Abstract
Background
Inappropriate initial antimicrobial therapy (IIAT) may increase the mortality rate of hematological malignancies (HMs) patients with Gram-negative bacteria bloodstream infections (GN-BSI). The aim of this study is to determine whether IIAT affects the prognosis in this patient population and recommend the appropriate antibiotic regimen to minimize IIAT.
Methods
We reviewed a retrospective cohort study of 361 HM patients with neutropenic fever from GN-BSI. The patients’ clinical characteristics and the results of the drug sensitivity test in vitro were analyzed.
Results
IIAT rate was 21.3% in HM patients with neutropenic fever caused by GN-BSI. There was a significant difference in 7-day mortality rate between patients treated with appropriate antibiotics and those with IIAT (7.7% vs 29.9%, p < 0.01). Multivariate analysis confirmed that IIAT was an independent risk factors for early mortality [4.860 (1.541–15.323)]. Drug sensitivity data of GN-bacteria suggested that carbapenems monotherapy or beta-lactamase inhibitors (BLBLI) combined with amikacin as the initial therapy can effectively reduce the IIAT rate. In the stratified antibiogram based on prior antimicrobial exposure, our results showed that BLBLI monotherapy could be initially used as an empirical treatment in patients without prior antimicrobial exposure. In those who had received prior antimicrobial exposure, BLBLI (especially piperacillin–tazobactam) combined with amikacin is recommended.
Conclusions
IIAT was a critical factor contributing to the mortality of HM patients with neutropenic fever from GN-BSI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the baseline immunodeficiency, mucosal barrier destruction and neutropenia by cytotoxic treatments, patients with hematological malignancies (HMs) are at higher risk for bloodstream infections (BSI). According to estimated data, 11–38% of HM patients develop BSI while undergoing chemotherapy [1,2,3], resulting in 12–42% mortality [1, 2, 4,5,6]. Our previous study showed that Gram-negative bacteria (GN-bacteria) were the main pathogen for BSI in HM patients [7]. This subgroup in particular carries high mortality [1]. Early use of appropriate antibiotics has been shown to be one of the most important factors in improving the outcome of these patients.
Inappropriate initial antimicrobial therapy (IIAT) refers to the antibiotic regimen prescribed and administered during the first 72 h after suspecting BSI that was not active against the pathogen identified by culture and in vitro susceptibility testing [8,9,10]. IIAT plays an important role in the prognosis of patients with severe infections [9]. Previous studies reported that IIAT increased the hospital mortality rate and length of stay in solid cancer patients and critically ill patients complicated by GN-BSI [8, 9]. Among patients with sepsis or septic shock, the mortality rate of those treated with inappropriate initial antibiotics was significantly higher than those treated with appropriate antibiotics from the beginning [8, 9]. However, little data exist about IIAT in hematologic cancer patients.
In order to increase appropriate antibiotic usage and reduce the incidence and mortality of IIAT, guidelines recommend the application of cephalosporins (e.g., cefepime), beta-lactamase inhibitors (BLBLI) (e.g., piperacillin and tazobactam), and carbapenems as first-line empirical drugs or other antibiotics according to the local epidemiological data in HM patients presenting with neutropenic fever [11, 12]. However, in China, as reported by the CHINET monitoring network, the rate of drug resistance to fourth-generation cephalosporins is relatively high at 21.6–28.1% [13]. Moreover, in our study on HM patients, resistance to fourth-generation cephalosporins was as high as 37.3% (Table 4). This means that in Chinese HM patients with suspected severe infections, cephalosporins are not suitable as initial empirical treatment. On the other hand, with increased use of carbapenems in recent years, resistance to carbapenems has also risen,especially in Klebsiella pneumoniae (2.9% in 2005 to 24% in 2017) [13].
Therefore, we are faced with the challenge to choose the effective antibiotic for HM patients while avoiding breeding more drug resistance. In this retrospective study, we analyzed clinical data in patients with HMs and GN-BSI in South Central China to determine whether IIAT affects the prognosis of this population and to identify the risk factors leading to IIAT. Combined with the results of in vitro drug sensitivity tests, we aimed to formulate an algorithm for selecting the appropriate antibiotic regimen for patients with HMs complicated by GN-BSI, in order to reduce the occurrence of antibiotic resistance and achieve effective infection control.
