Abstract
In the present study, cadmium tolerance potential of cold-tolerant and plant growth-promoting rhizobacteria (PGPR), namely Pseudomonas putida and Bacillus subtilis, and their effects on growth and Cd accumulation in wheat seedlings under mountain ecosystem have been studied. In petri-dish-based assays, P. putida and B. subtilis exhibited tolerance to Cd up to 18 mg l−1 and 20 mg l−1, respectively. In pot-based experiments, the bacterial inoculations that were carried out in two wheat cultivars (HPW-184 and HPW-236) under influence of Cd exposure (two doses @ 10 and 20 mg kg−1 soil) resulted in improved seed germination, plant-growth-related parameters including root–shoot length and root–shoot biomass accumulation and allocation, results being statistically significant (p < 0.05) in most instances. Overall, the bacterial inoculations were useful in reducing the Cd stress in the wheat cultivars through reducing Cd accumulation in roots and shoots. Cd accumulation in roots and shoots was found in the order: control > P. putida > B. subtilis. The capacity of tested PGPR, B. subtilis and P. putida, originally isolated from Indian Himalayan Region, to protect the wheat plants through reducing Cd accumulation, and consequent inhibitory effects may be utilized in preventing Cd accumulation in the food chain and in improving growth of wheat crop under mountain ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are known to cause toxicological effects on soil microbes that may lead to decrease their numbers and activities [16, 26]. Soil erosion, volcanic activities, weathering of minerals are the significant natural sources, whereas mining, smelting, electroplating, pesticides, phosphate fertilizers, biosolids, atmospheric deposition, etc., are man-made sources of heavy metals. Suppression in growth and yield of agricultural crops due to heavy metal contamination are very well documented [31,32,33]. Soil factors such as pH, organic matter, metals availability and cation exchange capacity have importance in the transfer of heavy metals from soil to plants. Seasonal changes and the presence of other heavy metals in soil are other relevant factors in this regard [25, 32].
Among the heavy metals, cadmium (Cd) is most easily taken up by roots of many plant species and its toxicity is generally considered 2 to 20 times higher than that of other heavy metals. Cd contamination causes deleterious effects on human health and also found in the food chain due to its greater solubility in water and application of phosphate fertilizers in agricultural land. Bacteria, that produce plant growth-promoting hormones, also possess an ability to reduce the level of heavy metals in soil [15]. Bacterial strains of Pseudomonas and Bacillus spp. have been reported for Cd resistance ranging between 0.5 and 1.0 mM of CdCl2 [19] and up to 4 mM [15]. Both strains were found resisting Cd accumulation in the plant parts and also raising the growth of plant parts and biomass due to plant growth-promoting activity [15, 17].
Seed germination is one of the highly sensitive physiological processes in plants and regulated by several hormonal interactions and environmental factors [38]. Germination process of a plant is also affected by metal contamination in soils. Many soil bacteria are tolerant to heavy metals and play an important role in mobilization or immobilization of heavy metals in the soil–plant system [10, 20, 27]. The rhizosphere of many hyperaccumulator plants such as Thlaspi caerulescens, Alyssum bertolonii and Alyssum murale grown on soil contaminated with Zn and Ni has been reported for a high portion of metal-resistant bacteria [1, 17, 23].
A long-term study conducted in high altitudes of Indian Himalayan Region (IHR) recommended the suitability of cold-tolerant and plant growth-promoting bacteria (PGPB), mainly species of Bacillus and Pseudomonas, for field application under mountain ecosystem [35, 36]. However, evidence on tolerance of these PGPB to heavy metals and their influence on seed germination and plant growth in Cd-polluted soils have not been investigated. Wheat (Triticum aestivum L.) is a major fasten crop in northern India and is grown over 26.4 million ha including colder regions of mountain ecosystem under IHR [22, 35]. A preliminary study carried out in Kullu Valley of Himachal Pradesh, India, further showed that Cd concentrations in soil exceeded the permissible limit set by PFA act 1954, whereas edible parts of radish and cauliflower had exceeded the permissible limit set by the European Union. Such findings are important for the scientific community interested in finding eco-friendly means to combat the heavy metal load in soil and further in the food chain.
