added to the carrier solution, followed by addition of the mixture into moulds and allowed the mixture to solidify again. But this process has some limitations for heat-labile compounds (Halliday et al. 2012). Alternatively, the carrier was dissolved in a suitable solvent rather than melted; drug is added to that carrier mixture and allowed to evaporate the solvent by leaving matrix systems behind by applying high electric charge is emerging now a days. This process allows unique matrices formation with nano to micrometer range fibers with diverse materials and numerous fabrication techniques (Meinel et al. 2012). Electrospinning process is such a technique (Larrondo and Manley 1981). The electrospinning of solvent mixture was done using an apparatus called electrospinning apparatus. The major three components of the equipment are high voltage supplier, drum/metal plate collector and a syringe pump connected with a pipette or a needle with low diameter. In this process high voltage is used to create electrically charged jet also called as Taylor cone, before it reaches to the collector the solvent in the droplet evaporates and material present in the droplet stretches, it is known as electrospinning fiber. The syringe is used to hold the solvent mixture and, droplet at the needle tip is formed by application of pressure on the syringe using syringe pump (Deng-Guang et al. 2009; Reyderman and Stavchansky 1995; Reneker and Yarin 2000). Figure 1 shows the schematic representation of electrospinning apparatus.
The principle phenomenon involved in the process of electrospinning is electrostatic force. When the solvent mixture comes through the pipette/needle it forms a tailor cone (Taylor 1964) at needle end. When high voltage potential is applied, the electric field is subjected to end of the needle that contains solution mixture held by its surface tension contains charge on it. A force developed directly opposite to the surface tension due to mutual charge repulsion and contraction of surface charges to counter electrode. During the process solvent present in droplet evaporates and solid material present in droplet stretches due to electrostatic force between needle and collector. The developed fiber was collected from surface of the collector or drum. There are several parameters which influence the fibers formation. Those are (Sill and Von Recum 2008; Pham et al. 2006) solution related factors such as viscosity, dielectric constant, surface tension, boiling point, conductivity and processing parameters such as flow rate, distance between tip to collector, electrical potential supply and needle internal diameter. These parameters have to be optimized to develop fiber due to distant effect on the process. Moreover all parameters were interrelated with each other.
The fiber formation depends mainly on viscosity of solvent used to spin. Viscosity of material plays major role on structural morphology and diameter of fiber. High and low viscous solutions produces beads in fibers at the time of spinning, this was due to high amount of material present in the droplet was not stretched successfully at spinning leads to beads formation in case of high viscous solutions and the flow rate will be high to form fiber in case of low viscous solutions, during high flow rate there was a chance to formation of beads. To produce bead less continuous fibers optimum viscosity must be maintained to the solution mixture. High dielectric constant solvents are always suitable for electrospinning process. This was because there is a relationship between dielectric constant and electrical conductivity. The solvents with high dielectric constant have more electrical conductivity. The best example is water. Water with 80.4 dielectric constant value has high electrical conductivity than most of other solvents. Surface tension was the factor which opposes repulsion in spinning process. When the droplet exceeds its surface tension then only forms fiber. Thus the surface tension of solvent must be low always due to have major impact on the spinning process. The application of electrical potential was also depends up on the surface tension of the solvent. A high boiling point solvent have capacity to form a stable droplet/taylor cone and prevents gel forming on the get surface leads to prevention of clogging on surface of the needle (Moghe and Gupta 2008). The electrospinning process fundamentally requires transfer of electric charge from one electrode to another to form fiber. For this the bridge between both the electrodes was solution conductivity. Minimal conductivity solvent was at least required to maintain the statistic charge. Further addition of spinning material leads to increase the conductivity required to stretch the material (Andrady 2008; Shen et al. 2011).
The structural morphology and diameter of fiber partly depends on flow rate of solvent mixture from the needle maintained by syringe pump. High viscous solutions can’t pump with a high flow rate due to chance of beads formation, also low viscous solution cannot maintain with less flow rate due to not fiber formation. The main parameter to optimize to get uniformity in fiber diameter was the distance between tip to collector. During the distance between two electrodes material present in drop gets stretched as fiber and the solvent evaporates. Applied electrical potential provides the droplet gets stretched. Increase in the potential leads stretching the material present in the droplet and formation of very less diameter fibers (Buchko et al. 1999). Mainly the diameter of the fiber depends on internal diameter of needle also. Smaller diameter of the fiber was possible with small tip of the needle, but in all cases it might have not possible especially pumping of high viscous fluids. Usually sharp diameter needle provides more efficient charge to the solution jet (Berkland et al. 2004).
