Abstract
The rhizosphere is an important region of microbial interactions where exudates released by plant roots are the main source of food for microorganisms, which play a vital role in increasing their population density and activities. An isolate of bacterium Pseudomonas sp., designated as KS51, that expressed plant growth-promoting traits and antagonistic activity was isolated from the rhizospheric soil of Calotropis sp., grown in the northern and central regions of India. The isolate was recognized as Gram negative rod (0.67 × 2.89 μm) shaped bacterium. It grew at optimum temperature ranging from 25 to 30 °C, exhibited tolerance to a wide pH range of 6–10 and tolerated salt concentrations up to 5 % (wt/vol). Though, it was sensitive to chloramphenicol, ampicillin, tetracycline, gentamicin, ceftriaxone, and erythromycin, it showed resistance to co-trimoxazole, cefuroxime, ciprofloxacin, penicillin G, augmentin, fusidic acid and vancomycin. The bacterium was biochemically characterized and examined in vitro for their plant growth-promoting traits like production of indole acetic acid (IAA) (8 μg ml−1 day−1), hydrogen cyanide (HCN), siderophore and phosphate solubilization (268 μg ml−1 after 144 h). The mean growth rate constant (K) of isolate was found to increase with successive increments in substrate concentration of naphthalene and anthracene (0.5–1.0 mg/50 ml). KS51 was also found to be a good degrader for naphthalene (78.44 %) and anthracene (63.53 %) as determined by HPLC analysis. Based on the 16S rRNA analysis, KS51 showed the maximum similarity with Pseudomonas sp. On the basis of its growth-promoting, biocontrol and bioremediation potential properties, KS51 could be applied in biotechnological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rhizosphere is an environment that the plant itself helps to create and where pathogenic and beneficial microorganisms constitute a major influential force on plant growth and health [13]. Many bacteria have a neutral effect on the plant, but are part of the complex food web that utilizes the large amount of carbon that is fixed by the plant and released into the rhizosphere (i.e. rhizodeposits) [10]. The bacterial community in the rhizosphere also harbours members that exert deleterious or beneficial effects on the plant. Bacteria that adversely affect plant growth and health are the pathogenic, whereas bacteria that are beneficial include nitrogen-fixing bacteria and plant growth-promoting rhizobacteria (PGPR) [34]. Pseudomonas sp. is one of the most important members of PGPRs showing all the three major group of PGPRs, that is having biofertilizer, phytostimulator and phytopathogen biocontrol activities [31]. Several Pseudomonas sp. have been extensively used for biological control against many soil borne plant pathogens [33].
It has been observed that polyaromatic hydro-carbons (PAH) degradation in soil is dominated by bacterial strains belonging to a very limited number of taxonomic groups such as Sphingomonas, Burkholderia, Pseudomonas [9]. Bacterial cell surface hydrophobicity is important in promoting cell adhesion to soil particles [23] and plays a critical role in facilitating biodegradation in situ [24]. However, the hydrophobicity and motility can potentially increase the ability of bacteria to access polycyclic aromatic hydrocarbons (PAHs) within soil [9]. Although, numerous studies have been conducted on Pseudomonas sp. and their possible role in plant growth promotion and other traits, the information regarding the association of Pseudomonas sp. in the rhizosphere niche of Calotropis plant possessing plant growth- promoting (PGP) traits, degradation abilities and hydrophobicity has not been yet been explored. Therefore, keeping in view the above constricts, the current study was designed to (i) isolate Pseudomonas sp. from the rhizosphere of Calotropis sp., growing in northern and central regions of India; (ii) screen the abilities of plant growth promoting attributes including the production of IAA hormone, siderophore, phosphate solubilization and the ability to inhibit pathogenic fungi Alternaria alternata, Fusarium oxysporum and Cladosporium oxysporum and (iii) screening with respect to ability to utilize PAHs, quantitative degradation by HPLC, growth study with varying concentration of anthracene and naphthalene and cell surface hydrophobicity of isolate. In addition, the morphological, physiological, biochemical and 16S rRNA analysis has been attempted to determine genus and species taxa of isolated strain.