Therefore, the present study aimed to assess the influence of Cd-tolerant and plant growth-promoting rhizobacteria on Cd accumulation and growth response of wheat seedlings under the mountain ecosystem.
Materials and Methods
Study Site
Pot-based experiments were carried out in the nursery of G. B. Pant National Institute of Himalayan Environment and Sustainable Development (GBPNIHESD), Himachal Regional Centre, Mohal-Kullu, Himachal Pradesh (Lat 31°54.914′; long 077°07.400′; and alt 1155 m amsl). Mean minimum and maximum temperature, rainfall, and minimum and maximum humidity during the experimental course were recorded as 10 °C and 25 °C, 20 cm, 67% and 85%, respectively. Soil pH ranged from 7.0 to 7.8.
Wheat Cultivars and Plant Growth-Promoting Rhizobacteria (PGPR)
Wheat (Triticum aestivum L. cv. HPW-184, HPW-236) seeds, commonly grown in Kullu Valley of Himachal Pradesh, India, during the winter season, were collected from Chaudhary Srawan Kumar Himachal Pradesh Krishi Vishwavidhyalaya (CSKHPKV), Hill Research and Extension Centre, Bajaura, Kullu, Himachal Pradesh. Pseudomonas putida and Bacillus subtilis (cold-tolerant PGPR) were obtained from Microbiology Laboratory of GBPNIHESD, Almora, Uttarakhand, India. The bacteria, originally isolated from the colder regions of IHR, have been reported for their plant growth promotion, biocontrol and carrier-based bioformulation properties, with reference to agricultural, forest and tissue culture-raised plant species [35, 36]. Fresh cultures of the two PGPR have been raised in tryptone–yeast extract (TY) broth (autoclaved at 121 °C for 15 min) for inoculation of wheat seeds.
Cadmium Tolerance Potential of the Bacterial Inoculants
For assessing Cd tolerance potential of bacterial inoculants, metal Cd (CdCl2·H2O, Merck, Analytical Grade, India) was added to sterilized TY agar media in concentrations ranging between 0 and 24 mg l−1. The bacterial suspension (P. putida and B. subtilis) was inoculated (separately) using streak culture on TY agar petri dishes containing different concentrations of Cd and incubated at 30 °C for 24 h. The minimum inhibitory concentration (MIC) of Cd was designated as the highest concentration of Cd at which the pure culture of bacterial strain showed their presence.
Pot Assay
Soils, collected from farmer’s agriculture field and mixed uniformly, were divided into two groups: One group uniformly mixed with Cd (CdCl2.H2O, Merck, Analytical Grade, India) @ 10 mg kg−1 dry soil, whereas the other group mixed with Cd @ 20 mg kg−1 dry soil. Thirty pots were filled with soil from each group and were further divided into two subgroups, each representing a wheat cultivar. Each subgroup was further divided into three sets and labeled as C, T1 and T2 for growing seeds of wheat without inoculants (control), inoculated with P. putida and B. subtilis, respectively. Healthy seeds of wheat cultivars (HPW-184 and HPW-236, surface-sterilized with 3% formaldehyde) were inoculated with 10–5 cells ml−1 broth of each PGPR and hand-sown in pots of respective treatments containing Cd-contaminated soils at 2 cm depth. Five pots (replicates) under each treatment were randomly placed in the nursery of GBPNIHESD, Himachal Regional Centre, under natural conditions. An equal volume of water was used to maintain the soil moisture. Weeding was also performed as per need during the course of an experiment.