All parameters discussed above are interrelated with each other in electrospinning process. Viscosity, conductivity, surface tension, boiling point and dielectric constant of a solvent play major role on fiber diameter. The diameter of the fiber was more when the viscosity of the solution is high and vice versa. If the solution doesn’t have enough viscosity the droplet will break and reach ground plate as drops due to more surface tension and the fiber doesn’t formed (Deitzel et al. 2001). So to maintain surface tension of the droplet at the needle tip, minimum viscosity is required to the solution. The lesser diameter fiber was formed when the solution have high conductivity (Baumgarten 1971). The conductivity of the solution is high when the solvent has high dielectric constant. Low boiling point solvents are quickly evaporated and this is the drawback in preparation of fibers. This is due to form a solid lump at needle tip during the droplet comes through needle. Flow rate is depends up on viscosity of the solution and internal diameter of the needle (Megelski et al. 2002). Low viscous solutions can pass through the needle faster and the electrical charge supply will be more to form fiber. It is opposite in case of high viscous solutions. The fiber diameter can be reduced if the tip to collector distance is more (Doshi and Reneker 1995).
Sustained drug delivery systems are the most convenient approaches to reduce the dosage frequency, dose, improve patient compliance, to maintain steady state plasma concentration, economical and so on. A variety of approaches has been utilized so far to prepare sustained release dosage forms among those recently electrospinning process is using widely as a novel approach for preparation of sustained release dosage forms. In the present investigation Simvastatin (Sim) was selected as a model drug for the development of sustained release electrospinning fiber. Simvastatin, BCS class II drug, with low elimination half life 1.5–2 h, is a lipid lowering agent, used in the treatment of dislipidemia, an abnormal amount of lipid in the blood. It inhibits 3 hydroxy-3 methyl glutaryl co-enzyme A (HMG COA), a rate limiting enzyme in cholesterol biosynthesis (McClelland et al. 1991).
To the best of our knowledge simvastatin loaded polyethylene oxide electrospinning fibers has not been reported. Due to consideration of the drug solubility characteristics, polyethylene oxide (PEO), non ionic, water soluble resign, was selected as a release rate retarding agent. Due to its characteristics such as, biodegradability, hydrophilicity, rate retarding capacity, filament forming property in electrospinning field, in recent years it attracts great interest by using as a carrier in the development of drug delivery systems. PEO forms gel immediately when exposed to aqueous environment and the drug present inside PEO matrix was diffusion controlled (Dhawan et al. 2005). Hence the objective of the present study was to develop simvastatin loaded polyethylene oxide electrospinning fibers for sustained release and also investigate the parameters that affect the fiber formation. The optimized formulation was characterized for SEM, XRD, DSC and FTIR. In vitro release studies were carried out to fibers and drug release mechanism and in vitro release kinetics were investigated by fitting the obtained data to available mathematical models.
Materials
Simvastatin was provided by Biocon Ltd, Bangalore, India. Polyethylene oxide WSR coagulant 301 (Mw = 4,000,000) was purchased from Aldrich, USA. Chloroform, sodium dihydrogne phosphate, sodium lauryl sulphate were purchased from Loba Chemie Laboratory agents Pvt Ltd, Mumbai, India. All other chemicals used in the study are analytical grade.
Electrospinning
To determine the optimum drug-carrier w/w ratio, electrospinning (Peco Spin Apparatus, Physics Equipments Co., India) was carried out with 1:1, 1:2, 1:3 and 1:4 w/w ratios of drug-carrier. Chloroform was selected as a common solvent due to solubility of both materials. A clear homogeneous viscous solution was formed after addition of drug and carrier to solvent. The spinning solution was transferred into a 3 ml syringe and fitted to syringe pump to control the flow rate. The distance between tip to collector and applied voltage were optimized and given in following tabular columns. The fibers were collected on the metal plate covered with aluminium foil, which was acts as a negative electrode also, and dried under hot air oven (Lab Equipments, Bangalore, India) at 50 °C to remove the traces of solvent if any prior to further experimentation. All the batches fibers development has been carried at room temperature.
