Abstract
Background
Increasing use of cardiovascular implantable electronic devices (CIED), as permanent pacemakers (PPM), implantable cardioverter defibrillators (ICD), or cardiac resynchronization therapy (CRT), is associated with the emergence of CIED-related infective endocarditis (CIED-IE). We aimed to characterize CIED-IE profile, temporal trends, and prognostic factors.
Methods
CIED-IE diagnosed at Rennes University Hospital during years 1992–2017 were identified through computerized database, and included if they presented all of the following: (1) clinical signs of infection; (2) microbiological documentation through blood and/or CIED lead cultures; (3) lead or valve vegetation, or definite IE according to Duke criteria. Data were retrospectively extracted from medical charts. The cohort was categorized in three periods: 1992–1999, 2000–2008, and 2009–2017.
Results
We included 199 patients (51 women, 148 men, median age 73 years [interquartile range, 64–79]), with CIED-IE: 158 PPMs (79%), 24 ICD (12%), and 17 CRT (9%). Main pathogens were coagulase-negative staphylococci (CoNS: n = 86, 43%), Staphylococcus aureus (n = 60, 30%), and other Gram-positive cocci (n = 28, 14%). Temporal trends were remarkable for the decline in CoNS (P = 0.002), and the emergence of S. aureus as the primary cause of CIED-IE (24/63 in 2009–2017, 38%). Factors independently associated with one-year mortality were chronic obstructive pulmonary disease (COPD: hazard ratio 3.84 [1.03–6.02], P = 0.03), left-sided endocarditis (HR 2.25 [1.09–4.65], P = 0.03), pathogens other than CoNS (HR 3.16 [1.19–8.39], P = 0.02), and CIED removal/reimplantation (HR 0.41 [0.20–0.83], P = 0.01).
Conclusions
S. aureus has emerged as the primary cause of CIED-IE. Left-sided endocarditis, COPD, pathogens other than CoNS, and no CIED removal/reimplantation are independent risk factors for one-year mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular implantable electronic devices (CIED) include permanent pacemakers (PPMs), implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT), with selected indications for the management of cardiac diseases since, respectively, the 1960s, 1980s, and 2000s [1, 2]. Over the years, despite the greater ease of device implantation, technical progress and experience, the numbers of cardiac device-related infections have increased out of proportion to rates of new device implantation [3,4,5,6,7]. These complications mostly affect generator pocket (up to 83% of CIED-related infections), but may also involve the leads and endocardial structures, with CIED-related infective endocarditis (CIED-IE) representing 10% of all cases of infective endocarditis in contemporary cohorts [8,9,10,11].
For the latter, mortality rates of 17.4–36% have been reported in recent series [2, 12,13,14,15], depending on several factors, including comorbidities, management, and pathogens. In addition, CIED-related infections have enormous economic implication, with estimated infection-related costs of 62,638 US$ per episode in patients who required CIED removal and implantation of a new device, and 50,079 US$ in patients who required CIED removal, but no reimplantation [16]. Although CIED-IE is an emerging infectious disease, with substantial morbidity and mortality, it remains poorly characterized. We aimed to report CIED-IE profile and management in our institution, with a focus on temporal trends, and prognosis factors.
Methods
Study design
We performed an observational, retrospective study of all adult patients diagnosed with CIED-IE in Rennes University Hospital from 1992 to 2017. This tertiary centre serves as a referral centre for a catchment area of 1.5 million inhabitants. In this area, all patients who undergo CIED implantation are educated about CIED-related risks, including infections, and advised to consult in case of symptoms potentially associated with CIED-related infections. All patients are routinely followed-up at least once a year after implantation. A multidisciplinary Endocarditis team, as defined by the European Society of Cardiology guidelines [17], prospectively evaluated all suspicion of CIED-related infections. Investigations and treatment were decided on a case-by-case basis. Data for clinical, microbiological, echocardiographic variables, as well as management of CIED-IE, and follow-up, were collected on a standardized questionnaire from medical charts.