Samplings and Analysis
Seed Germination
Germination of wheat seeds was recorded from the date of sowing to till maximum seed germination was achieved. Percent seed germination was calculated by using the following formulae:
Growth, Biomass Accumulation and the Root–Shoot Ratio (RSR)
Ten plants were randomly sampled from five pots of respective treatments at the age of 45 days for the analysis of growth performance of test plants in terms of length of roots and shoots. Each plant was considered as a replicate. The samples were kept in sieves and washed in running tap water to remove the adhered soil particles and other unwanted plant materials. Root length and shoot length were measured manually. The above plant samples were then separated into root and shoot, and their fresh weight was measured. The root and shoot were then oven-dried at 80 °C till a constant weight was achieved. The dry weight of root and shoot was measured separately. Root–shoot ratio (RSR) for test plants was calculated by dividing the dry weight of root with that of a shoot.
Cadmium Accumulation in Wheat Tissue
One gram of air-dried soil, oven-dried roots, and shoots of plants were separately digested in 15 ml of a tri-acid mixture (HNO3/H2SO4/HClO4 in the ratio of 5:1:1) at 80 °C till a transparent solution was obtained [3]. The concentration of Cd in the filtrate was determined using an atomic absorption spectrophotometer (Model AAnalyst 800, PerkinElmer) using blanks and the standard solution of Cd (RANKEM, India). Standard reference material (SRM-1570) obtained from the National Institute of Standard and Technology (NIST) was also analyzed to ensure the precision and accuracy of the method. The obtained results were found within ± 2% of the certified values.
Bioconcentration Factor (BCF)
Bioconcentration refers to the process of uptake and buildup of a chemical in living organisms and was calculated as the ratio of Cd concentration in plant tissues to that in the soil.
Plant Response to Stress (PRS)
The response of PGPB-inoculated cultivars of wheat crops was assessed in terms of germination, lengths, fresh and dry weight of roots and shoots, RSR, Cd accumulation in roots and shoots, and BCF of roots and shoots. To assess the relative response of cultivars to different bioinoculants, plant response to stress (PRS) was calculated using the following equation:
where Rwi and Ri are plant response to seeds without bioinoculant and with bioinoculant, respectively. Higher PRS value refers to higher growth-promoting and preventing Cd accumulation ability of a bioinoculant as the test PGPB are capable to reduce Cd accumulation and consequently improved the growth of test cultivars of wheat plants.
Statistical Analysis
Data were analyzed for mean ± SE. The treatment means were separated using Duncan’s multiple range tests (DMRT) at p < 0.05. Data were further subjected to multiple-way (m-way) ANOVA test to assess the effects of different variables, namely cultivars (C), contamination levels (Cd) and bioinoculants (B), as well as their interactions on different tested traits. All the statistical analysis was performed using SPSS software (SPSS Inc., Version 16.0).
Results and Discussion
Heavy Metal Tolerance of PGPR
In the present study, the PGPB, P. putida and B. subtilis, isolated from the colder regions in IHR are demonstrated for the first time for their possible application in bioremediation of heavy-metal-polluted soil. The tolerance level of P. putida and B. subtilis against Cd concentrations ranging between 0 and 24 mg l−1 is presented in Fig. 1. Both the PGPB were found to resist Cd concentration from 5 to 18 mg l−1. These results indicated that B. subtilis showed a higher level of Cd tolerance (22 mg l−1) in comparison with P. putida (18 mg l−1). In earlier studies, Pseudomonas spp. has been reported for the maximum frequency of resistance up to 4 mM for Cd [9]. Dell’ Amico et al. [9] and Sun et al. [34] reported four bacterial strains, namely P. tolaasii, P. fluorescens, Mycobacterium sp. and Alcaligenes sp., with high Cd resistance, and MIC values varying from 0.5 to 2.5 mM.