Patients and methods
Setting and study design
This retrospective cohort study was evaluated by the Ethics Committee of the Central South University and deemed exempt from a formal review as no personally identifiable information would be collected. The requirement for informed consent from patients was waived. Data were extracted from the medical records of patients admitted to three university-affiliated tertiary care hospitals in Hunan Province, China, from January 2010 to April 2015.
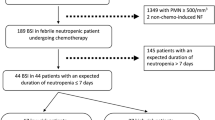
Figure 1 shows the process of case selection. A total of 520 blood culture samples were collected from febrile patients during the study period, 361 (69.4%) of which were GN-bacterial. The utilization of anti-infection therapies was performed according to the guidelines [11, 14]. For patients who had more than one positive culture with the same speciation and sensitivity, only the first one was included for analysis. Blood culture samples which showed different bacterial strains within 48 h of each other were defined as polymicrobial bacteremia and were excluded from this study due to the limited sample size. Information about patients with positive blood cultures was then obtained and the utilization of antimicrobial therapy examined. Exclusion criteria for patients were: (1) no diagnosis of hematologic malignancy or diagnosed with other malignancies; (2) blood culture contamination; (3) loss to follow-up; (4) < 16 years old; (5) history of hematopoietic stem cell transplant (HSCT). The primary outcome was all-cause mortality within 7 days after BSI onset.
Definitions
The following data were collected for each patient: demographic information, malignancy diagnosis, disease status, comorbidities, causal species, presence of multi-drug resistance (MDR) infection, vital sign trends, laboratory data, and history of antibiotic therapy. BSI was defined by the isolation of infectious organisms from blood culture specimens in patients with compatible clinical signs and symptoms [15, 16]. Patient must have had either at least two blood cultures positive for bacteria other than skin contaminants (e.g., diphtheroids, Bacillus species., Propionibacterium acnes, coagulase-negative Staphylococcus, micrococci) or the presence of any bacterial species in at least one blood culture in patients with an central intravascular catheter (e.g., port-a-cath, central venous catheter, or peripherally inserted central catheter) [17]. In addition initiation of antimicrobial therapy was required as well as at least one of the following findings: temperature of > 38.0 °C or < 36.0 °C, chills, and systolic blood pressure < 90 mmHg. When single blood culture yielded skin contaminants and without compatible clinical signs and symptoms, the blood cultures were considered as contaminants and theses cases were not included [17]. Neutropenia and profound neutropenia were defined as an absolute neutrophil count (ANC) of < 500 cells/mm3 and < 100 cells/mm3, respectively [15]. Pitt bacteremia score was calculated at the time of fever. Pitt bacteremia score is a simple score evaluation by calculating values of temperature, blood pressure, mental status, the presence or absence of mechanical ventilation and cardiac arrest [18]. The date of collection of the first positive blood culture (index culture) was regarded as the date of BSI onset. BSI were classified as nosocomial if the index blood culture was drawn more than 48 h after hospital admission [11]. MDR was defined as non-susceptibility to at least one agent in three or more antimicrobial categories [19]. Disease status was assessed by the most recently available bone marrow biopsy and categorized as remission, relapsed, or uncontrolled malignancy, as previously defined [20]. According to our population characteristics and cutoff value, sustained neutropenia exceeding 21 days was defined as prolonged neutropenia. Acute respiratory failure and acute renal failure was defined as previously described [20]. Antibiotic exposure was defined as any antimicrobial therapy for more than 48 h in the previous month [21]. Our hospitals performed susceptibility testing using standard bacteriological methods and an automated system (Vitek II System; bioMerieux, Durham, NC, USA) according to Clinical & Laboratory Standards Institute (CLSI) guidelines [22].
Statistical analysis
Statistical analysis was done in SPSS version 19.0 for Windows. Chi-square tests were used for bivariate, and logistic regression was used for multivariate analyses. The cutoff value for continuous variables were set according to clinical practice or laboratory references by use of receiver operating characteristic curve (ROC). When fewer than 5% of data were missing, missing values for continuous variables were imputed to the mean and for categorical variables to the mode. All p values were two sided, and p ≤ 0.05 was considered significant.
