Abstract
Low-input organic agriculture is preferred over conventional agriculture for its sustainability and environmentally friendly nature. In this investigation, a field trial experiment was conducted to unravel the effect of conventional and organic farming practices on soil health and productivity under rice–wheat cropping system. Moreover, the dynamics of microbial communities was analyzed under both the farming systems using denaturant gradient gel electrophoresis (DGGE) and qRT-PCR techniques. This study found that the soil organic carbon was significantly higher under the organic farming system (0.63 ± 0.3% in wheat and 0.88 ± 0.2% in rice) than the conventional farming system (0.2 ± 0.1% in wheat and 0.63 ± 0.3% in rice). Quantification of 16S rDNA and nifH genes revealed higher abundance of total bacteria (2.52 × 1011 in rice and 2.40 × 1011 in wheat) as well as diazotrophs (8 × 106 in rice and 1.8 × 107 in wheat) under the organic farming system. Therefore, higher copy number of nifH genes in organic soil indicated that the diazotrophs played a significant role in supplying N for plant growth. DGGE band pattern authenticated that the bacterial diversity was higher under organic farming system. This study also showed that conventional practice gives comparatively more yield i.e. 0.29 t ha−1 extra rice and 2.86 t ha−1 more wheat than in organic farming. All other plant growth parameters are found higher in organic except nitrate reductase activity. This study signifies that organic farming is sustainable and can substitute the conventional practice for cost-effective, beneficial soil health and environmental sustainability for the long term.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Population explosion around the globe, especially in India, since last decades demands increased productivity from the agricultural sector. Moreover, to feed the projected 11 billion people by the end of twenty-first century is one among the great challenges facing humanity [35]. Modernization of agricultural farming systems tends to generate adequate food supply to the world’s existing population, and this feat inevitably led to the conventional farming practices which require high capital, high input, rationalization and uses of agrochemicals [16]. However, it is imperative to manage the contradictions between agricultural modernization, social equitability, agroecological sustainability and resilience for over the long term. Under conventional farming systems, applications of extensive synthetic pesticides and growth fertilizers have a negative impact on environmental health and also lead to long-term degradation of soil resources, which is a global concern [7, 31].
In India, the production and consumption of chemical fertilizers have shown substantial growth over the years. Whereas pesticides consumption was 0.29 kg ha−1 (gross cropped area) in the year 2014–2015, this was 50% higher than the use in 2009–2010 [9]. The intensive use of these agrichemicals poses potential threats to surrounding ecosystems and causes a human health hazard. Ever since from the dawn of the Green Revolution, it is estimated that approximately 800,000 people have lost their lives due to pesticides in developing countries [8]. Thus, at the present scenario, modern agriculture is facing a twinning challenge that is augmentation in agricultural production and environmental sustainability. Therefore, the shift from conventional agriculture to alternative sustainable low-input agriculture system is required for prosperous and environmentally friendly growth.
Among alternative agriculture systems, organic farming has achieved tremendous thrust around the globe due to its agroecological, socioeconomic and cost-effective concerns [2]. Organic agriculture is the holistic approach which provides sustainability, low-input and environmentally friendly system and is considered as a suitable substitute for conventional agriculture system. Furthermore, it involves the use of biofertilizers, biopesticides, composts and manures, which besides increasing soil fertility, enhances natural processes, improves soil organic matter status and also nurtures soil biological function, thus reclaiming degraded soil properties for long-term soil fertility [26]. Moreover, organic foodstuffs have increasing high demand as they are produced according to specified standards, which control the use of chemicals in crop with minimal environmental impact. Nevertheless, organic food has many defense-related secondary metabolites which are missing in conventional food [5]. At present, the maximum percentage of certified organically farmed area is occupied by Australia than any other country, with around 23 million hectares under cultivation. However, India has the greatest absolute number of recognized organic farmers [34]. Organic farming is more relevant to the Indian scenario where the government put special emphasis on policies like “per drop, more crop” and “zero budget natural farming” in which farming takes place without using any credit or chemicals but with natural means [15].
However, there are reports which suggest that currently, conventional agriculture contributes to 98.9% of the total world’s food [36]. But the proponents of organic agriculture often identify various disadvantages of conventional agricultural practices [4, 6, 22]. Discrepancies between reports may be due to interactive effects of several farming practices, soil quality, crop varieties, time of harvesting, etc. [27]. The genuine overview of wholesome sustainability of organic farming systems, however, continues to face many challenges. Such concerns require a greater understanding of the long-term productivity of organic farming system and its feasibility as an alternative to the conventional practices, for sustainable use of natural resources.