Solid mixtures prepared by kneading method
Sold mixtures were prepared with same ratios mentioned above by kneading method as a conventional approach. Initially drug and carrier were triturated in mortar with pestle by addition of solvent until complete mixing has been done. Then the semisolid mass was transferred through sieve no 18 to form uniform mixture and then dried under hot air oven for 30 min (Lab Equipments, Bangalore, India).
Drug content
Known amount of the fiber was dissolved in dissolution medium and kept in orbital shaker (Model-Cis 24 BL Remi Udyog, India) for overnight. The absorbance was recorded at λmax = 239 nm spectrophotometrically (LabIndia, UV 3000). The drug content was calculated by using following formula
FTIR studies
FTIR studies were performed to find out the interaction between drug and carrier. The pellet was prepared by grinding the drug and carrier with KBR and analyzed at wave number range from 4,000 to 500 cm−1 (BRUKER Optics, Model-Alpha 200218).
DSC studies
Thermal analysis was performed on pure drug, physical mixture and drug loaded fibers to study the physical state of the substances by differential scanning calorimetry (Perkin Elmer DSC 4000). In an aluminium pan the samples were placed and heated at 10 °C/min from 20 to 160 °C by placing pinch of iridium in aluminium pan as reference sample.
XRD studies
X-ray diffraction has been performed to pure drug and optimized formulations to study the physical state of the components in the drug loaded fibers (Panalytical, Model-Xpert Pro) with copper radiation in the 2θ range of 0°–80° at 40 mA and 45 kV.
SEM studies
Analysis were performed to pure drug and optimized formulation to find out the surface morphology studies by scanning electron microscopy (Jeol, Model-JSM 6610 LV).
In vitro release studies
The fiber was forcibly inserted into empty capsule shell and used for in vitro release studies (Verreck et al. 2003). The release studies were carried out using USP type II apparatus (LabIndia Model No: DISSO-8000). Sodium phosphate buffer (pH 7.0) containing 0.5 % sodium lauryl sulphate was used as dissolution medium maintaining at 37 ± 0.5 °C of bath temperature and paddle rpm at 50. Each time 5 ml of aliquot was withdrawn at predetermined intervals with a syringe fitted with prefilter and replaced with the same medium (maintained at 37 ± 0.5 °C). The sample was filtered through a 0.22 µm membrane filter disc (Millipore) and at λmax 239 nm absorbance was determined using double beam UV–Visible Spectrophotometer (LabIndia, UV 3000).
In vitro release kinetics
To explain the release rate of Sim, model dependent approach was chosen. The obtained in vitro release data was fitted to four popular mathematical equations such as zero order, first order, diffusion and Peppas-Korsemeyer equations. The order of drug release from electrospun fibers were described by zero order kinetics or first order kinetics. Diffusion and Peppas-Korsemeyer equations are useful to find out the drug release mechanism from matrix systems. The equations are as follows:
At time ‘t’ the fractional amount of drug release is ‘Q’ is and K0 is the zero order release rate constant.
The first order release rate constant is K1
The drug release mechanisms were explained by (Higuchi 1963) and Korsmeyer–Peppas equations (Peppas 1985)
where Mt/M∞ is the fraction of drug released at time t, K is the release rate constant. The n value was used to find out the drug transport mechanism. The value of n ≤ 0.45, means fickian diffusion, 0.45 < n < 0.85 follows non-fickian diffusion, n = 0.89 follows zero order kinetics (case II mechanism) and n > 0.89 follows super case II mechanisms.
Stability studies
The optimized formulation was kept for stability. The fibers were packed in HDPE screw caped bottles and kept in humidity chambers maintained at 25 ± 2 °C/60 ± 5 % RH and 40 ± 2 °C/75 ± 5 % RH as per ICH guidelines. In each case the samples stored in 25 ± 2 °C/60 ± 5 % RH storage conditions were withdrawn after 3 and 6 months and in case of samples stored in 40 ± 2 °C/75 ± 5 % RH storage conditions were withdrawn at 1, 2, 3 and 6 months. These samples were analyzed for appearance, in vitro release rate studies and content uniformity.