Results
Patient characteristics
We reviewed the clinical data of 361 HM patients complicated by GN-BSI, all of whom were admitted for neutropenic fever (Table 1). Over half of the patients were diagnosed with AML (55.4%), followed by ALL (34.3%). A majority (69.8%) was in relapse or uncontrolled disease states. Almost all (93.9%) had profound neutropenia, and 30.5% had prolonged low counts for more than 21 days. The most common pathogenic GN-bacteria was Enterobacter spp. (51.8%), followed by K. pneumoniae (19.9%), P. aeruginosa (14.1%), and Enterobacter cloacae (4.7%). Seventy-seven patients (21.3%) had received appropriate antibiotics within 72 h of BSI onset, and 45 patients (12.5%) died within 7 days.
IIAT effect on early mortality
To determine whether IIAT affected the prognosis of the patients, we compared the mortality patients who were initially treated with sensitive antibiotics and those who were treated with resistant antibiotics (Fig. 2). Statistically significant increased mortality rates with IIAT was seen as early as 24 h (7.8% vs 18.7%) and the difference escalated with time at 72 h (7.7% vs. 29.9%).
In multi-variable analysis, IIAT remained an independent risk factor for 7-day mortality (Table 2, p = 0.013). Other patient characteristics contributing to the worse outcome included elderly age, presence of acute respiratory failure, disease state, platelet count, and Pitt score (supplementary Table 1, for reviewers’ information only). These results suggest that IIAT significantly affects the mortality of HM patients with bloodstream infection.
Risk factors of IIAT
The preceding results showed that IIAT was an independent risk factor for early mortality. To find out what contributed to IIAT in our sample, we compared the clinical condition and therapeutic history of IIAT patients with non-IIAT patients (Table 3). In univariate analysis (supplementary Table 2, for reviewers’ information only), the risk of IIAT increased in patients with ALL, previous antibiotic exposure, prolonged neutropenia, and respiratory failure. IIAT was not associated with age, gender or disease status (p > 0.05). In multivariate analysis, MDR bacterial infection, prolonged neutropenia and ALL remained independent risk factors for IIAT (p < 0.05).
Antibiotic sensitivity in HM patients with GN-BSI
Table 4 lists the antibiotic susceptibility test results of different pathogens isolated from our sample population. Across all species, resistance to cephalosporins was high, up to 37.3%. Resistance to fluoroquinolones was also high at 45.9%. On the other hand, resistance to carbapenems, BLBLI (piperacillin–tazobactam and cefoperazone–sulbactam) and amikacin was relatively low at around 10%. MDR bacteria accounted for 73.4% of the resistance species. Nearly half (48.7%) of MDR bacteria were resistant to cephalosporins, and the resistance rates to BLBLI, carbapenems, and amikacin were relatively low, at 18.4%, 8.2% and 12.8%, respectively. Our results confirm high proportion of drug-resistant bacteria in HM patients with HM complicated by GN-BSI. The overall resistance rates of GN-bacteria were the lowest to carbapenems and aminoglycosides (such as amikacin), followed by BLBLI.
Appropriate antibiotics recommendation based on drug sensitivity and prior antibiotics exposure
As seen above, our patients often received IIAT when they were treated with fourth-generation cephalosporins recommended by established guidelines [11]. Therefore, we attempted to generate a recommendation for the combination of antibiotics according to the results of in vitro drug sensitivity of pathogens (Table 5). The sensitivity rate to carbapenem and cefoperazone–tazobactam monotherapy was nearly 90.0%. The results also indicated that only adding amikacin significantly improved appropriateness to cephalosporins and BLBLI, making antibiotics combinations more than 95% sensitive in vitro, which were significantly higher than those of monotherapies. Thus, carbapenem and cefoperazone–tazobactam monotherapy or combination of antibiotics selected based on antibiogram can reduce IIAT rate to less than 15%.
Our previous results showed that prior antimicrobial exposure was an independent factor in affecting IIAT, which can be explained by the previous utilization of antibiotics may lead to increased resistance of bacteria to subsequent antibiotics. To further explain this phenomenon we performed a statistical analysis on the effect of prior antimicrobial exposure (see Table 6). Our results showed that prior antimicrobial exposure may lead to a significant decrease in multiple antibiotic susceptibility except cefoperazone–tazobactam and carbapenems (imipenem or meropenem). For example, the sensitivity of cefepime for whom had received prior antimicrobial exposure decreased from 76.5 to 49.7% (p < 0.001), while the sensitivity of cefoperazone–sulbactam for whom had received prior antimicrobial exposure remain stable (p = 0.761). In addition, for patients who had not received prior antimicrobial exposure, the antibiotic sensitivity of cefepime, fluoroquinolones, aminoglycosides, piperacillin–tazobactam remain unsusceptible to most antibiotics (susceptibility rate was less than 85%), for example, the sensitivities of cefepime was 76.5%. The sensitivities of carbapenems, BLBLI (cefoperazone–sulbactam and piperacillin–tazobactam)and amikacin were above 85%.