Therefore, the present investigation was conducted for comparing conventional and organic management systems with major emphasis on soil health, soil microbial diversity along with plant growth and productivity.
Materials and Methods
Study Site and Experimental Design
A field trial experiment was conducted in organic agriculture technology block at G.B. Pant University of Agriculture and Technology, Pantnagar (29° 01′ 12.0″ N and 79° 29′ 16.8″ E at 243.84 m altitude), with Pusa Basmati 1 variety of rice and UP 2565 variety of wheat (Online resource). The two plots—one of organic and one of conventional farming system (of 408 m2 each), were split into three subblocks for conducting the experiments by using randomized block design (RBD). Organic and conventional fields were separated with a tract of Sesbania as a buffer zone in rice and with flax in wheat. Sesbania was used for green manuring in organic rice field before rice transplantation, while in wheat farmyard manure was used during the sequential period. Chemical fertilizers (NPK) were used only in the conventional plot at regular interval. Soil order was described as Mollisol with suborder udoll and great group Hapludoll (USDA classification). The soil was silt clay loam in texture with pH 7.25 (± 0.4) and electrical conductivity 0.22 (± 0.1) dS m−1 (in a soil/water ratio of 1:2.5, weight/volume). Further, soil organic carbon (% oxidizable carbon) was 0.63 (± 0.3) % of soil mass, nitrate–nitrogen was 10.00 (± 0.8) kg ha−1 and phosphorus (as P2O5) was 65.00 (± 1.1) kg ha−1. The climate of the experimental site was subtropical and humid with 1000–2000 mm annual rainfall. The temperature ranges from ~ 48 °C in summer to < 0 °C in winters. The relative humidity was recorded highest in July–August, while it was recorded lowest in April and May.
Soil and Plant Sample Collection
Soil samples were collected at different time intervals, i.e. before plowing, after transplantation/sowing in rice/wheat, at 45 days after transplantation/sowing (DAT/DAS) (at maximum tillering), 60 DAT/DAS (panicle initiation) and 90 DAT/DAS (at maturity). Soil samples from each study site, i.e., conventional and organic fields, for both crops, i.e., rice and wheat, respectively, were collected from different points between the rows of the crop at the depth of 0–15 cm and 15–30 cm. All the collected samples from each farming system were quickly transferred to sterile zip plastic bags and transported to the laboratory and stored at 4 °C for subsequent soil analysis. For metagenomic analysis, soil samples were stored at − 20 °C. All the analyses were performed in triplicates.
Soil Chemical Analysis
Sampled soil was divided into two parts: one part was kept for microbial community analysis, and another part was taken for soil pH, total organic carbon (TOC), nitrate–nitrogen (NN) and available phosphorus (P) analysis, respectively. All soil testing was performed by the K054 soil testing Kit, Himedia Laboratories Pvt Ltd India, as recommended by the manufacturer.
Microbial Community Analysis
Soil DNA Extraction
Total soil DNA was extracted from 0.50 g (fresh weight) soil sample by using the Powersoil™ DNA isolation kit (Mobio Lab. Inc., USA), according to the manufacturer’s instructions. DNA was quantified spectrophotometrically at 260 nm and stored in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) at − 80 °C till further use [33].
Quantification of 16S rDNA and nifH Copy Number in Soil
Copy numbers of 16S rDNA and nifH genes from collected soil samples were quantified using iCycleriQ™ Multicolor (Bio-Rad Lab, Hercules, USA) real-time polymerase chain reaction (qPCR) machine as described in previous studies [17, 32].
Denaturant Gradient Gel Electrophoresis (DGGE) Analysis
PCR for DGGE analysis was performed as per earlier studies [17, 32]. DGGE was performed on a Dcode system (Bio-Rad Lab, Hercules, CA, USA), and the products were separated on 8% (w/v) acrylamide–bisacrylamide gel with a 40–70% denaturing gradient of urea and formamide at 60 °C at 90 V in 1X TAE for 16 h. The gels were stained for 30 min in ethidium bromide in 1X TAE (Invitrogen, Paisley, UK) and visualized with a Gel Documentation System (Bio-Rad Lab, Hercules, CA, USA).
Plant Growth Parameters
Plant shoot length, root length, plant dry weight, number of tillers per hill and number of spikelet per panicle were studied at 45, 60 and 90 DAT for rice/DAS for wheat. Yield in tonne per hectare (t ha−1) and harvest index (HI) were also studied after the harvesting in both rice and wheat.
Plant Biochemical Parameters
Total leaf chlorophyll content and leaf nitrate reductase activities were studied by using previously mentioned methods of Hiscox and Israelstam [13] and Hageman and Hicklesley [11], respectively.