Definitions
For this study, CIED-IE cases diagnosed from 1992 to 2017 were identified through computerized databases and independently validated by three experts. To ensure that most cases were enrolled, three databases were used, from the cardiology, the microbiology, and the infectious diseases/intensive care unit departments. Patients were included if they presented with all of the followings: (1) clinical signs of infection, either local (swelling, redness, or discharge in the pocket region), or systemic (sepsis, fever); (2) microbiological documentation through blood and/or lead culture(s); (3) lead or tricuspid/pulmonary valve vegetation, or definite IE according to the Duke University criteria [18]. Long-term follow-up was evaluated through medical charts review, and through systematic contact with the patient’s general practitioner or cardiologist. The main endpoints were one-year, and long-term survival. Characteristics, management and outcome of patients with CIED-IE were compared between three periods: 1992–1999, 2000–2008, and 2009–2017. The management of CIED-IE cases in our centre during the first period (1992–1999) has been previously reported [19].
A threshold of 2 months was selected to differentiate early-, and late-onset CIED-IE, in the absence of consensus on the optimal threshold [20, 21]. A new admission for CIED-IE was categorized as a relapse if the same pathogen was isolated (i.e., same species, same susceptibility profile), or a new episode otherwise. Since 1992, transesophageal echocardiography (TEE) has been performed in all patients suspected of CIED-IE, if not contraindicated. A vegetation was defined as circumscribed masses or clumps or echoes that arose from leaflet tips or electrode leads, confirmed by imaging in more than one echocardiographic plane.
Management of CIED-IE
The decision to remove CIED was based on the judgement of the endocarditis team. Total device removal was attempted during the active phase in most cases of CIED-IE, whenever feasible [22]. However, in the management of bloodstream infections in CDIE patients, removal of the device was considered as optional in our centre for cases with all of the 3 following criteria: no signs of generator pocket infection, sterilization of blood cultures within the first 48 h of appropriate antibiotic therapy, and no lesion suggestive of lead or right-sided vegetation on TEE [19]. For patients in whom CIED could not be removed, due to technical reasons, severe comorbidities, and/or failure of extraction attempt(s), chronic suppressive antimicrobial therapy was considered, on a case-by-case basis.
Since 2000, percutaneous extraction has been the primary procedure [2, 16], whatever the vegetation(s) size, and the microorganisms involved. Surgical extraction during cardiopulmonary bypass is performed when percutaneous extraction failed, or when cardiac surgery is indicated for additional reasons (e.g., valve replacement). No antibiotic prophylaxis is administered for percutaneous extraction. Samples are sent to the microbiology laboratory only when infection is suspected, either clinically or according to preoperative findings. Microorganisms are identified using standard criteria, and antimicrobial susceptibility testing is performed by the disk diffusion method. The need for CIED reimplantation is reassessed for each patient. When indicated, reimplantation is performed at a new site, preferably contralateral, once patients are no longer septic, with at least one set of sterile blood cultures post-CIED removal.
Statistical analysis
For patients with multiple CIED-IE during the study period, only the first episode was included. Data were processed with StatView software (SAS, Chicago, USA). To assess temporal trends, the variables were compared between the three periods, or between the second and the third periods for variables not available during the first period. Quantitative variables were expressed as median and interquartile range 25–75 [IQR], and were compared using chi-square test and Fisher’s exact test when appropriate. Qualitative variables were analysed using Mann–Whitney or Kruskal–Wallis test, as appropriate. Survival curves were constructed according to the Kaplan–Meier method and analysed by the logrank test. Prognostic factors were identified by univariate analysis using a Cox model. Variables with a P value < 0.20 were included in the multivariate analysis. All tests were two-sided, and a P value ≤ 0.05 was considered statistically significant.
Results
Patients (Supplementary Table 1)
During the study period, 199 patients with CIED-IE fulfilled inclusion criteria: 148 men, 51 women (male-to-female ratio, 2.9), with a median age of 73 years (64–79). Main comorbidities included chronic obstructive pulmonary disease (COPD: n = 18, 9%), cancer diagnosed during the previous year (n = 15, 8%), and diabetes mellitus (n = 14, 7%). Median baseline left ventricular ejection fraction (LVEF) was 50% (35–60). Cardiac device included 158 PPMs (79%), 24 ICD (12%), and 17 CRT (9%).