Discussion
This study examined issues related to IIAT in HM patients presenting with neutropenic fever caused by GN-BSI. The rate of IIAT was 21.3% among our patients. IIAT was associated with significant increase in mortality, by as much as sixfold in 7 days. IIAT remained an independent risk factor for early mortality in these patients after accounting for other demographic and clinical characteristics in our multivariate analysis.
It is generally believed that early use of appropriate antibiotics can improve prognosis, but controversy exists on the definitions of “early”. Most studies suggest within 24–72 h of BSI onset [23, 24]. Our previous results demonstrated that the absence of appropriate antibiotics within 72 h, rather than 24 h, had a more significant impact on mortality rate. Therefore, in the current study, we adopted the 72-h time frame to assess the usage of sensitive antibiotics and IIAT.
The incidence of IIAT varies according to the underlying diseases, pathogenic bacteria species, and the degree of drug resistance [25, 26]. However, published data on IIAT in HM patients has been limited. Here we reported IIAT rate of 21.3% in HM patients with GN-BSI. In a previous study of 760 ICU patients with severe sepsis from GN-BSI, IIAT rate was 31.3% [25]. Other study focused on P. aeruginosa bacteremia reported an IIAT rate of 34.3% [26]. A much higher IIAT rate of 65% was observed in patients suffering from Acinetobacter baumannii bacteremia, most of whom were also on mechanical ventilation and had high APACHE II scores [27]. In comparison, the IIAT rate seen in our study might appear relatively low. One reason could be that our patients often (65.1%) received carbapenems or other broad-spectrum antibiotics as part of the initial regimen, following treatment protocols for neutropenic fever (supplementary material 3, for reviewers’ information only). However, increasing use of carbapenem and other broad-spectrum agents aggravates drug resistance, which may in turn translate into higher IIAT rates in the future. Moreover, despite the lower IIAT rate in our population, it still had a significant adverse effect on patient mortality, compelling clinicians to aim for a lower IIAT in practice. We propose an institutionalized protocol based on local antibiogram and drug resistance rates of common pathogenic organisms to tackle this challenge.
In treating HM patients presenting with neutropenic fever caused by GN-bacteremia, the initial antibacterial therapy should cover the common pathogenic bacteria in order to reduce the occurrence of IIAT. This approach would help reduce patient mortality. Based on the data at our hospitals, carbapenems monotherapy or amikacin with BLBLI would be able to reduce the rate of IIAT to < 10%. However, due to the frequent use of carbapenems in recent years, antibiotics resistance of carbapenems is on the rise [13]. Carbapenems resistance is especially prominent among GN-bacteria and Enterobacter species (CRE, carbapenems-resistant Enterobacteriaceae), increasing from 3 to 20.9% in the last 10 years [13]. How to reduce the occurrence of IIAT while avoiding selecting for carbapenems resistance is one of the urgent issues in current anti-infection treatment. In this study, in vitro drug sensitivity test results showed that the rates of resistance to carbapenems, amikacin, and BLBLI, were less than 10%. Resistance to other drugs such as cephalosporins and fluoroquinolones was high. Moreover, according to the result of Table 6, we believe that either carbapenems, BLBLI could be used when patients with HMs had no prior antimicrobial exposure. Although amikacin has a low drug resistance rate, it is prone to generating resistant strains and should not be used alone [31]. In our analysis (Tables 5, 6) that amikacin combined with BLBLI are effective against more than 95% of pathogenic bacteria, which might be a strategy to reduce IIAT. Therefore, in order to reduce the incidence of carbapenems resistance, we suggested that BLBLI monotherapy could be used as the priority choice when patients with HMs had no prior antimicrobial exposure. In those who had received prior antimicrobial exposure, BLBLI (especially piperacillin–tazobactam) combined with amikacin is recommended. On a cautionary note, amikacin’s nephrotoxicity and ototoxicity would limit its utility to short term treatment. Renal function and blood concentration of vancomycin should be monitored in time to minimize the occurrence of nephrotoxicity when BLBLI and amikacin combination when used concomitantly with empirical vancomycin for neutropenic fever prior to blood culture results. The combined therapy of amikacin and BLBLI was reported to have excellent effect against sepsis due to GN-bacteria [25], but few studies have been conducted in HM patients. A Turkey analysis showed that combination therapy of sulbactam–cefoperazone plus amikacin and imipenem–cilastatin monotherapy were equally effective empiric therapy for febrile granulocytopenic cancer patients [28]. However, since this study were older and the epidemiology of pathogenic bacteria is constantly evolving, the clinical significance of its conclusion could be debated.