Statistical Analysis
Three replicates of each treatment were taken for statistical analysis, and resulting values were expressed as mean ± SEM. Further, the results were statistically evaluated using a Statistical Package for Social Science (SPSS) tool comparing the mean values of two independent assortments. Student's t test (P ≤ 0.05) was carried out to assess any significant differences between the means of traits under the organic and conventional mode of crop production.
Results
Soil Chemical Properties
The initial soil pH before plowing was 7.25 (± 0.4) and had increased slightly under both farming systems after transplantation/sowing. At 45 and DAT, it was observed that the pH of conventionally and organically managed soil was around 8 and 7, respectively. Moreover, at 90 DAT, the soil pH was found to decrease under both the farming systems (Table 1).
In rice field, the TOC before plowing, after transplantation and at 45 DAT was 0.40 (± 0.10) % and 0.20 (± 0.10) % under organic and conventional conditions, respectively. At 60 DAT and 90 DAT, the organically managed TOC ranged from 0.63 (± 0.30) %, whereas there was no change in the organic carbon in conventional farming. In contrast, TOC in wheat field before plowing was 0.40 (± 0.10) % and 0.63 (± 0.30) % at 45 days and 0.88 (± 0.20) % at 90 DAS for organically managed soil. Then, it was 0.40 (± 0.10) % in conventional soil before plowing and increased to 0.63 (± 0.30) % at 45 DAS and remained the same thereafter (Table 1). All the differences were significant at P ≤ 0.05.
The available P content of the rice soil was 7.30 mg kg−1 [22.00 (± 0.90) kg ha−1] before plowing, and after transplantation, it was significantly increased to 13.00 mg kg−1 [39.00 (± 0.90) kg ha−1] in both practices and then decreased in the organic soil and remained constant in conventional soil. On the other hand, the available P content of the organically managed wheat soil at sowing time was the same with rice soil. At 45 DAS, it was increased to 13.00 mg kg−1 [39.00 (± 0.90) kg ha−1] and thereafter remained the same till 90 DAS. Under conventionally managed wheat soil, the initial available P content was 13.00 mg kg−1 [39.00 (± 0.90) kg ha−1]. At 45 DAS and 60 DAS, it significantly increased up to 21.70 mg kg−1 [65.00 (± 1.10) kg ha−1] and thereafter at 90 DAS came down to 13.00 mg kg−1 [39.00 (± 0.90) kg ha−1] (Table 1), subsequently.
Soil NN content of the rice before plowing and after transplantation was near 4.00 (± 1.10) kg ha−1. No general trend was observed for subsequent time intervals of 45 and 60 DAT for both the soils. In wheat soil, initially, the NN was about 4.00 (± 1.10) kg ha−1 for both management systems. Further, at 45, 60 and 90 DAS, it was 10.00 (± 0.80), 20.00 (± 1.30) and 10.00 (± 0.80) kg ha−1, respectively, for organic soil (Table 1). Similar trend was recorded in conventional soil at all stages of growth. All the differences observed were significant at P ≤ 0.05.
Total Bacterial and Free-living Diazotrophic Count
Real-Time Quantification of 16S rDNA and nifH genes in soil revealed that the copy numbers of 16S rDNA and nifH genes were significantly higher in organic soil over conventional soil for both rice and wheat at all subsequent days (Fig. 1). In both rice and wheat soil, nifH copy number increased at all stages (DAT/DAS) and reached a maximum at 90 DAT/DAS in organic soil at 0–15 cm depth. Under conventional soil, nifH copy number was almost the same in all stages (DAT/DAS) at 0–15 cm. Copy number of both 16S rDNA and nifH was found lower in the soil at 15–30 cm as compared to 0–15 cm depth (Fig. 1). At 15–30 cm depth, 16S rDNA copy number was the same in both management practices. However, nifH abundance was higher at 15–30 cm depth for organic soil and also increased slightly temporally. These defined shifts in microbial communities with depth would likely to be governed by contemporary physiochemical factors resulting from strong environmental gradients such as surface nutrient input and abundant TOC [30].
Dynamics of 16S rDNA and nifH gene copy numbers in soil at different time intervals under the organic and conventional farming practices where a 16S rDNA copy number in rice soil, b nifH gene copy numbers in rice soil, c 16S rDNA copy number in wheat soil and d nifH gene copy numbers in wheat soil. BP, AT and AS represent before plowing, after transplantation and after sowing stages, O and C represent organic and conventional soil from 0–15 and 15–30 cm depth, and DAT and DAS represent days after transplantation and days after sowing, respectively
Moreover, lower nifH gene abundance in conventionally managed soil may be due to the application of synthetic fertilizer which provides higher levels of mineral N in the soil, thus suppressing the activity of N-fixing bacteria. Further, diazotrophic communities are also sensitive to the pesticides which might be considered as a major factor contributing to the low nifH copy number [23].