Results and discussion
The process is a multi factorial dependent initially fibers were developed only with carrier solution in order to optimize the processing parameters and once optimized the drug loaded fibers were developed with same optimized parameters. The optimized parameters for development of fiber were given in Table 1. It was observed that the flow rate, distance between tip to collector, applied voltage, viscosity and conductivity of the materials play major role on the development of fibers.
Figure 2 showed FTIR spectra of simvastatin and physical mixture. The narrow peak at 3,549.67 cm−1 indicates phenol OH stretching; the short peak obtained at wave number 3,373.66 cm−1 corresponds to N–H stretching of secondary amine group, the peak observed at 2,955.43 represents the C–H stretching of alkane group, the peaks found at 1,724.75 and 1,697.91 cm−1 belongs to C=O stretching respectively were observed with pure drug. The same characteristic bands were also observed in the physical mixture of simvastatin with PEO. From the obtained data it can conformed that the drug and carrier are compatible with each other and used for further experimental purpose.
The DSC studies were performed to find out physical state of the drug. The studies were done to pure drug, physical mixture and drug loaded fibers and thermograms were shown in Fig. 3, The DSC exhibited sharp peak at 140.54 °C corresponding to the drug melting point, also the sharp peak obtained at 140.65 °C corresponding to simvastatin in physical mixture, was similar to pure drug simvastatin, indicating that there was no interaction found between drug and polymer. There was no drug peak observed in the fiber DSC thermogram indicated that the drug was completely converted into amorphous from crystalline state. This was due to solubility of drug in the solvent chloroform and at the time of spinning of drug carrier mixture, soluble drug and PEO mixture forms matrix with amorphous form of the drug in it. The broad peak obtained at 69.34 and 70.15 °C corresponds to PEO in physical mixture and optimized fiber. The broadened peak of the carrier was due to loss of moisture present in it. There was no evidence of drug polymer interaction based up on DSC thermogram results.
The powder X ray diffractogram of simvastatin and optimized fiber were shown in Fig. 4. The XRD of simvastatin shown distinct number of sharp peaks at diffraction angle of 9.36, 10.94, 15.6, 16.5, 17.3, 18.8, and 19.3 indicated that the drug was in crystalline nature. The intensity of peaks was reduced in the range of diffraction angle of 9.53-18.8 and was completely minimized but sharp large peaks were observed only at the diffraction angle of 19.3 and 23.5 indicated that the negligible amount of drug was slightly in crystalline state on the surface of fibers (from SEM images) in comparison with that of pure simvastatin.
The morphology of pure drug and drug loaded fibers were shown in Fig. 5. The drug was in a crystalline state in pure form. No beaded fibers were obtained during the development. There were long, continuous with rough surfaces and non porous fibers can be observed.
Drug content in all the developed electrospun fibers was estimated in triplicate and given in Table 1. The uniformity of drug content in each batch was found and this was confirmed by low relative standard deviation values. There was no significant loss of drug during the preparation of electrospun fibers. The electrospinning process has shown no effect on the drug content uniformity. It was also confirmed that solvent used in the electrospinning process has no effect on content uniformity. Also almost 100 % of drug content was found in solid mixtures prepared by kneading method due to no chance of drug loss in preparation process.