Infection is the major cause of neutropenic fever in HM patients. 13–60% of HSCT recipients develop bloodstream infection (BSI), which are associated with 12–42% mortality [11]. Due to limitations on the timing of blood collection and blood culture technology, the yield of pathogenic isolates is often low. Our previous data show that the positive rate of blood culture was only 11.3% [7]. In this study, we included only patients with documented positive blood cultures in order to assess IIAT. When HM patients are admitted with neutropenic fever, they are often in urgent need of early antibiotic treatment, but etiological evidence cannot be obtained immediately, so they receive empirical antibiotics. We suggest that institution and geographic drug resistance analysis of known common pathogens be extended to the HM patient population and the collective information used to guide empirical antibiotic administration. In addition, the epidemiological data of GN-bacteria in HM patients in Central and Southern China appear similar to those in other regions of the country (Northeast China) [29]. We speculate that the results of this study may be also applied to patients with neutropenic fever in those epidemiologically similar regions.
In the last, we want to say, although the result of blood culture is the golden standard to guide clinical medication, but the blood culture often takes long time. The results of pathogenic microorganisms can be obtained in a few hours by rapid diagnostics, but the results of drug sensitivity cannot be obtained [30]. Therefore, rapid diagnostics combined with existing bacterial susceptibility may allow patients with HMs to use appropriate antibiotics within 72 h.
This study has several limitations. First, as a retrospective study, we could not control the timing of antibiotic administration or blood culture collection. Delays in appropriate treatment caused by provider or organizational factors such as drug shortage could not be accounted. Second, the therapeutic regimen recommended in this paper was based strictly on in vitro drug sensitivity test. Amikacin combined with BLBLI is rarely used in actual clinical practice, and its specific efficacy remains to be confirmed by future prospective studies.
In summary, we showed that inappropriate initial antimicrobial therapy (IIAT) was one of the critical factors contributing to the mortality of HM patients with neutropenic fever from GN-bacteria bloodstream infections. In order to reduce the rate of carbapenems resistance, BLBLI monotherapy could be used as the priority choice when patients with HMs had no prior antimicrobial exposure. In those who had received prior antimicrobial exposure, BLBLI (especially piperacillin–tazobactam) combined with amikacin is recommended, thus could reduce the IIAT rate while preventing increase in carbapenem resistance.
References
Norgaard M, Larsson H, Pedersen G, Schonheyder HC, Sorensen HT. Risk of bacteraemia and mortality in patients with haematological malignancies. Clin Microbiol Infect. 2006;12:217–23.
Tumbarello M, Spanu T, Caira M, Trecarichi EM, Laurenti L, Montuori E, et al. Factors associated with mortality in bacteremic patients with hematologic malignancies. Diagn Microbiol Infect Dis. 2009;64:320–6.
Madani TA. Clinical infections and bloodstream isolates associated with fever in patients undergoing chemotherapy for acute myeloid leukemia. Infection. 2000;28:367–73.
Chen CY, Tsay W, Tang JL, Tien HF, Chen YC, Chang SC, et al. Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiol Infect. 2010;138:1044–51.
Attman E, Aittoniemi J, Sinisalo M, Vuento R, Lyytikainen O, Karki T, et al. Etiology, clinical course and outcome of healthcare-associated bloodstream infections in patients with hematological malignancies: a retrospective study of 350 patients in a Finnish tertiary care hospital. Leuk Lymphoma. 2015;56:3370–7.
Trecarichi EM, Pagano L, Candoni A, Pastore D, Cattaneo C, Fanci R, et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect. 2015;21:337–43.