Materials and Methods
Isolation
The soil used for bacterial isolation was collected from the rhizosphere of Calotropis sp. found to grow in four different zones of the northern and central India. The rhizospheric soil was collected in sterile polythene bags and stored at the 4 °C until further use. The pure culture was maintained on slants at 4 °C and in 10 % glycerol at 20 °C.
Identification and Characterization of the Bacterial Isolate
Phenotypic characterization of the isolate was carried out by subjecting the bacterial isolate to cultural (oxygen requirement), morphological (colony morphology and pigmentation), microscopic (Gram staining, cell shape, size and arrangement of cells), biochemical (utilization of different carbon sources and enzyme activity) and physiological characterization (temperature, pH, salt tolerance and antibiotic sensitivity) following standard procedures [7].
Qualitative and Quantitative Estimation of PGP Attributes
Salkowski’s reagent was used to examine the indole acetic acid (IAA) production [19]. Quantitative estimation of IAA production was carried out by the standard method [8]. The amount of IAA produced in culture medium was calculated by comparing it with standard curve in μg/ml.
The potential to solubilize relatively insoluble calcium phosphate was checked by point inoculation on Pikovaskaya’s agar and the plates were incubated at 28 °C for 4–5 days [25]. The zone was measured and phosphate-solublizing index (PSI) was calculated by means of the formula:
Quantitative estimation of phosphate solubilization for selected isolate was carried out by chlorostanus-reduced molybdo-phosphoric acid blue method [29]. HCN production was checked by culturing the organism on nutrient agar medium supplemented with glycine (0.44 %). Filter paper dipped in 0.5 % picric acid was attached to the lid of culture tube. After incubation for 48 h at 28 ± 1 °C, colour change was observed for HCN production [4]. Siderophore production was detected qualitatively on Chrome-Azurol S agar (CAS) medium [21].
Growth Profile of Isolates in Anthracene or Naphthalene and HPLC Analysis
Growth profile of isolates in anthracene or naphthalene amended medium was also determined. Minimum salt basal medium was supplemented with different concentrations (0.5, 0.8 and 1.0 mg/50 ml) of anthracene or naphthalene. Mean growth rate (K) was calculated by formula given as follows:
where K is the mean growth rate constant, Z t is final growth at time t, Z 0 is initial growth at time 0, and ΔT is difference in time.
Residual amounts of anthracene and naphthalene were determined by high performance liquid chromatography (HPLC) analysis in culture medium for quantitative estimation of PAH degradation.
Antagonistic Properties Due to Diffusible and Volatile Compounds
For examining the antagonism due to diffusible compounds produced by the bacterial isolate, fungal culture of test fungi (F. oxysporum, A. alternata, and C. oxysporum) were individually inoculated on potato dextrose agar (PDA) plate. The inoculation of bacterial culture was made about 2 cm away from fungal inoculation. The plates were incubated in inverted position at 28 °C in the incubator. The percentage growth inhibition (PGI) was calculated using the formula:
where R 1 represents the radius of the test fungus in the direction with no bacterial colony, and R 2 is the radius of the fungal colony in the direction of the bacterial colony. The antagonism due to volatile compounds was evaluated by preparing a bacterial lawn on nutrient agar plates. After incubation for 24 h, the lid was replaced by a plate containing an agar block of the test fungus grown on potato carrot agar (PCA). The two plates were sealed together with parafilm. Control sets were prepared in similar manner, without bacteria in the bottom plate. Such sealed sets of petri dishes were incubated at 28 °C. The PGI was calculated using the formula:
where R 1 represents the radius of the test fungus in the control, and R 2 is the radius of the fungal colony with the bacterial colony [22].