Management Impact on Bacterial Community Structure
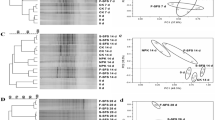
DGGE analysis has shown that the intensity of the bands in organic soil was comparatively higher suggesting the increase in abundance of the respective group of bacteria (Fig. 2). It justifies the increment in bacterial copy numbers under organic soils due to selective increase of some dominant bacterial communities. These results of DGGE were supported by the RT-PCR results, which showed higher bacterial count in all studied stages in rice under organic cultivation practices. However, in wheat-cultivated soil, there was comparatively more difference in the community fingerprint under both organic and conventional soils. The more number of DNA bands was observed in organic soil as compared to the conventional soil at all studied stages of wheat, which indicates higher bacterial diversity in the earlier (Fig. 2). Therefore, the shift from the waterlogged condition in rice to comparatively dry soil in wheat could cause differential changes in soil microbial community structure. Moreover, this difference could also arise from the establishment of a wheat plant, which enriches different bacterial communities through different root exudates as compared to the rice crop.
DGGE profile of 16S rDNA under organic and conventional management practices, where a 16S rDNA DGGE profile of rice soil and b 16S rDNA DGGE profile of wheat soil, where BP, AT and AS represent before plowing, after transplantation and after sowing stages, DAT and DAS represent days after transplantation and days after sowing, and O and C represent organic and conventional soil from 0–15 and 15–30 cm depth, respectively
Plant Growth Parameters
Studies of plant growth parameters suggest that the organic practices showed significant and positive response in a number of vegetative characteristics. Plant shoot length, root length, dry weight and number of tillers were found significantly higher for organically grown rice at all subsequent stages (Table 2). However, numbers of spikelet per panicle were comparatively lower for organically grown rice (Table 2). Similarly, in the case of wheat, shoot length, root length and dry weight were found higher, while the number of tillers and number of spikelets per panicle were significantly lower for the organic field (Table 2).
The yield of organically and conventionally managed rice was 4.31 ± 1.54 ton per hectare (t ha−1) and 4.6 ± 1.78 t ha−1, respectively, with a net increase of 0.29 t ha−1 in conventional farming practice. Harvest index (HI) was found to be 0.42 and 0.39, respectively (Fig. 3). On the other hand, yield in the organically and conventionally managed wheat was 3.26 ± 1.98 t ha−1 and 6.12 ± 1.86 t ha−1, respectively, with the large difference of 2.86 t ha−1, and HI was 0.45 and 0.37, respectively (Fig. 3).
Although the comparative yield of rice is higher in conventional farming, monetary return from 0.29 tons of rice grain will be lesser than money invested on the extra inputs in conventional farming practice. Nevertheless, for organic wheat cultivation, the significantly low yield was observed which may be due to noninclusiveness of the other factors affecting organic agriculture.
Plant Biochemical Properties
The trend of leaf nitrate reductase (NR) activity and leaf total chlorophyll content was found similar in both rice and wheat. Both the studied parameters were significantly higher for conventionally grown rice and wheat in all subsequent days. Temporally, NR activity was found maximum at 45 DAT/DAS in both the crops—rice (0.57 ± 0.2 and 0.49 ± 0.1 μ mol NO2 g−1 fr.wt in conventional and organic systems, respectively) and wheat (3.15 ± 0.2 and 1.75 ± 0.2 μ mol NO2 g−1 fr.wt in conventional and organic systems, respectively). After 45DAT/DAS, it was then subsequently decreased (Fig. 4). Total chlorophyll content was found maximum at 60 DAT/DAS in both the crops—rice (4.90 ± 0.1 and 3.85 ± 0.2 mg g−1 fr.wt in conventional and organic systems, respectively) and wheat (2.00 ± 0.1 and 1.63 ± 0.2 mg g−1 fr.wt in conventional and organic systems, respectively) and thereafter decreased (Fig. 4).