Cadmium tolerance potential of B. subtilis [off white color streak line] and P. putida [yellow color streak line] cultured on tryptone–yeast extract agar (i) representing control plate of P. putida and B. subtilis, (ii) representing up to 10 µg ml−1 Cd tolerance potential of both strains, (iii) representing up to 20 µg ml−1 Cd tolerance potential of both strains (color figure online)
The Cd tolerance potentials of the test PGPB in the present study are, however, lower as compared to the earlier reported values in the literature [9, 34]. This may be ascribed to their long-term exposure to non-metal contaminated and cold environment of IHR. Higher resistance to the metals, namely Zn and Cd, in bacteria isolated from leaves and roots of Elsholtzia splendens has been reported by Abou-Shanab et al. [2]. Sun et al. [34] have also reported that species of Bacillus were predominant among Cu-resistant endophytic bacteria in a Cu accumulator E. splendens plant species. These results are indicative of the better performance of the test PGPB in terms of their application in treating the Cd-contaminated soils and reduction in the Cd-induced toxicity in plants through reducing Cd accumulation in plant tissues under mountain ecosystem or a moderate Cd-contaminated soil.
Cd Accumulation in Wheat Tissues and Bioconcentration Factor (BFC)
The effects of P. putida and B. subtilis inoculation on Cd accumulation in root and shoot tissues of 45-day-old cultivars of T. aestivum plants were found significantly lower as compared to control at both the levels of Cd contamination (Fig. 2). The percent reduction in Cd accumulation in roots and shoots of HPW 184 over control were recorded as 17.7% and 17.0%, respectively, at low Cd and 48.5% and 61.8%, respectively, at high Cd exposure due to B. subtilis inoculation. Similarly, P. putida inoculation reduced Cd accumulation by 10.5% and 13.0% in roots and 11.4% and 15.9% in shoots, respectively, at low and high Cd exposure (Fig. 2). The results further showed that inoculation of HPW 236 with B. subtilis and P. putida had the higher percent reduction in Cd accumulation in roots and shoots as compared to those of cv. HPW 184 (Fig. 2). Results of m-way ANOVA test showed that different test variables and their interaction had significant effects on Cd accumulation in roots and shoots (Table 1). However, C, C × Cd and C × Cd × B had no significant effect on Cd accumulation in shoots and C × B on Cd accumulation in roots of T. aestivum cultivars (Table 1). The results further showed that the reduction in Cd accumulation in wheat tissue by B. subtilis was higher than those of P. putida (Fig. 2).
Effect of bacterial inoculation on Cd accumulation in roots and shoots of 45-day-old wheat cultivars exposed to Cd-contaminated soil. Bars are mean ± SE of three replicates. Bars with different letters in each group are significantly different from each other at < 0.05 (Duncan’s multiple range test)
The results of the effects of inoculation with P. putida and B. subtilis on BCF of Cd accumulation in roots and shoots of two wheat cultivars exposed to two levels of Cd-contaminated soil under mountain ecosystem are summarized in Table 2. The results showed that BCF of Cd accumulation in both roots and shoots of both test wheat cultivars exposed to low and high levels of Cd-contaminated soil was significantly (p < 0.05) lowest in B. subtilis inoculation followed by P. putida and were highest in control (Table 2). The percent change in BCF values was found highest in HPW 236 as compared to HPW 184 due to B. subtilis inoculation, whereas vice versa results were observed in the case of P. putida inoculation (Table 2). M-way ANOVA test showed that test variables and their interaction except in C and C × Cd had significant effects on BCF of Cd accumulation in both roots and shoots of T. aestivum plants (Table 1).