Characteristics of CIED-IE (Supplementary Table 2)
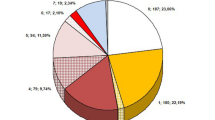
Most cases (n = 172, 86%) were documented by positive blood culture(s). The source of CIED-IE was the generator pocket in 55 cases (28%), other infectious site in 59 (30%), and unknown in 85 (43%). Fig. 1 illustrates the microorganisms distribution through the three study periods. Overall, main pathogens were coagulase-negative staphylococci (CoNS: n = 86, 43%), Staphylococcus aureus (n = 60, 30%), other Gram-positive cocci (n = 28, 14%), and Gram-negative rods (n = 18, 9%). Metastatic infectious foci were found in 44 cases (22%).
Management of CIED-IE (Supplementary Table 3)
Antibiotic regimen mostly consisted of penicillins (n = 141, 71%), and glyco/lipopeptides (n = 28, 14%), for a median duration of 35 days [28.5–45]. Most patients received a combination with aminoglycoside (n = 140, 70%). CIED was removed in 185 cases (93%): percutaneously (n = 156, 84% of all extraction), or with cardiopulmonary bypass (n = 29, 16%). During the years 2000–2017, the removal of the device was complete in 111/135 patients and near-complete (distal lead fragment could not be extracted) in 24/135 (18%). This information was not available for the period 1992–1999. Five patients with left-sided endocarditis required surgery for both CIED removal and valve replacement.
Temporal trends (Supplementary Tables 1–3)
Although slight differences were observed between patients diagnosed during 1992–1999, 2000–2008, and 2009–2017 in terms of age, and comorbidities, the most striking difference was the decline of CoNS, representing 30/50 (60%) of pathogens responsible for CIED-IE in 1992–1999, 39/86 (45%) in 2000–2008, and 17/63 (27%), in 2009–2017 (P = 0.002), along with the emergence of S. aureus as the primary cause of CIED-IE during the most recent period (24/63, 38%). A significant increase over time was also observed for the proportion of CIED-IE for which the portal of entry was identified (32–70%, P = 0.006), and with metastatic infectious foci (12% to 37%, P = 0.003), while the proportion of patients with vegetations (any localization, 100% to 93%, P = 0.01; on leads, 94% to 75%, P = 0.02), and vegetations size (median, 15 mm [11,12,13,14,15,16,17,18,19,20,21,22,23], to 10 mm [6.8–13.5], P = 0.006), decreased. Regarding management and outcome, the proportion of patients in whom the CIED was extracted decreased (from 100 to 83%, P < 0.001), while the proportion of device extraction performed percutaneously increased (from 64 to 90%, P < 0.001).
Outcome
Patients have been followed for a median duration of 2.3 years [0.7–4.7] after their first episode of CIED-IE. Of the 199 patients enrolled, 18 (9%) died during the index hospital stay: death was directly attributable to CIED-IE in 16 (89%), and particularly to the persistence of sepsis in 7 (38.9%). Seventy-three patients (37%) died after hospital discharge. The one-year and five-year mortality rates were significantly different between periods (Supplementary Table 3). Because the comparison of deaths distributions between the periods 2000–2008 and 2009–2017 did not meet the proportional hazard assumption, the Cox model was not used to compare survival between the three periods. Survival was better in the earliest period compared to the last two periods taken altogether (P = 0.01, Fig. 2a, b). By univariate analysis (Table 1), risk factors for one-year mortality were age (per one-year increment), COPD, cancer diagnosed during the year before CIED-IE, diabetes mellitus, infection source other than the generator, left-sided endocarditis, micro-organism other than CoNS (Fig. 3, supplemental Fig. 1), no removal/reimplantation of CIED, and study period. On multivariate analysis, factors independently associated with one-year mortality were COPD (hazard ratio, HR 3.84 [1.03–6.02], P = 0.03), left-sided endocarditis (HR 2.25 [1.09–4.65], P = 0.03), and microorganisms other than CoNS (HR 3.16 [1.19–8.39], P = 0.02) whereas removal/reimplantation appeared protective (HR 0.41 [0.20–0.83], P = 0.01). Factors independently associated with five-year mortality were age (HR per one-year increment, 1.03 [1.01–1.06], P = 0.008), COPD (HR 2.80 [1.37–5.75], P = 0.005), cancer during the year before CIED-IE (HR 2.05 [1.01–4.20], P = 0.05), microorganisms other than CoNS (HR 1.93 [1.02–3.63], P = 0.04), and removal/reimplantation (HR 0.56 [0.33–095], P = 0.03), whereas left-sided endocarditis did not reach statistical significance (HR 1.73 [0.92–3.25], P = 0.09). The study period was not independently associated with mortality (Table 1).