Comparative biochemical indicators in rice and wheat at different time intervals, where a and c represent leaf nitrate reductase activity in rice and wheat, respectively. Further, b and d represent total leaf chlorophyll content in rice and wheat, respectively. Each vertical bar represents standard error of mean. DAT and DAS represent days after transplantation and days after sowing
Discussion
This study suggests that the organic cultivation is sustainable and positively affects soil nutrients, soil microbial community including beneficial nitrogen-fixing bacteria and also plant growth. It was found that soil OC content was higher in organic soil in both crops and remained medium to high at all studied stages (DAT/DAS) (Table 1). Previous studies have shown that the several years of contrasting soil management have a minute difference in soil OC content [18]. In this study, addition of the green manure in rice and farmyard manure in wheat under organic practice increases soil OC content. Therefore, these findings highlight the role of organic management practices and the type of manure used on the status of soil OC. Further, the pH was found acidic in organic soil and alkaline in conventional soil. This showed that green manure acts as a soil-acidifying agent and decreases the alkalinity of soil by generating humic and acetic acid [21]. Moreover, P and N content in rice and wheat soil was found variable in both agricultural practices during the experiment. Applications of N and P fertilizer in conventional soil and green manure and farmyard manure under organic regime are responsible for the increase. Previous results [25] suggested that the soil nitrate N and phosphate P were significantly higher in organic soil than their conventional counterpart. This inconsistency might be the result of increased doses of fertilizers used in conventional soil which will always increase soil P and NN.
During the investigation, a relatively high copy number of nifH genes in organic soil clearly indicates that the nitrogen-fixing bacterial communities are increasing in organic soil. This increase under the organic system is attributed to the implication of green manure and farmyard manure as its decomposition creates a nutrient flush, which influences the distinct heterotrophic microbial assemblage by significantly increasing soil microbial count and biomass, thereby restoring the microbial communities [28]. Moreover, it has been already reported [3] that biological nitrogen fixation (BNF) is sufficient to fulfill nitrogen requirements in agricultural land without the involvement of synthetic fertilizers. Therefore, organic farming has enough potential to supply N and P in soil besides secondary and trace elements, usually lacking in conventional farming systems [1, 24].
Microbial community structure in the soil is affected by agricultural management, such as crop rotation, tillage, compost, manure, chemical fertilizers and pesticides [10]. Besides it, vegetation also plays a crucial role by releasing different carbon sources in the soil, which decides the community structure of microorganisms in the soil. In this study, DGGE and qPCR were used to compare the bacterial community in both management practices under the combined effects of these factors. Quantification of 16S rDNA and nifH gene in both rice and wheat soil showed that the copy numbers of these genes were high in organic soil and decreased with depth (Fig. 3). In the case of rice, copy numbers of 16S rDNA at 0–15 cm depth were found increasing at all DAT for organic soil. However, at 15–30 cm, 16S rDNA copy numbers were drastically reduced from the initial count taken before plowing in both managements but comparatively found higher in organic soil at 60 and 90 DAT. Water-logged condition in rice cultivation could be the reason for this decrease in the total bacterial count at 15–30 cm. Similar trend was observed in wheat soil, and bacterial abundance was slightly increased at 45 DAS and thereafter remained almost constant.
Copy number of nifH genes in rice was found to increase with time and reached a maximum at 90 DAT in organic soil at 0–15 cm depth (Fig. 1). Under conventional practice, temporal dynamics of nifH was following the same trend as organic but the copy number was comparatively less than organic soil. Comparatively, in wheat-cultivated soil, nifH gene copy number was high as compared to rice in both practices. Nonetheless, in conventional soil, nifH gene was almost the same at all DAS but in organic it was profoundly increasing at 45, 60 and 90 DAS at both 0–15 and 15–30 cm depth. This suggests that previously established diazotrophs in rice soil were working as founder/pioneer population for wheat agroecosystem and further extending their populations under organic condition [17, 23].
Increased nifH gene abundance was not always related to the increased NN content in soil for both rice and wheat. This indicates that the expression of nifH gene is not always consistent with the copy number of nifH in soil, but changes according to the plant nitrogen requirement and growth stages. Further, NR activity and soil NN were found maximum in 45 DAS/DAT, suggesting that the diazotrophic community was most functional at 45 DAS/DAT. High levels of chemical fertilizers in conventional practice maintain soil NN level as sufficient and also high to suppress the growth of diazotrophs, thus contributing to comparatively low nifH abundance.