In vitro drug release studies were performed and the data were given in Table 2. Initially burst release was observed in fibers developed with 1:1 drug carrier w/w ratio. This could be due to conversion of drug from crystalline to amorphous state during spinning process, as well as the effect of hydrophilicity of polymer on amorphous drug makes burst release in F1. The initial burst release was reduced to half in comparison with previous formulation in F2 and 5 h time was consumed to release 100 % of drug. In 1:3 drug-carrier w/w ratio 18.85 ± 0.04 % of drug was released in 1 h and 100.84 ± 0.47 drug was released at the end of 9th hour. Where the drug release was sustained in 1:4 drug-carrier w/w ratio and it meets objective of the study. 10.45 ± 0.2 % of drug was released in 1 h and 98.86 ± 0.42 % of drug from the formulation was released at the end of 12th hour. So from the above results it can be observed that even the drug was in amorphous state its release can be sustained by employing more quantity of the carrier. Here hydrophilic carrier was employed in the study for dual purpose i.e. to enhance the solubility of insoluble drug simvastatin and to sustain the drug release over a period of 12 h. The release pattern was shown in Fig. 6. When the hydrophilic matrix systems are exposed to aqueous environment or biological medium, the solvent penetrates into free space between the macromolecule chains of the polymer and may undergo relaxation process due to stress of penetrated solvent; the polymer chain become more flexible (Siepmanna and Peppas 2001; Brannon 1990) and decrease in glass transition temperature results conversion of glassy polymer into rubbery phase leads to swelling and form a gelatinous layer on the surface of the system (Flory 1953; Colombo et al. 2000). The gel layer becomes thicker and it depends up on the time with continuous penetration of medium into the system. The thickness of this region is a critical factor in drug release process and essentially depends on the viscosity of the polymer (Pham and Lee 1994). The drug release from the gelatinous layer is much slower and longer due to lengthy diffusion path of swelling matrix (Colombo et al. 2000) and decreased surface area at the penetrating solvent front (Joseph R. Robinson). As the gel layer becomes thicker, the distance that the drug must cover increases, which decreases its release rate. Exactly this was happen in formulations F3 and F4. Due to high drug-carrier ratios the drug release rate was sustained over a period of 12 h.
The release profile of solid mixtures prepared by kneading method with same drug-carrier w/w ratios were studied in same buffer solution for comparison purpose. Interestingly burst release was observed in all the formulations and within span of less than 1 h 100 % drug release was achieved. No effect on the drug release rate was observed even further increase in drug-carrier w/w ratios. This could be due to conversion of drug from crystalline state to amorphous state and wettability of drug soon after contact to release medium due to small individual particles.
The samples were withdrawn frequently from the stability chamber as described above and analysed for appearance, drug content and drug release rate studies. There was no significant change was observed in the appearance of the product, drug content as well as drug release profile. Hence this method is suitable for development of sustained release dosage forms. The obtained data from in vitro drug release studies were fitted to mathematical models and represented in Fig. 7. From those plots it was found that the drug release was followed zero order release rate kinetics with non fickian diffusion process as drug release mechanism.
Conclusion
In conclusion sustained release simvastatin fibers were successfully developed by using electrospinning apparatus as a tool, and the processing parameters which effect the fiber formation were also studied. The effect of polyethylene oxide, as carrier, on the drug release rate of electrospinning fibers and solid mixtures prepared by kneading method was evaluated and found that the fibers developed with 1:4 drug-carrier ratio was sustained over a period of 12 h only. Stability studies were also performed to optimized electrospinning fibers and no significant difference in comparison with freshly developed fiber in the name of appearance, drug content and in vitro drug release rate was observed. Finally it can be conclude that the electrospinning process is an alternative method of approaches for preparation of sustained release dosage forms and have future applications in drug delivery effectively.
References
Andrady AL (2008) Science and technology of polymer nanofibers. Wiley, New York, pp 81–96
Baumgarten P (1971) Electrostatic spinning of acrylic microfibers. J Colloid Interface Sci 36(1):71–79. doi:10.1016/0021-9797(71)90241-4