In the present study, transfer of Cd from soil to roots and shoots of test plants was also affected by the inoculation of PGPR. Accumulation of heavy metals in different plant tissues due to bacterial inoculation has been studied by various researchers [8, 33]. Influence of heavy-metal-resistant bacteria on metal transport and translocation in the rhizosphere with the high toxicity of metals has also been studied [17]. A number of PGPR, which are known to stimulate the growth of different plants, contain the enzyme 1-aminocyclopropane 1-carboxylate (ACC) deaminase, which hydrolyzes ACC. Roots or seeds of some plants exude ACC, cleaved by ACC deaminase to ammonia and alpha-ketobutyrate [21]. The bacteria utilize the ammonia evolved from ACC as a source of nitrogen and thereby decreased ACC within the plants [5, 29, 30]. The application of plant growth-promoting rhizobacterial isolates could primarily be attributed to their ACC deaminase activity. This might be able to reduce endogenous biosynthesis of ethylene (C2H4) in developing roots of seedlings, resulting in the formation of healthy and longer roots for efficient nutrient uptake. Indigenous C2H4 biosynthesis is increased during seed germination and root growth [4]. PGPR containing ACC deaminase reduce the effect of ACC. Reduction in the endogenous biosynthesis of C2H4 in etiolated seedlings by hydrolysis of ACC through the action of ACC deaminase of PGPR was recorded in various studies [5, 28, 29]. Studies have also shown the correlation of ACC deaminase with plant growth-promoting activity of bacterial strains [5, 29].
While the examples of the direct mechanism(s) are growth promotion by providing fixed nitrogen to the host plant, production of phytohormones and phosphate solubilization, the indirect mechanisms mainly involve biological control of plant pathogens assisted through antibiosis and production of antimicrobial substances, including siderophores and lytic enzymes [13]. In the present study, root growth in both the test cultivars was increased due to PGPR inoculation. Generally, the elevated levels of heavy metals in soil interfere with the uptake of nutrients such as phosphorus leading to plant growth retardation. Such nutritional deficiency can be compensated by the phosphate-solubilizing ability of PGPR [12, 24]. The production IAA by PGPR further promotes root growth by directly stimulating plant cell elongation or cell division [11]. Xie et al. [37] have reported that low level of IAA produced by rhizospheric bacteria promotes primary root elongation, whereas a high level of IAA stimulates lateral and adventitious root formation and inhibits primary root growth.
The higher root growth in HPW184 due to B. subtilis inoculation can be attributed to the solubilization of phosphate and production of IAA [18]. Dell’Amico et al. [9] have shown that inoculation with four ACC-utilizing bacteria proved to be an efficient method for protecting Brassica napus seeds from the growth inhibition caused by Cd concentration. Overall, the results of the present study support the previously documented findings [6, 18, 34] that metal-resistant bacteria can improve the growth of plants exposed to soil contaminated with heavy metals. The capacity of test PGPR, B. subtilis and P. putida, isolated from the cold region of IHR to protect the plants against the inhibitory effects of Cd resulting from lowering its accumulation may be utilized in the bioremediation of Cd-contaminated soil under mountain ecosystem.
Seed Germination and Growth Performance
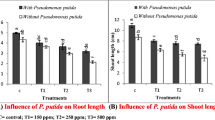
Inoculation with P. putida and B. subtilis increased germination of seeds of two wheat cultivars exposed to Cd-contaminated soil significantly as compared to the control; the results are presented in Fig. 3. B. subtilis inoculated seeds gave maximum germination of 88.3% and 72.7% in HPW-236 and 59.7% and 49.7% in HPW-184 underexposure of low and high Cd contamination, respectively. In the case of P. putida inoculation, values for germination were recorded as 85.3% and 65.3% in HPW- 236 and 54.3% and 45% in HPW-184 exposed to low and high Cd contamination, respectively (Fig. 3). The results further showed that the positive effects of bacterial inoculation on germination of tested wheat cultivars exposed to both low and high Cd contamination were significant at < 0.05. The percent increase in germination of seeds of test cultivars inoculated with B. subtilis and Cd-contaminated soil was higher as compared to that of P. putida-inoculated seeds (Fig. 3). The results of m-way ANOVA test further showed that test variables and their interaction affect germination significantly (Table 1).