Phylogenetic Analysis
Molecular characterization was carried out by 16S ribosomal RNA gene sequencing of the isolate. It was performed using universal eubacterial primers fD1 and rp2 [32]. The phylogenetic tree was constructed by the neighbour joining method using the distance matrix from the alignment [26].
Statistical Analysis
The data presented have been analysed statistically with the relevant standard deviation and standard error (SE) method.
Results
Isolation and Biochemical Characterization
On the basis of morphological and biochemical characterization, the bacterial isolate was found to be aerobic, pigmented (yellow), Gram-negative, rod-shaped and motile. It was also found that the isolate was positive for glucose fermentation, casein, gelatine, starch, methyl red, nitrate reduction, urea hydrolysis, catalase and oxidase and showed negative results for indole, voges proskauer, mannitol and citrate utilization test. However, it was found to be sensitive to chloramphenicol, ampicillin, tetracycline, gentamicin, ceftriaxone and erythromycin, and it showed resistance to co-trimoxazole, cefuroxime, ciprofloxacin, penicillin G, augmentin, fusidic acid and vancomycin (Table 1). The strain showed optimum growth between 25 and 30 °C, exhibited tolerance to a wide pH range between 6 and 10 and salt concentrations up to 5 % (wt/vol). On the basis of morphological, biochemical and physiological characteristics, the isolate was grouped under Pseudomonas sp. as described in Bergey’s Manual of Determinative Bacteriology.
Plant Growth Promoting Traits of Test Isolates
After 24, 48 and 72 h incubation time, the isolate was able to produce 8, 13 and 18 μg ml−1 day−1, IAA, respectively (Table 2). It was interesting to observe that the isolate was able to retain its functional traits even after 72 h. Phosphate solubilization was evidently visible on the Pikovaskya agar plate where isolate form a clear halo zone around it. The zone of solubilization around the bacterial colony was found to increase with the increase of incubation time. Quantitative estimation of phosphate solubilization, estimated after incubation for duration ranging from 24 to 144 h, is presented in Fig. 1a. The bacteria solubilized 71 μg ml−1 of phosphorus on 24 h of incubation, and maximum solubilization (268 μg ml−1 of P) was recorded after 144 h. The pH of the phosphate solubilization in broth was found to decline because of bacterial activity and lowering of pH coincided with increase in the efficiency of phosphate-solubilizing activity. The pH was found to decline from 6.3 to 4.5 (Fig. 1b).
Formation of an orange halo zone around bacterial colony on Chrome azurole S agar medium indicates the siderophore production. A moderate siderophore production was witnessed after 24 h, and strong orange halo zones were observed after 48 and 72 h. However, the test isolates was found negative for HCN production (Table 2).
Growth Profile and HPLC Analysis
It was found that the growth rate constant increased with the increase in concentration of substrate. The K values of the isolate KS51 in medium amended with 0.5, 0.8 and 1 mg/50 ml anthracene was obtained as 0.33, 0.38 and 0.46 h−1, respectively. Similar results were also obtained for 0.5, 0.8,s and 1 mg/50 ml naphthalene, where K values was found to be 0.26, 0.37 and 0.44 h−1, respectively (Fig. 2).
Growth profile of isolate KS51 in medium supplemented with different concentrations of anthracene or naphthalene. Control (filled square), 0.5 mg/50 ml Ant (filled circle), 0.8 mg/50 ml Ant (up-pointing triangle), 1 mg/50 ml Ant (down-pointing triangle), 0.5 mg/50 ml Nap (left- and right-pointing triangles), 0.8 mg/50 ml Nap (left-pointing triangles), 1 mg/50 ml Nap (right-pointing triangles)
The isolate was found to considerably reduce PAH concentration in medium as estimated by HPLC analysis. The isolate KS51 showed 63.53 % degradation of anthracene, while 78.44 % decrease in naphthalene concentration was recorded after 6 days.