Because data were missing in the early period, outcome was analysed separately among patients from the 2000–2017 period (Supplementary Table 4). Factors independently associated with reduced one-year and five-year mortality were removal/reimplantation (respectively P = 0.004, and P = 0.01), and LVEF (per 1% increment, P = 0.01 for both). CIED-IE due to pathogens other than S. aureus had reduced one-year mortality (P = 0.02). Endocarditis relapse was reported in 17 patients during follow-up, and was the cause of death in three.
Discussion
The major findings of this single-centre, retrospective study, are the following: i) S. aureus has emerged as the primary pathogen responsible for CIED-IE, while the proportion of cases related to CoNS has declined (P = 0.002); ii) The three independent risk factors for one-year and five-year mortality were COPD, pathogens other than CoNS, and no removal/reimplantation of CIED, while left-sided endocarditis was associated with one-year, but not with 5-year mortality. Although S. aureus has gradually replaced streptococci as the primary pathogen for IE overall in different cohorts [8, 9, 23], CoNS is still the main pathogen responsible for CIED-IE, more common than S. aureus in most cohorts [1, 2, 4, 13, 20, 24,25,26,27,28,29,30], but not all [12, 14, 31, 32]. Data from other contemporary cohorts will tell whether S. aureus is emerging as the main pathogen responsible for CIED-IE in other sites as well [23]. This evolution may have consequences, given that CIED-IE related to CoNS have been associated with better prognosis, in our study as in others [14, 28, 29, 33]. Interestingly, three independent factors predictive of one-year mortality in our study were markers of the three main determinants of outcome in infectious diseases: the host (comorbidities, i.e. COPD), the pathogen (i.e. other than CoNS, S aureus in the period 2000–2017), and the extent of the infectious process (i.e. left-sided endocarditis). Our cohort is also remarkable for the increasing proportion of CIED-IE deemed to be related to generator pocket infection: 9/50 (18%) in 1992–1999, 23/86 (27%) in 2000–2008, and 23/63 (37%) in 2009–2017 (P = 0.006), while the median time from last intervention to CIED-IE diagnosis remained unchanged, between 1.5 and 1.8 years. The proportion of CIED-IE classified as early endocarditis remained low, at 16% (31/199) overall, in line with most recent cohorts [1, 2, 7, 12, 20, 27, 29, 34], which would suggest that most cases are not directly related to interventions on CIED.
Results in light of existing literature
Few studies have analysed risk factors for one-year, and long-term mortality, in patients with CIED-IE. Baman et al. found that systemic embolization (HR 7.11 [2.74–18.48]), moderate or severe tricuspid regurgitation (HR 4.24 [1.84–9.75]), abnormal right ventricular function (HR 3.59 [1.57–8.24]), and abnormal renal function (HR 2.98 [1.17–7.59]) were the four independent factors associated with six-month mortality [35]. For Kim et al., only methicillin-resistant S. aureus infection (odds ratio for survival 0.158 [0.047–0.534], P = 0.003), and concomitant valve endocarditis (OR 0.141 [0.041–0.491], P = 0.002) independently predicted mortality [14]. In the largest study on CIED-IE published to date, from the International Collaboration on Endocarditis (ICE), the only factor independently associated with one-year survival was CIED removal (HR for death, 0.42 [0.22–0.82]) [12], and this was also the only factor predictive of survival in smaller sample size-studies [36, 37]. In our study, CIED removal/reimplantation was associated with better one-year and five-year survival on multivariate analysis. Of note, when removal was indicated, but could not be performed due to patients condition or technical issues, most patients were prescribed chronic suppressive antimicrobial therapy in our centre as in others [1, 2, 38, 39], which may limit the consequences of CIED retention. In a large case–control study, Deharo et al. found that the long-term survival of patients with CIED-related infections was similar to matched-patients with non-infected CIED, which suggests that, when CIED infection are appropriately managed, life expectancy is not affected [40].