DGGE pattern in organic and conventional rice soil suggested that there is a slight difference in bacterial community structure with high band intensities in organic soil. Increase in the abundance of single bacterial genera or species, attributing single band in DGGE profile, is justified for the increased intensity of bands. Thus, organic agriculture favored some bacterial groups over others in rice soil, which leads to an increase in overall bacterial count as also evident by increased 16S rDNA copy number in organic soil. On the other hand, the DGGE pattern of wheat showed the comparatively high bacterial diversity in organic soil. High OC in organic farming could be the reason for this rich bacterial diversity, which favors the growth of specific microbial guilds which have the capacity to degrade complex organic compounds such as manure and compost [12, 20]. Further, it is evident from the DGGE analysis that bacterial community structure is not the same in rice and wheat under organic practices. Therefore, plant species, type of manure, seasonal variation and duration of organic practice are the major factors affecting the bacterial community structure in the soil. From these findings, it can be concluded that the organic agricultural practices are positively increasing soil microbial flora which in turns benefits plant health, and also, the crop rotation has an influential role in establishing soil microbial structure.
The average organic-to-conventional-yield ratio in this study was 0.93 and 0.53 for rice and wheat, respectively. However, in a global meta-analysis of comparative management, this ratio was found o be 0.75 [29]. Rice yield in organic farming was at par with conventional farming with 0.29 t ha−1 extra productions in conventional practice. Thus, it can be concluded that organic farming is proved to be sustainable for rice cultivation. These findings were in accordance with earlier studies [19], which showed that lower productivity of organic rice was balanced out by lower variable production costs, thus making rice production system considerably more profitable, healthy and sustainable under the organic regime. Furthermore, a large difference in wheat yield was observed with 2.86 t ha−1 more productions under conventional practice.
These results were consistent with the global study [29], which documented the yield difference under different management systems and revealed that organic yields are typically lower than conventional yields. However, these differences were highly contextual and depend on the site of farming practices and management systems. Organic farming with poor pest management would enhance soil nutrient status and restore soil fertility but this will reduce yield severely in comparison with conventional farming where chemical pesticides are used for pest management. Moreover, many comparative studies suggest that under good management conditions yield from organic agriculture meets the conventional practice [29]. Therefore, all the major aspects affecting soil fertility, plant health and inputs like plant varieties, type of manure, N/P biofertilizers and pest management should be considered with technical skills for better yield in organic agriculture.
Contrary to plant growth parameters, the HI was found to be increased in both rice (0.42 ± 0.1) and (0.45 ± 0.1) wheat under organic practices than that of conventional farming (0.39 ± 0.1 and 0.37 ± 0.2, respectively). This increase signifies that the photosynthates are partitioned well to the grains, which suggest that under organic conditions these genotypes are having a better source to sink conversion which proved to be an economical affair.
Total leaf chlorophyll and NR activities were observed higher under conventional farming in all stages of rice and wheat (Fig. 4). Chlorophyll which is a nitrogen-containing compound is positively correlated with available nitrogen content in soil and supposed to be low under nitrogen stress. NR is an inducible enzyme and is positively correlated with nitrogen input in soil [17]. Thus, higher NR activity in conventional soil is the result of the application of synthetic nitrogen fertilizers. On the other hand, NR activity was comparatively less under organic farming because nitrogen is provided by diazotrophs and applied manure which releases nitrogen slowly which is sufficient for plant requirements. However, over-fertilization in conventional agriculture causes imbalance of nutritional elements in plants and also reduces resistance to insect pests [14]. Therefore, this study established the relationship between soil-available nitrogen and NR activity in the crop under conventional and organic management systems.
Lastly, the comparative results of both conventional and organic management practices under rice–wheat cropping systems are setting an example for promoting new sustainable and environmentally friendly alternatives. It is worthy to mention that in previous studies, organic agriculture had emerged as one such alternative but this practice is criticized by the advocates of conventional agriculture for its low yield. However, a global study [3] concluded that organic methods are sufficient to produce enough food without increasing the agricultural land to sustain the current and even larger human population. Thereafter, continuous efforts are made to study the comparative effect of organic and conventional management practices. In this continuation, the present study was aimed to analyze comparative conventional and organic agricultural practice with major emphasis on soil health, microbial community structure and plant growth during the initial first year of successive rice and wheat cultivation.
Conclusion
In conclusion, organic farming practices were significantly enhancing soil health and associated microbial communities including diazotrophs. Moreover, a positive effect of organic management system has also been observed on the crop productivity. The soil phosphate availability and nitrogen content were most determining factor among both the farming systems. Furthermore, diazotrophic population was found most sensitive toward different management practices with better adaptation in organic farming during the first year of study, which will help in reclaiming long-term soil fertility in upcoming years. Conclusively, it is evident that organic management system is both sustainable and economical and must be adopted for long-term benefits.