Antifungal Activity of the Test Isolates
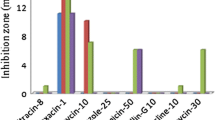
Biocontrol activity of the isolate was checked against F. oxysporum, A. alternata and C. oxysporum. The test isolate showed good antifungal activity fungi through the production of volatile metabolite but relatively less antifungal activity was detected through the diffusible metabolite (Table 3).
Phylogenetic Analysis
Based on the 16S ribosomal RNA gene sequence, isolate KS51 showed maximum similarity with Pseudomonas sp. (Accession No. FM173664.1). The phylogenetic tree (Fig. 3) constructed using 16S rRNA gene sequences of other related members of the Pseudomonad group revealed that the isolate formed a close cluster with Pseudomonas xanthomarina (Accession No. HQ202840.1).
Discussion
Rhizosphere is a rich source of microbes and should be explored for obtaining potential PGPRs, which might be good bio-inoculants for improving yields of crop plants. The exact mechanisms by which PGPR promote plant growth are not fully understood, but are thought to include (i) the ability to produce or change the concentration of plant growth regulators like IAA, gibberellic acid, cytokinins and ethylene; (ii) asymbiotic N2 fixation; (iii) antagonism against phytopathogenic microorganisms by production of siderophores, antibiotics and cyanide; and (iv) solubilization of mineral phosphates and other nutrients [1]. In the present investigation, isolate KS51 of Pseudomonas sp. was screened in vitro for its PGP activities. Though the results of the present investigations are in conformity with earlier recorded observations, based on several species of Pseudomonas that produce secondary metabolites, mobilize nutrients and promote plant growth [31].
Production of phytohormone like auxin (IAA) is a desirable characteristic of a PGPR [20]. In the current investigation, IAA production was detected by the test isolate. Our findings of IAA production by the Pseudomonas isolate are in agreement with other researchers [11]. There was an increase in the concentration of IAA with the increasing incubation time. Similar trend of IAA production with the increasing concentration of incubation time has also been reported [27]. Such findings may have direct practical application, although intrinsic ability of bacteria to produce IAA in the rhizosphere depends on the availability of precursors and uptake of microbial IAA by plant [3].
The ability of the isolate to solubilize tricalcium phosphate in vitro is another important means of achieving plant growth promotion [14]. From the current study, it was evident that the isolate liberated maximum 268 μg/ml of available phosphorus after 144 h and the pH declined from 6.3 to 4.5. These results draws support from an earlier study which highlights production of a variety of organic acids by ten bacterial and three fungal strains and subsequent drop in pH [16]. The mechanism(s) of microbial solubilization of insoluble phosphate has received attention, and phosphate solubilization is considered an important attribute of plant growth-promoting rhizobacteria. Detailed studies including phylogenetic relationships on strains of phosphate-solubilizing Pseudomonas sp. isolated from different ecological niches have also been carried out [17].
Siderophore is one of the biocontrol mechanisms belonging to PGPR groups, and PGPRs produce a range of siderophore which have a very high affinity for iron [31]. Therefore, the low availability of iron in the environment would suppress the growth of pathogenic organisms including plant pathogenic fungi [33]. From this study, it was found that the isolate was a very good siderophore producer while the isolate displayed no HCN production.
In plant disease management programmes, the use of a rapid method for screening efficient biocontrol agents is a prerequisite [2]. Antagonistic activity possessed by biocontrol agents is often evaluated by measuring the inhibition zones developed under in vitro agar based assays [30]. In the present study, isolate was examined for its antagonistic activities against three phytopathogenic fungi, viz., Alternaria alternata, Fusarium oxysporum and C. oxysporum, and it showed inhibition in fungal growth (40 % in F. oxysporum and 36 % in A. alternata) because of diffusible metabolites, while the maximum inhibition in fungal growth (70.37 % in A. alternata and 65 % in C. oxysporum) due to volatile metabolite was observed after 120 h of incubation. Similar results on the effectiveness of Pseudomonas corrugata against plant pathogenic fungi like A. alternata and F. oxysporum [30], Fusarium, Rhizoctonia, Sclerotium, Pythium [18] were also reported. Our results are in conformity with the studies of Trivedy et al. [30] which prove that the effect(s) of inhibitory volatile metabolite(s) produced by P. corrugata had a predominant inhibitory role in the antagonism of the test fungi, A. alternata and F. oxysporum, and the diffusible metabolite(s) played only a subsidiary role in the antagonism. In addition, some other studies are in contrast with the current study where the effect of inhibitory volatile metabolite(s) received less importance than the inhibitory diffusible metabolite(s) [28].