Strengths and limitations
Our study has limitations: First, due to the design (observational, retrospective, single-centre, over a long period of time), this study carries potential biases, and its findings may not apply to other settings. In addition, although we used three different databases to identify cases, some may have been missed. Second, as we only enrolled patients admitted in our tertiary care centre, this study is subject to referral bias, i.e. complicated cases may be over-represented in this cohort, as compared to all cases of CIED-IE in the geographical area. Third, no standardized protocol was in use for anti-infective treatment during the study period, which implies that the management of CIED-IE could differ from one patient to another, especially given the long study period (1992–2017). However, our study also has significant strengths, including the strict definition of CIED-IE, which ensures that we enrolled homogenous patients, with definite endocarditis. A large number of previous studies merged all cases of CIED-associated infections, including pocket generator infections, which jeopardizes their interpretation. In addition, the cases presented herein were managed following similar basic principles over a long period of time, with multidisciplinary management, and prospective collection of relevant data in medical files, which allowed the inclusion of a large number of variables for the analysis of prognostic factors, and temporal trends, with a low proportion of missing data. Finally, to the best of our knowledge, this is the largest study on CIED-IE to date, even in comparison with the ICE cohort [12].
Conclusions
This study suggests that CoNS have declined and S. aureus has emerged as the main pathogen responsible for CIED-IE over the last decade. Four variables independently predict one-year mortality: COPD, pathogens other than CoNS, left-sided endocarditis and the absence of CIED removal/reimplantation.
References
Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70(2):325–59.
Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–51.
Loffler S, Kasper J, Postulka J, Cornak V, Bohunicky F, Zelenay J, et al. Septic complications in patients with permanent pacemakers. Cor Vasa. 1988;30:400–4.
Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–77.
Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J. 2004;147:582–6.
Deshmukh A, Patel N, Noseworthy PA, Patel AA, Patel N, Arora S, et al. Trends in use and adverse outcomes associated with transvenous lead removal in the United States. Circulation. 2015;132:2363–71.
Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythm Electrophysiol. 2016;9(8):e003929.
Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–73.
Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230–9.
Tleyjeh IM, Abdel-Latif A, Rahbi H, Scott CG, Bailey KR, Steckelberg JM, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132:1025–35.
Gould PA, Gula LJ, Yee R, Skanes AC, Klein GJ, Krahn AD. Cardiovascular implantable electrophysiological device-related infections: a review. Curr Opin Cardiol. 2011;26:6–11.
Athan E, Chu VH, Tattevin P, Selton-Suty C, Jones P, Naber C, et al. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA. 2012;307:1727–35.
Cecchi E, Chirillo F, Castiglione A, Faggiano P, Cecconi M, Moreo A, et al. Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): focus on age, intravascular devices and enterococci. Int J Cardiol. 2015;190:151–6.
Kim DH, Tate J, Dresen WF, Papa FC Jr, Bloch KC, Kalams SA, et al. Cardiac implanted electronic device-related infective endocarditis: clinical features, management, and outcomes of 80 consecutive patients. Pacing Clin Electrophysiol. 2014;37:978–85.
Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60:1540–5.
Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with cardiovascular implantable electronic devices infection. Pacing Clin Electrophysiol. 2018;41(5):495–503.
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the european society of cardiology (ESC). endorsed by: european association for cardio-thoracic surgery (EACTS), the european association of nuclear medicine (EANM). Eur Heart J. 2015;36(44):3075–128.
Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings Duke Endocarditis Service. Am J Med. 1994;96:200–9.