Tang Y, Cheng Q, Yang Q, Liu J, Zhang D, Cao W, et al. Prognostic factors and scoring model of hematological malignancies patients with bloodstream infections. Infection. 2018;46:513–21.
Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596.
Ramphal R. Importance of adequate initial antimicrobial therapy. Chemotherapy. 2005;51:171–6.
Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–6.
Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukemia. Haematologica. 2013;98:1826–35.
Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58:3799–803.
Fu-pin HU, Guo Y, Zhu D, et al. CHINET China bacterial resistance surveillance in 2017. Chin J Infect Chemother. 2018;2018:241–251.13.
de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F. Management of febrile neutropenia: ESMO clinical practice guidelines. Ann Oncol. 2010;21:v252–6.
Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:794–810.
Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–713.
Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19:788–802.
Ghafur A, Devarajan V, Raja T, Easow J, Raja MA, Sreenivas S, et al. Monotherapy versus combination therapy against carbapenem-resistant Gram-negative bacteria: a retrospective observational study. Indian J Cancer. 2016;53:592–4.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Liu J, Cheng Q, Yang Q, Li X, Shen X, Zhang L, et al. Prognosis-related factors in intensive care unit (ICU) patients with hematological malignancies: a retrospective cohort analysis in a Chinese population. Hematology. 2015;20:494–503.
Marin M, Gudiol C, Ardanuy C, Garcia-Vidal C, Jimenez L, Domingo-Domenech E, et al. Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumours with bloodstream infection. Clin Microbiol Infect. 2015;21:583–90.
Agrawal GN, Shevade SU. Comparison of Clinical and Laboratory Standards Institute 2008 and 2010 guidelines in interpreting susceptibility of enterobacteriaceae isolates. Indian J Pathol Microbiol. 2014;57:518–9.
De Rosa FG, Pagani N, Fossati L, Raviolo S, Cometto C, Cavallerio P, et al. The effect of inappropriate therapy on bacteremia by ESBL-producing bacteria. Infection. 2011;39:555–61.
Vasudevan A, Chuang L, Jialiang L, Mukhopadhyay A, Goh EY, Tambyah PA. Inappropriate empirical antimicrobial therapy for multidrug-resistant organisms in critically ill patients with pneumonia is not an independent risk factor for mortality: results of a prospective observational study of 758 patients. J Glob Antimicrob Resist. 2013;1:123–30.
Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54:1742–8.
Su TY, Ye JJ, Hsu PC, Wu HF, Chia JH, Lee MH. Clinical characteristics and risk factors for mortality in cefepime-resistant Pseudomonas aeruginosa bacteremia. J Microbiol Immunol Infect. 2015;48:175–82.
Erbay A, Idil A, Gozel MG, Mumcuoglu I, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. 2009;34:575–9.
Ozyilkan O, Yalcintas U, Baskan S. Imipenem-cilastatin versus sulbactam-cefoperazone plus amikacin in the initial treatment of febrile neutropenic cancer patients. Korean J Intern Med. 1999;14:15–9.
Zhai W, Zhang X, Wei J, Deng Q, Dong X, Zhang X, et al. A prospective observational study of antibiotic therapy in febrile neutropenia patients with hematological malignances from multiple centers in Northeast China. Int J Infect Dis. 2015;37:97–103.
Cendejas-Bueno E, Romero-Gomez MP, Mingorance J. The challenge of molecular diagnosis of bloodstream infections. World J Microbiol Biotechnol. 2019;35:65.
Cha MK, Kang CI, Kim SH, et al. In vitro activities of 21 antimicrobial agents alone and in combination with aminoglycosides or fluoroquinolones against extended-spectrum-β-lactamase-producing Escherichia coli isolates causing bacteremia. Antimicrob Agents Chemother. 2015;59:5834–7.
Acknowledgements
We thank all those who helped us in this study, in particular, the Department of Hematology and the Department of Clinical Laboratory for making this study possible. Qing Yang helped us modify the grammar of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (General Program), no. 81870165 and Fundamental Research Funds for the Central Universities of Central South University, no. 2017zzts837.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. All authors meet the ICMJE authorship criteria.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, Y., Wu, X., Cheng, Q. et al. Inappropriate initial antimicrobial therapy for hematological malignancies patients with Gram-negative bloodstream infections. Infection 48, 109–116 (2020). https://doi.org/10.1007/s15010-019-01370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-019-01370-x