Growth, biomass accumulation and allocation (RSR) of wheat cultivars inoculated with PGPB along with the exposure to Cd-contaminated soil, studied in terms of root and shoot lengths, fresh and dry weights of roots and shoots, RSR, respectively, are presented in Table 3. These traits were decreased slightly with increasing Cd concentration in soil but, increased significantly over control due to inoculation of B. subtilis and P. putida. No significant differences in above traits were recorded between inoculation of P. putida and B. subtilis. The percent change in root and shoot length of HPW-184 and HPW- 236 was found higher in P. putida inoculation as compared to B. subtilis inoculation at low and high Cd-contaminated soil, whereas percent change in shoot length of HPW-236 was higher in B. subtilis as compared to that of P. putida-inoculated seeds (Table 3). Percent change in fresh weight of roots and shoots of wheat plants exposed to both levels of contamination varied between 13.9–29.2% and 7.2–21% in HPW-184 and 4.8–101.2% and 5.2–49.6% in HPW-236, respectively. Similarly, dry weights ranged between 16.7–101.2% and 17.1–33.9% in HPW-184 and 16.7–55.8% and 10.9–58.5% in HPW0236, respectively (Table 3).
The results further showed inoculation with P. putida and B. subtilis had potential to translocate photosynthate in aboveground parts from low-ground parts, which results in higher biomass accumulation. Significant differences between different inoculations were not consistent for both the tested wheat cultivars (Table 3). Further, the results further also showed that the above traits were significantly affected by the test variables such as cultivars, concentrations of Cd and bioinoculants as well as their interactions (Table 3). But, no significant effects of variable interactions were found on root and shoot lengths, and shoot fresh weights. Cultivars also did not affect the change in fresh weight of roots and shoots. Increasing Cd concentration in soil has insignificant effects on shoot dry weight (Table 3).
In the present study, the growth of both the test cultivars of wheat increased due to inoculation of Cd-resistant P. putida and B. subtilis. These PGPB were effective in protecting test plants from growth inhibition caused by Cd contamination. Inoculation with rhizosphere or endophytic bacteria in hyperaccumulator plants has been reported for improved growth and biomass production [7, 9, 14, 39]. The metal-resistant rhizospheric bacteria are known to have the ability to protect the host plants from the metal toxicity by several possible mechanisms. Utilization of 1-aminocyclopropane-1-carboxylate (ACC) by the rhizospheric bacteria is the best-known mechanism for reducing the metal toxicity in plants. In the present study, both tests PGPB were capable to reduce Cd accumulation in root and shoot tissues of both the test wheat cultivars, whereas growth promotion and the consequent increase in Ni accumulation in Brassica juncea plants have also been reported [24]. Belimove et al. [6] used a chromium-resistant Pseudomonas sp. strain that produced indole acetic acid (IAA) in a higher amount to promote the plant growth and enhanced accumulation of Zn in the roots and shoots of B. juncea. Belimove et al. [6] have further reported a remarkable increase in root growth of Indian mustard in response to microbial inoculation as compared with un-inoculated control in Cd-contaminated soil.
Plant Response to Stress
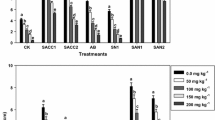
Plant response to stress (PRS) was calculated to assess the relative growth-promoting and preventing Cd accumulation ability of PGPB under the influence of Cd. Higher PRS value indicates more growth-promoting and preventing Cd accumulation ability of bacteria as compared to its lower value. The overall response of test cultivars of wheat plants inoculated with PGPB and Cd-contaminated soil in terms of PRS is shown in Fig. 4. In the present study, the results showed that B. subtilis had higher PRS values as compared to those of P. putida. The results further showed that the growth-promoting ability of both the test PGPB increased with increasing Cd concentrations in soil. Overall, the results indicate that both test PGPB can be used to improve the growth and to prevent the Cd accumulation in wheat crops grown under Cd-contaminated soil under mountain ecosystem.