Dumont E, Camus C, Victor F, de Place C, Pavin D, Alonso C, et al. Suspected pacemaker or defibrillator transvenous lead infection. Prospective assessment of a TEE-guided therapeutic strategy. Eur Heart J. 2003;24(19):1779–87.
Osmonov D, Ozcan KS, Erdinler I, Altay S, Yildirim E, Turkkan C, et al. Cardiac device-related endocarditis: 31-Years’ experience. J Cardiol. 2013;61:175–80.
Welch M, Uslan DZ, Greenspon AJ, Sohail MR, Baddour LM, Blank E, et al. Variability in clinical features of early versus late cardiovascular implantable electronic device pocket infections. Pacing Clin Electrophysiol. 2014;37:955–62.
Victor F, De Place C, Camus C, Le Breton H, Leclercq C, Pavin D, et al. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart. 1999;81:82–7.
Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21.
Baddour LM, Cha YM, Wilson WR. Clinical practice Infections of cardiovascular implantable electronic devices. N Engl J Med. 2012;367:842–9.
Jan E, Camou F, Texier-Maugein J, Whinnett Z, Caubet O, Ploux S, et al. Microbiologic characteristics and in vitro susceptibility to antimicrobials in a large population of patients with cardiovascular implantable electronic device infection. J Cardiovasc Electrophysiol. 2012;23:375–81.
Ferraris L, Milazzo L, Rimoldi SG, Mazzali C, Barosi A, Gismondo MR, et al. Epidemiological trends of infective endocarditis in a single center in Italy between 2003–2015. Infect Dis (Lond). 2018;50(10):749–56.
Rohacek M, Baddour LM. Cardiovascular implantable electronic device infections: associated risk factors and prevention. Swiss Med Wkly. 2015;145:w14157.
Gandhi T, Crawford T, Riddell J. Cardiovascular implantable electronic device associated infections. Infect Dis Clin North Am. 2012;26(1):57–76.
Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL, et al. Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species. Am J Cardiol. 2012;110:1143–9.
Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–9.
Greenspon AJ, Rhim ES, Mark G, Desimone J, Ho RT. Lead-associated endocarditis: the important role of methicillin-resistant Staphylococcus aureus. Pacing Clin Electrophysiol. 2008;31:548–53.
Smit J, Korup E, Schonheyder HC. Infections associated with permanent pacemakers and implanted cardioverter-defibrillator devices. A 10-year regional study in Denmark. Scand J Infect Dis. 2010;42(9):658–64.
Viola GM, Awan LL, Ostrosky-Zeichner L, Chan W, Darouiche RO. Infections of cardiac implantable electronic devices: a retrospective multicenter observational study. Medicine (Baltimore). 2012;91:123–30.
Uslan DZ, Gleva MJ, Warren DK, Mela T, Chung MK, Gottipaty V, et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE Registry. Pacing Clin Electrophysiol. 2012;35:81–7.
Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol. 2009;2:129–34.
del Rio A, Anguera I, Miro JM, Mont L, Fowler VG Jr, Azqueta M, et al. Surgical treatment of pacemaker and defibrillator lead endocarditis: the impact of electrode lead extraction on outcome. Chest. 2003;124:1451–9.
Massoure PL, Reuter S, Lafitte S, Laborderie J, Bordachard P, Clementy J, et al. Pacemaker endocarditis: clinical features and management of 60 consecutive cases. Pacing Clin Electrophysiol. 2007;30:12–9.
Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci. 2001;322:209–12.
Sekiguchi Y. Conservative therapy for the management of cardiac implantable electronic device infection. J Arrhythm. 2016;32:293–6.
Deharo JC, Quatre A, Mancini J, Khairy P, Le Dolley Y, Casalta JP, et al. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart. 2012;98:724–31.
Acknowledgements
Eric Dumont who collected and analysed data from the first cohort (1992-1999), and Marie Guinoiseau for her tremendous help in data collection for years 2000-2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Urien, JM., Camus, C., Leclercq, C. et al. The emergence of Staphylococcus aureus as the primary cause of cardiac device-related infective endocarditis. Infection 49, 999–1006 (2021). https://doi.org/10.1007/s15010-021-01634-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-021-01634-5