Abstract
Purpose
The management of early- (EOS) and late-onset sepsis (LOS) and neonatal intensive care units (NICUs) has not been extensively evaluated.
Methods
231 highly specialized level 1 and level 2 NICUs in Germany were asked to participate in an internet-based survey.
Results
The final analysis of anonymized datasets from 80 NICUs (response rate 34.6 %) compared university hospitals and regional neonatal referral centers. The survey describes potential areas of improvement concerning empirical treatment of infants with LOS with vancomycin and 3rd generation cephalosporins, minimal volume of blood sampling for aerobic culture, consideration of lumbar tap in any child with blood culture positive LOS and drug monitoring details for gentamicin and vancomycin.
Conclusion
In summary, this survey reveals a significant gap between recent national German guidelines and daily practices in German NICUs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial early-onset sepsis (EOS) and late-onset sepsis (LOS) still represent severe complications in neonatal intensive care with high morbidity, substantial mortality and important long-term consequences for the neurodevelopmental outcome of affected neonates [1,2,3]. Surveillance and prevention of LOS are important issues in NICUs [4,5,6]. Clinical management is aggravated by an increasing proportion of bacterial pathogens which display in vitro resistance against commonly used first and second line antibiotics for the treatment of LOS [7, 8]. The most appropriate and effective mode to diagnose and treat these infections is controversially debated. Recently, dosing of antimicrobials and recommendations for drug monitoring of aminoglycosides, e.g., gentamicin and vancomycin have been reevaluated [9,10,11,12,13]. Previous studies have demonstrated high heterogeneity of treatment schedules comparing different NICUs unrelated to differences in their case mix index [14,15,16,17,18].
In 2012, the German Commission for Hospital Hygiene and Infection Prevention (KRINKO), affiliated at the Robert Koch Institute, Berlin, decided to recommend weekly microbiologic colonization screening in NICUs [19]. In 2013, the KRINKO published additional details how to perform such a screening, in which (facultative or opportunistic) bacteria should be included. Details were also given on how neonatologists and infection control personnel should react when detecting certain pathogens colonizing patients in the NICU [20, 21]. In case of LOS, knowledge about the colonization of an infant with certain multidrug-resistent organisms (MDROs) may lead to alterations of the empiric therapeutic schedule [8, 22, 23].
Recently, the Association of the Scientific Medical Societies in Germany (AWMF) released a consensus guideline about diagnostics and treatment of sepsis in neonates, including premature infants treated in NICUs [24]. The aims of the survey presented here were to evaluate important aspects of the current clinical practice concerning blood culture diagnostics, lumbar puncture and antimicrobial treatment of EOS and LOS in German NICUs and to compare the results with the recently published AWMF guideline.
Methods
Two of the authors (AS, CH) and the KRINKO working group “Neonatal Intensive Care” (see acknowledgment) developed an internet-based survey (Survey Monkey™; San Mateo, USA). The authors invited 231 level 1 and 2 NICUs in Germany to participate from January to June 2017. It should be mentioned here that—differing from other countries where it is the other way around—in Germany the intensity of care is classified from 4 to 1 with level 1 and 2 NICUs providing specialized neonatal intensive care to premature infants with a birth weight below 1500 g (very-low-birth weight infants, VLBWI). German level 1 units are capable to perform early postnatal surgical interventions in neonates with severe malformations (e.g., congenital heart disease, gastroschisis, and diaphragmal herniation).
With the help of comments and feedback of the working group “Neonatal Intensive Care” of the KRINKO (see acknowledgement) the survey and its layout were validated internally. The authors then sent an invitation to the head of the respective department by e-mail. The German Society for Pediatric Infectious Diseases (DGPI) posted the invitation on its website, and the German Neonatal Network (GNN) informed its members about the survey. In addition, all NICUS participating in the NEO-KISS module for the surveillance of nosocomial infections in premature neonates were contacted by email (Prof. Dr. Geffers, National Reference Center for Nosocomial Infections, Charité, Berlin, Germany). The survey comprised 8 questions (see online supplement). The participating neonatologists provided contact data such as their name and affiliation and whether their institution is a University Hospital (including teaching hospitals associated to a University Hospital) or a regional neonatal referral center. Collection of data and analysis of anonymized data took place according to German data protection laws. Datasets were checked for duplicates and ruled out (from analysis) where the participating neonatologists answered less than 70% of the questions.
To compare results from institutions with different resources and experiences, we stratified data according to whether the NICU is located at a university hospital or a regional neonatal referral center, and depending on the number of admissions with a birth weight below 1500 g in 2015. Differences in type of hospital, e.g., university hospital versus regional neonatal referral center, or number of admissions per year (VLBWI ≤ 30, > 30 to < 50, and ≥ 50, respectively) were investigated with standard statistical methods using SPSS (Version 24 IBM SPSS Statistics). The number of missing values, e.g., questions without answer, is always outlined (100% refers to all complete datasets of the individual question; mv = missing values).
In Germany, Gram-negative bacteria with in vitro resistance to certain antibiotic classes used for the treatment of severe systemic infections are described as multidrug-resistant Gram-negative bacteria (MRGN) [25]. Since the survey did not contain individual patient data, participation was voluntary, and the participating neonatologist consented to the anonymous cumulative analysis, so, an approval by the competent ethics committee was not necessary.