Conclusions
In the present time, heavy metal contamination of soil is one of the most important challenges related to environmental management. Use of microbial inoculants technology with respect to improved plant productivity and biocontrol has been well recognized. One way to reduce the deleterious effects of heavy metals taken up from the environment on some plants involves the use of PGPR. This approach is aesthetically low and need minimum maintenance and presents no major need of recycling. The microbial community contains many different microbes, but the species of Pseudomonas and Bacillus bacteria contribute the major community with active metabolism in the rhizospheric region of plant species which tend to improve the growth and yield of a plant by having the ability of plant growth-promoting activities. Some pollutants such as diesel, parathion, polycyclic aromatic hydrocarbons and polychlorinated biphenyls are remediated using Pseudomonas spp. Arthrobacter spp. and actinomycetes in the different rhizospheric region. On the basis of the results obtained in the present study, it is concluded that due to the cold tolerance and Cd resistance traits, inoculation with the PGPR (B. subtilis and P. putida) can be of additional benefit, i.e., the reduction in the adverse effects of Cd on growth and yield of wheat cultivars grown in Cd-contaminated soil in the low-temperature environment of mountain ecosystem.
References
Abou-Shanab RA, Angle JS, Delorme TA, Chaney RL, Van Berkum P, Moawad H, Ghanem K, Ghozlan HA (2003) Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytol 158:219–224
Abou-Shanab RA, Angle JS, Chaney RL (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soil. Soil Biol Biochem 38:2882–2889
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Champman SB (eds) Methods in plant Ecology. Blackwell, Oxford, pp 285–344
Arshad M, Shaharoona B, Mahmood T (2008) Inoculation with plant growth promoting rhizobacteria containing ACC-deaminase partially eliminates the effects of water stress on growth, yield and ripening of Pisum sativum L. Pedos. 18:611–620
Belimov AA, Safranova VI, Mimura T (2002) Response of spring rape (Brassica napus L.) to inoculation with PGPR containing ACC-deaminase depends on nutrient status of plant. Can J Microbiol 48:189–199
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cd-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern). Soil Biol Biochem 37:241–250
Cabello-Conejo MI, Centofanti T, Kidd PS, Prieto-Fernandez A, Chaney RL (2013) Evaluation of plant growth regulators to increase nickel phytoextraction by Alyssum species. Int J Phytrem 15:365–375
Chen YH, Sheng ZG, Li XD (2004) The use of vetiver grass (Vetivera zizanioides) in the phytoremediation of soils contaminated with heavy metals. Appl Geochem 19:1553–1565
Dell’Amico E, Cavalaca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem 40:74–78
Delorme TA, Gagliardi JV, Angle JS, Chaney RL (2001) Influence of the zinc hyperaccumulator Thlaspi caerulescens J. & C. Presl. and the nonmetal accumulator Trifolium pretense L. on soil microbial population. Can J Microbiol 67:190–197
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:109–117
Gupta CP, Dubey RC, Maheshwari DK (2002) Plant growth enhancement and suppression of Macrophomina phaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol Fertil Soils 35:399–405
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Li WC, Ye Z, Wong M (2007) Effects of bacteria on enhanced metal uptake of the Cd/Zn hyper accumulating plant, Sedum alfredii. J Exp Bot 58:4173–4182
Liu X, Zhang S, Shan X, Zhu YG (2005) Toxicity of arsenate and arsenite on germination seedling growth and amylolytic activity of wheat. Chemosphere 61:293–301
Malayeri BE, Chehregani A, Yousefi N, Lorestani B (2008) Identification of the hyper accumulator plants in copper and iron mine in Iran. Pak J Biol Sci 11:490–492
Mengoni A, Barzanti R, Gonneli C, Gabbrielli R, Bazzicalupo M (2001) Characterization of nickel-resistant bacteria isolated from serpentine soil. Environ Microbiol 3:691–708