Abbreviations
- SOC:
-
Soil organic carbon
- NPK:
-
Nitrogen phosphorous potassium
- NN:
-
Nitrate nitrogen
- DGGE:
-
Denaturant gradient gel electrophoresis
- NR:
-
Nitrate reductase
References
Altieri MA, Nicholls CI (2003) Soil fertility management and insect pests: harmonizing soil and plant health in agroecosystems. Soil Tillage Res 72(2):203–211. https://doi.org/10.1016/S0167-1987(03)00089-8
Araujo AD, Santos VB, Monteiro RT (2008) Responses of soil microbial biomass and activity for practices of organic and conventional farming systems in Piauí state, Brazil. Eur J Soil Biol 44(2):225–230. https://doi.org/10.1016/j.ejsobi.2007.06.001
Badgley C, Moghtader J, Quintero E, Zakem E, Chappell MJ, Aviles-Vazquez K, Samulon A, Perfecto I (2007) Organic agriculture and the global food supply. Renew Agric Food Syst 22(2):86–108. https://doi.org/10.1017/S1742170507001640
Borel BR (2017) When the pesticides run out. Nature 543:302–304
Brantsaeter AL, Ydersbond TA, Hoppin JA, Haugen M, Meltzer HM (2017) Organic food in the diet: exposure and health implications. Annu Rev Public Health 38:295–313. https://doi.org/10.1146/annurev-publhealth-031816-044437
Chagnon M, Kreutzweiser D, Mitchell EA, Morrissey CA, Noome DA, Van der Sluijs JP (2015) Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ Sci Pollut Res 22(1):119–134. https://doi.org/10.1007/s11356-014-3277-x
Desai P, Patil A, Veeresh K (2017) An overview of production and consumption of major chemical fertilizers in India. J Pharmacogn Phytochem 6(6):2353–2358
Devi PI, Judy T, Raju RK (2017) Pesticide consumption in India: a spatiotemporal analysis. Agr Econ Res Rev 30(1):163–172
FAOSTAT (2017) Pesticides. Food and Agriculture Organization, Rome
Grantina L, Kenigsvalde K, Eze D, Petrina Z, Skrabule I, Rostoks N, Nikolajeva V (2011) Impact of six-year-long organic cropping on soil microorganisms and crop disease suppressiveness. Zemdirbyste 98(4):399–408
Hageman RH, Hicklesly DP (1971) Nitrate reductase from higher plants. In: Methods in enzymology, vol 23. Academic Press, pp. 491–503. https://doi.org/10.1016/S0076-6879(71)23121-9
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9(5):1177–1194. https://doi.org/10.1038/ismej.2014.210
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334. https://doi.org/10.1139/b79-163
Hu XF, Cheng C, Luo F, Chang YY, Teng Q, Men DY, Liu L, Yang MY (2016) Effects of different fertilization practices on the incidence of rice pests and diseases: a three-year case study in Shanghai, in subtropical southeastern China. Field Crops Res 196:33–50. https://doi.org/10.1016/j.fcr.2016.06.004
Khadse A, Rosset PM, Morales H, Ferguson BG (2018) Taking agroecology to scale: the zero budget natural farming peasant movement in Karnataka, India. J Peasant Stud 45(1):192–219. https://doi.org/10.1080/03066150.2016.1276450
Knickel K, Ashkenazy A, Chebach TC, Parrot N (2017) Agricultural modernization and sustainable agriculture: contradictions and complementarities. Int J Agric Sustain 15(5):575–592. https://doi.org/10.1080/14735903.2017.1373464
Kumar S, Suyal DC, Dhauni N, Bhoriyal M, Goel R (2014) Relative plant growth promoting potential of Himalayan Psychrotolerant Pseudomonas jesenii strain MP1 against native Cicer arietinum (L.)., Vigna mungo (L.) Hepper; Vigna radiata (L.) Wilczek., Cajanus cajan (L.) Millsp. and Eleusine coracana (L.) Gaertn. Afr J Microbiol Res 8(50):3931–3943. https://doi.org/10.5897/ajmr2014.7035
Leifeld J, Fuhrer J (2010) Organic farming and soil carbon sequestration: what do we really know about the benefits? Ambio 39(8):585–599. https://doi.org/10.1007/s13280-010-0082-8
Lin HC, Fukushima Y (2016) Rice cultivation methods and their sustainability aspects: organic and conventional rice production in industrialized tropical monsoon Asia with a dual cropping system. Sustainability 8(6):529. https://doi.org/10.3390/su8060529
Lori M, Symnaczik S, Mäder P, De Deyn G, Gattinger A (2017) Organic farming enhances soil microbial abundance and activity—a meta-analysis and meta-regression. PLoS ONE 12(7):e0180442. https://doi.org/10.1371/journal.pone.0180442