Berkland C, Pack DW, Kim KK (2004) Controlling surface nano-structure using flow-limited field-injection electrostatic spraying (FFESS) of poly(D, Llactide-co-glycolide). Biomaterials 25(25):5649–5658. doi:10.1016/S0142-9612(04)00054-7
Brannon LP (1990) Preparation and characterization of crosslinked hydrophilic networks. In: Brannon-Peppas L, Harland RS (eds) Absorbent polymer technology. Elsevier, Amsterdam, pp 45–66
Buchko CJ, Chen LC, Shen Y, Martin DC (1999) Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer 40(26):7397–7407. doi:10.1016/S0032-3861(98)00866-0
Colombo P, Bettini R, Santi P, Peppas NA (2000) Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol Today 3(6):198–204
Deitzel JM, Kleinmeyer J, Harris D, Tan NCB (2001) The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42(1):261–272. doi:10.1016/S0032-3861(00)00250-0
Deng-Guang Y, Li-Min Z, White K, Branford-White C (2009) Electrospun nano fiber based drug delivery systems. Health 1(2):67–75. doi:10.4236/health.2009.12012
Dhawan S, Dhawan K, Varma M, Sinha VR (2005) Applications of polyethylene oxide in drug delivery systems—Part II. Pharm Technol 29:82–96
Doshi J, Reneker DH (1995) Electrospinning process and applications of electrospun fibers. J Electrost 35(2–3):151–160. doi:10.1016/0304-3886(95)00041-8
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, New York
Halliday AJ, Moulton SE, Wallace GG, Cook MJ (2012) Novel methods of antiepileptic drug delivery polymer-based implants. Adv Drug Deliv Rev 64:953–964. doi:10.1016/j.addr.2012.04.004
Higuchi T (1963) Mechanism of sustained action medication theoretical analysis of rate of release of solid matrices. J Pharm Sci 51:1145–1149. doi:10.1002/jps.2600521210
Larrondo L, Manley RSJ (1981) Electrostatic fiber spinning from polymer melts. I. Experimental observations on fiber formation and properties. J Polym Sci Polym Phys Ed 19:909–920. doi:10.1002/pol.1981.180190601
McClelland CA, Stubbs RJ, Fix JA, Pogany SA, Zentner GM (1991) Enhancement of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductage inhibitor efficacy through administration of a controlled osmotic pump dosage form. Pharm Res 8(7):873–876. doi:10.1023/A:1015899328105
Megelski S, Stephens JS, Chase DB, Rabolt JF (2002) Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 35(22):8456–8466. doi:10.1021/ma020444a
Meinel AJ, Germershaus O, Luhmann T, Merkle HP, Meinel L (2012) Electrospun matrices for localized drug delivery: current technologies and selected biomedical applications. Eur J Pharm Biopharm 81:1–13. doi:10.1016/j.ejpb.2012.01.016
Moghe AK, Gupta BS (2008) Co-axial electrospinning for nanofiber structures: preparation and applications. Polym Rev 48:353–377. doi:10.1080/15583720802022257
Peppas NA (1985) Analysis of Fickian and Non-Fickian drug release from polymers. Pharma Acta Helv 60:110–111
Pham AT, Lee PI (1994) Probing the mechanisms of drug release from hydroxypropylmethyl cellulose matrices. Pharm Res 11(10):1379–1384. doi:10.1023/A:1018975318805
Pham QP, Sharma U, Mikos AG (2006) Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng 12:1197–1211. doi:10.1089/ten.2006.12.1197
Reneker DH, Yarin AL (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys 87(9):4531–4547. doi:10.1063/1.373532
Reyderman L, Stavchansky S (1995) Electrostatic spraying and its use in drug-delivery-cholesterol microspheres. Int J Pharm 124:75–85. doi:10.1016/0378-5173(95)00078-W
Robinson JR, Lee VHL (1987) Controlled drug delivery: fundamentals and applications. 2nd edn. 390. Dekker
Shen X, Dengguang Y, Zhu L, Branford-White C, White K, Chatterton NP (2011) Electrospun diclofenac sodium loaded Eudragit® L 100-55 nanofibers for colon-targeted drug delivery. Int J Pharm 408:200–207. doi:10.1016/j.ijpharm.2011.01.058
Siepmanna J, Peppas NA (2001) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 48:139–157. doi:10.1016/S0169-409X(01)00112-0
Sill TJ, Von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006. doi:10.1016/j.biomaterials.2008.01.011
Taylor GI (1964) Electrically driven jets. Proc R Soc Lond 313A:453. doi:10.1098/rspa.1969.0205
Verreck G, Chun I, Peeters J, Rosenblatt J, Brewster ME (2003) Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm Res 20(5):810–817. doi:10.1023/A:1023450006281
Acknowledgments
This article dose not contain any studies with human and animal subjects performed by any of the authors. All authors (S. Betha, B. P. Reddy, M. M. Varma, D. B. Raju, and V. R. M. Kolapalli) declare that they have no conflict of interest. The authors are very much thankful to Biocon Ltd, for providing Simvastatin as gift sample.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Betha, S., Pamula Reddy, B., Mohan Varma, M. et al. Development of simvastatin electrospun fibers: a novel approach for sustained drug delivery. Journal of Pharmaceutical Investigation 45, 13–22 (2015). https://doi.org/10.1007/s40005-014-0140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0140-5