The growth rate of the isolate was found to increase with increase of substrate concentration. This further confirmed that anthracene and naphthalene concentrations are significant in growth physiology of the isolates and may also act as limiting nutrients in rhizosphere [6]. However, in this study, the quantitative estimation of residual amount of PAH in culture medium showed that 78.44 % of anthracene was degraded by the isolate [15] have also reported the degradation of PAH in liquid and solid medium by Pseudomonas sp. in their study. In the present study, the bacterium was isolated from the rhizosphere of Calotropis sp., found to grow in north and central part of India. They are highly prone to grow in extreme environmental conditions with temperature 0–50 °C, low rainfall 5–10 ml/annum, low relative humidity, high salinity and deposition of kankar or hardpan in the soil [12]. Therefore, it is assumed that the isolated bacterium has naturally adapted to variable extremities of temperatures and would retain its beneficial plant growth-promoting traits when inoculated in similar conditions.
The phylogenetic tree constructed on the 16S rRNA gene sequence revealed its close identity with Pseudomonas sp., a Gram-negative bacterium, which was first described from the rhizosphere of grasses [5]. The study carried by [31] support the present investigation to screen the Pseudomonas sp. isolated from rhizosphere of Soybean plant as plant growth promoter and biocontrol agent on the basis of 16S rRNA gene sequence analysis.
Thus, it can be concluded that rhizosphere of Calotropis sp. is a source of Pseudomonas sp. possessing potent PGP attributes, PAH degradation and biocontrol activities against phytopathogenic fungi. Further studies are underway to confirm their effectiveness in field conditions.
References
Ahmad F, Iqbal A, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Anith KN, Radhakrishan NV, Manomohandas TP (2003) Screening of antagonistic bacteria for biological control of nursery wilt of black pepper (Piper nigrum). Microbiol Res 158:91–97
Arshad M, Frankenberger JWT (1993) Microbial production of plant growth regulators. Plant Soil 133:1–3
Bakker AW, Schipper B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas sp. meditated plant growth stimulation. Soil Biochem 19:451–457
Behrendt U, Andreas U, Peter S, Jean-marie M, Cathrin S (2007) Pseudomonas lurida sp. nov., a fluorescent species associated with the phyllosphere of grasses. Int J Syst Evol Microbiol 57:979–985
Bisht S, Pandey P, Sood A, Sharma S, Bisht NS (2010) Biodegradation of naphthalene and anthracene by chemo-tactically active rhizobacteria of Populus deltoides. Braz J Microbiol 41:922–930
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Gordon S, Weber RP (1951) The colorimetric estimation of IAA. Plant Physiol 26:192–195
Johnsen AR, Wick LY, Harms H (2005) Principles of microbial PAH-degradation in soil. Environ Pollut 133:71–84
Jos MR, Timothy CP, Christian S, Claude A, Yvan ML (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Karnwal A (2009) Production of indole acetic acid by fluorescent Pseudomonas in the presence of l-tryptophan and rice root exudates. Journal of Plant Pathology 91:61–63
Kumar A (1995) Cultivation of hydrocarbon yielding plants in Rajasthan as alternative energy source. J Environ Pollut 2:67–70
Lynch J (1990) The rhizosphere. Wiley, London
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate solubilising bacteria. Curr Microbiol 43:51–56