Owolabi JB, Hekeu MM (2014) Heavy metal resistance and antibiotic susceptibility pattern of bacteria isolated from selected polluted soils in Lagos and Ota, Nigeria. IJBAS-IJENS 14:06
Oyetibo GO, Ilori MO, Adebusoye SA, Obayori OS, Amund OO (2010) Bacteria with dual resistance to elevated concentrations of heavy metals and antibiotics in Nigeria in contaminated systems. Environ Monit Assess 16:305–314
Pal A, Dutta S, Maikhuri PK, Paul AK (2005) Occurrence of heavy metal-resistance in microflora from serpentine soil of Anadama. J Basic Microbiol 45:207–218
Penrose DM, Glick BR (2001) methods for isolating and characterizing ACC deaminase-containing plant growth promoting rhizobacteria. Physiol Plant 118:10–15
Rai R, Agrawal M, Agarwal SB (2007) Assessment of yield losses in tropical wheat using open chambers. Atmos Environ 41:9543–9554
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71:834–842
Rajkumar M, Nagendran R, Lee KJ, Lee WH (2005) Characterization of a novel Cr6+ reducing Pseudomonas sp. with plant growth promoting potential. Curr Microbiol 50:266–271
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of PGPB and Cr6+ on the growth of Indian mustard. Chemosphere 62:741–746
Sanchez-Chardi A, Marques CC, Nadal J, Mathias ML (2007) Metal bio-accumulation in the greater white-toothed shrew, Crocidura russula inhabiting an abandoned pyrite mine site. Chemosphere 67:121–130
Sevgi E, Coral G, Gizir AM, Sangun MK (2010) Investigation of heavy metal resistance in some bacterial strains isolated from industrial soils. Turk J Biol 34:423–443
Shaharoona B, Arshad M, Khalid A (2007) Differential response of etiolated pea seedlings to inoculation with rhizobacteria capable of utilizing 1-aminocyclopropane-1-carboxylate or l-methionine. J Microbiol 45:15–20
Shahzad SM, Khalid A, Arshad M, Tahir J, Mahmood T (2010) Improving nodulation, growth and yield of Cicer arietinum L. through bacterial ACC-deaminase induced changes in root architecture. Eur J Soil Biol 46:342–347
Shahzad SM, Khalid A, Arshad M, Rehman KU (2010) Screening rhizobacteria containing ACC-deaminase for growth promotion of chickpea seedlings under axenic conditions. Soil Environ 29:38–46
Sharma RK, Agrawal SB (2010) Responses of Abelmoschus esculentus L. (lady’s finger) to elevated level of zinc and Cd. Trop Ecol 51:389–396
Sharma RK, Agrawal M, Marshall FM (2007) Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 66:258–266
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and Cd uptake by Cd-resistant bacteria. Chemosphere 6:1036–1042
Sun LN, Zhang YF, He YL, Chan ZJ, Wang YQ, Qian M, Sheng FX (2010) Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Biores Technol 101:501–509
Trivedi P, Pandey A (2008) Recovery of plant growth promoting rhizobacteria from sodium alginate beads after three years following storage at 4 °C. J Ind Microbiol Biotechnol 35:205–209
Trivedi P, Pandey A, Palni LMS (2012) Bacterial inoculants for field applications under mountain ecosystem: present initiatives and future prospects. In: Maheshwari DK (ed) Bacteria in agrobiology: plant probiotics. Springer, Berlin, pp 15–44
Xie H, Pasternak JJ, Glick BR (1996) Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR 12-2 that overproduce indoleacetic acid. Curr Microbiol 32:67–71
Xiong ZT, Wang H (2005) Copper toxicity and bioaccumulation in Chinese cabbage (Brassica pekinensis Rupr.). Environ Toxicol 20:188–194
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997
Acknowledgements
The authors are thankful to the Director (GBPNIHESD; Project no. 9) for extending the facilities, Ministry of Environment, Forest and Climate Change, Govt. of India, New Delhi, for providing the financial support and Dr. S.S. Samant for the moral support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khatri, S., Sharma, R.K. & Shridhar, V. Influence of Cadmium-Tolerant and Plant Growth-Promoting Rhizobacteria on Cadmium Accumulation and Growth Response of Wheat Seedlings Under Mountain Ecosystem. Agric Res 9, 56–65 (2020). https://doi.org/10.1007/s40003-019-00407-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-019-00407-9