Manna MC, Swarup A, Wanjari RH, Mishra B, Shahi DK (2007) Long-term fertilization, manure and liming effects on soil organic matter and crop yields. Soil Tillage Res 94(2):397–409. https://doi.org/10.1016/j.still.2006.08.013
Mie A, Andersen HR, Gunnarsson S, Kahl J, Kesse-Guyot E, Rembiałkowska E, Quaglio G, Grandjean P (2017) Human health implications of organic food and organic agriculture: a comprehensive review. Environ Health 16(1):111. https://doi.org/10.1186/s12940-017-0315-4
Orr CH, Leifert C, Cummings SP, Cooper JM (2012) Impacts of organic and conventional crop management on diversity and activity of free-living nitrogen fixing bacteria and total bacteria are subsidiary to temporal effects. PLoS ONE 7(12):e52891. https://doi.org/10.1371/journal.pone.0052891
Paulsen HM, Köpke U, Oberson A, Rahmann G (2016) Phosphorus—the predicament of organic farming. In: Schnug E, De Kok L (eds) Phosphorus in agriculture: 100% zero. Springer, Dordrecht, pp 195–213. https://doi.org/10.1007/978-94-017-7612-7_10
Ramos MR, Favaretto N, Dieckow J, Dedeck RA, Vezzani FM, Almeida L, Sperrin M (2014) Soil, water and nutrient loss under conventional and organic vegetable production managed in small farms. J Agric Rural Dev Trop 115(1):31–40
Reganold JP, Wachter JM (2016) Organic agriculture in the twenty-first century. Nat Plants 2(2):15221. https://doi.org/10.1038/nplants.2015.221
Reganold JP, Andrews PK, Reeve JR, Carpenter-Boggs L, Schadt CW, Alldredge JR, Ross CF, Davies NM, Zhou J (2010) Fruit and soil quality of organic and conventional strawberry agroecosystems. PLoS ONE 5(9):e12346. https://doi.org/10.1371/journal.pone.0012346
Riggs CE, Hobbie SE (2016) Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol Biochem 99:54–65. https://doi.org/10.1016/j.soilbio.2016.04.023
Seufert V, Ramankutty N, Foley JA (2012) Comparing the yields of organic and conventional agriculture. Nature 485(7397):229–232. https://doi.org/10.1038/nature11069
Seuradge BJ, Oelbermann M, Neufeld JD (2017) Depth-dependent influence of different land-use systems on bacterial biogeography. FEMS Microbiol Ecol 93(2):fiw239. https://doi.org/10.1093/femsec/fiw239
Sheoran HS, Phogat VK, Dahiya R, Dhull S, Kakar R (2018) Comparative Effect of Organic and Conventional Farming Practices on Micronutrient Content in Different Textured Soils of Haryana, India. Int J Curr Microbiol App Sci 7(4):3399–3407
Soni R, Goel R (2010) Triphasic approach for assessment of bacterial population in different soil systems. Ekologija 56(3–4):99–104. https://doi.org/10.2478/v10055-010-0014-8
Suyal DC, Yadav A, Shouche Y, Goel R (2015) Bacterial diversity and community structure of Western Indian Himalayan red kidney bean (Phaseolus vulgaris) rhizosphere as revealed by 16S rRNA gene sequences. Biologia 70(3):305–313. https://doi.org/10.1515/biolog-2015-0048
Tal A (2018) Making conventional agriculture environmentally friendly: moving beyond the glorification of organic agriculture and the demonization of conventional agriculture. Sustainability 10(4):1078. https://doi.org/10.3390/su10041078
United Nations Convention to Combat Desertification (2017) The global land outlook, 1st edn. UNCCD, Bonn
Willer H, Lernoud J (2017) Organic agriculture worldwide 2017. In: Current statistics. Research Institute of Organic Agriculture (FiBL), Frick
Acknowledgements
We are extremely grateful to G.B. Pant University of Agriculture and Technology, Pantnagar (Uttarakhand), for providing financial assistance and space to conduct experiments. The authors PDB, DCS and SK also acknowledge the University Grants Commission (F1-17.1/2015-16/NFST-2015-17-ST-TRI-1657), the Science and Engineering Research Board-Young Scientist Award (455/2015/001214) and Council of Scientific & Industrial Research-Senior Research Fellowship (09/171/0126/2015-EMR-I) during the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goel, R., Debbarma, P., Kumari, P. et al. Assessment of Soil Chemical Quality, Soil Microbial Population and Plant Growth Parameters Under Organic and Conventional Rice–Wheat Cropping System. Agric Res 10, 193–204 (2021). https://doi.org/10.1007/s40003-020-00499-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-020-00499-8