Na KS, Kuroda A, Takiguchi N, Ikeda T, Ohtake H, Kato J (2005) Isolation and characterization of benzene tolerant Rhodococcus opacus strains. J Biosci Bioeng 99:378–382
Pandey A, Trivedi P, Kumar B, Palni LMS (2006) Characterization of a phosphate solubilising and antagonistic strain of Pseudomonas putida (Bo) isolated from a sub-alpine location in the central Himalaya. Curr Microbiol 53:102–107
Peix A, Rivas R, Santa RI, Mateos PF, Martinez ME, Rodriguez BC, Velazquez E (2004) Pseudomonas lutea sp. nov., a novel phosphate-solubilizing bacterium isolated from the rhizosphere of grasses. Int J Syst Evol Microbiol 54:847–850
Rao VS, Sachan IP, Johri BN (1999) Influence of fluorescent Pseudomonads on growth and nodulation of lentil (Lens esculentus) in Fusarium infested soil. Indian J Microbiol 39:23–29
Rodriguez H, Mendoza A, Cruz MA, Holguin G, Glick BR, Bashan Y (2006) Pleiotropic physiological effects in the plant growth-promoting bacterium Azospirillum brasilense following chromosomal labeling in the clpX gene. FEMS Microbiol Ecol 57:217–225
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Schwyn B, Neilands JB (1986) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 140:47–56
Skidmore AM, Dickinson CH (1976) Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans Br Mycol Soc 66:57–64
Stenstrom TA (1989) Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl Environ Microbiol 55:142–147
Streger SH, Vainberg S, Dong HL, Hatzinger PB (2002) Enhancing transport of Hydrogenophaga flava ENV735 for bioaugmentation of aquifers contaminated with methyl tert-butyl ether. Appl Environ Microbiol 68:5571–5579
Subba RNJ (1982) Advance in agricultural microbiology. In: Subba Rao NS (ed) Studies in the agricultural and food sciences. Butterwirth Scientific, London, pp 298–303
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequences alignment aided by quality analysis tools. Nucl Acids Res 25:4876–4882
Ting Y, Jishuang C, Huangping L, Xiaodong Z (2009) Indole-3-acetic acid improves postharvest biological control of blue mold rot of apple by Cryptococcus laurentii. Biol Control 99:258–264
Tripathi M, Johri BN (2002) In vitro antagonistic potential of fluorescent Pseudomonas and control of sheath blight of maize caused by Rhizoctonia solani. Indian J Microbiol 42:207–214
Trivedi P, Kumar B, Pandey A, Palni LMS (2007) Growth promotion of rice by phosphate solubilizing bioinoculants in a Himalayan location. ***First Int Meeting on Microbial Phosphate Solubilization Developments in Plant and Soil Sciences 102:291–299
Trivedi P, Pandey A, Palni LMS (2008) In vitro evaluation of antagonistic properties of Pseudomonas corrugate. Microbiol Res 163:329–336
Wahyudi AT, Astuti RI, Giyanto (2011) Screening of Pseudomonas sp. isolated from rhizosphere of soybean plant as plant growth promoter and biocontrol agent. Am J Agric Biol Sci 6:134–141
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Woyessa D, Assefa F (2011) Effects of plant growth promoting rhizobaceria on growth and yield of tef (Eragrostis tef Zucc. Trotter) under greenhouse condition. Res J Microbiol 6:343–355
Acknowledgments
The authors are grateful to the Director, Motilal Nehru National Institute of Technology, Allahabad (UP), India, for providing necessary facilities for the execution of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shukla, K.P., Sharma, S., Singh, N.K. et al. Deciphering Rhizosphere Soil System for Strains Having Plant Growth Promoting and Bioremediation Traits. Agric Res 1, 251–257 (2012). https://doi.org/10.1007/s40003-012-0028-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-012-0028-4