Results
Number and characteristics of participating NICUs
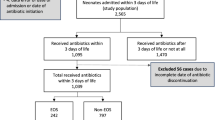
A total of 101 units out of 231 invited NICUs participated in this survey and out of this only 80 datasets met the requirements for analysis (final complete response rate 80 of 231, 34.6%). 34% of these 80 datasets were entered by university hospitals or teaching hospitals cooperating with a university hospital and 66% were from regional neonatal referral centers. In terms of VLBWI admissions, 24% units had ≤ 30, 30% > 30 to < 50, and 61% more than 50 admissions in 2015. The mean number of inpatient treatment beds per NICU was 16.9; 13% had ≤ 10 cots, 69% 10 to 20 beds and 19% more than 20 beds. The mean number of all admissions was 426; 36% of the units stated ≤ 300, 37% between 301 and 500, and 27% stated more than 500 admissions per year.
Empirical antimicrobial treatment of EOS
Figure 1 shows the distribution of preferred antibiotics for the empiric treatment of EOS. In 89% (n = 70), neonatologists from the participating NICUs administer a combination of a beta-lactam (e.g., ampicillin) with an aminoglycoside (e.g., gentamicin or tobramycin). Some preferably or occasionally use piperacillin (11%), ampicillin-sulbactam (11%), cefotaxime (10%) or piperacillin–tazobactam (4%) instead of ampicillin.
Empirical antimicrobial treatment of LOS
Figure 2 shows the distribution of preferred antibiotics for the empiric treatment of LOS. The related question did not differentiate between infants with LOS as first or second infection (after previous treatment of EOS). Here, ampicillin (29%) and gentamicin (33%) were still marked as eligible by some participants. Results concerning LOS seem much more heterogeneous. Many centers prefer third generation cephalosporins (altogether 52%; cefotaxime 44%, ceftazidime 8%) and 48% use vancomycin empirically before any methicillin-resistant Gram-positive pathogen is detected and reported in blood cultures or from the tip of a removed central venous catheter. In contrast to EOS, piperacillin-tazobactam scores are higher (13%), and a carbapenem (e.g., meropenem) appears as first choice in nearly one-fifth of all participating NICUs (18%).
Empiric treatment of LOS in infants colonized with MDROs
In total, neonatologists from 79 NICUs answered to the question, whether a previously known colonization of an infant with MDRO affects the decision on how to treat a case of LOS (mv n = 1). The great majority (94%; n = 74) regularly recommend to adjust antimicrobial treatment to recent results of colonization screening; 4% (n = 3) integrate this information into individual treatment consideration (without regularly recommending an empirical use of broader spectrum antibiotics in any case), and 2% (n = 2) just ignore this information.
Blood culture diagnostics in infants with suspected infection
In an infant with suspected systemic infection, 33% (n = 26; mv = 2) regularly collect at least 1 ml of blood for one aerobic blood culture from an infant with suspected LOS (Fig. 3). The majority (59%; n = 46) take at least 0.5 ml per aerobic blood culture, and 8% (n = 6) take 0.5 ml each for aerobic and anaerobic blood cultures, respectively. One of the questions was about the clinical relevance of anaerobic blood cultures in infants with suspected LOS. Here, nineteen (23%; mv n = 2) participants stated, that an additional anaerobic blood culture is essential, 53% (n = 41) take an anaerobic blood culture only at special indications (such as necrotizing enterocolitis), and 24% (n = 18) state that additional anaerobic blood cultures are never needed in case of suspected LOS.
Examination of cerebrospinal fluid in infants with LOS and positive blood culture
One question aimed to elucidate whether attending neonatologists consider performing a lumbar tap to exclude meningitis in a clinically stable infant with LOS and positive blood culture (infections with Coagulase negative staphylococci, CoNS, were excluded). The majority (65%, n = 50; mv = 3) perform a lumbar tap only in infants with clinical signs of meningitis; 30% (n = 23) always try to collect CSF in this situation and 5% (n = 4) decide about this investigation depending on the bacterial species detected in blood culture.
Drug monitoring
Of all participating NICUs, 94% (n = 74; mv = 1) perform any kind of drug monitoring in infants with LOS treated with gentamicin; in 4% (n = 3), this decision depends on the individual clinical situation, and in 3% (n = 2), no drug monitoring is accomplished. Seventy-three NICUs (mv = 7) provided details concerning the accepted gentamicin trough level. In 60% (n = 44), the targeted level is < 2 µg/mL, and in 40% (n = 29) < 1 µg/mL. Concerning drug monitoring in infants with LOS treated with vancomycin (mv = 3), 96% (n = 74) do, and 4% (n = 3) do not perform a drug monitoring (at least one trough level). Again, 73 NICUs provided details (mv = 7). The targeted vancomycin trough level is 5–10 µg/ml in 79% (n = 58), 10–20 µg/ml in 14% (n = 10), and < 5 µg/ml in 7% (n = 5).
Statistical analysis
Statistical analysis (see tables in online supplement) revealed some significant differences. Gentamicin for the treatment of EOS was significantly more often used in regional referral NICUs than in NICUs located at university hospitals (p = 0.046). Tobramycin for the treatment of LOS was significantly more often used in centers with ≤ 30 admissions with a birth weight < 1500 g/year (p = 0.018). NICUs who state that a lumbar tap is obligate in an infant with LOS and a positive blood culture (except CoNS) significantly more often use a third generation cephalosporin empirically in infants with suspected LOS (p = 0.013).
Discussion
This survey provides an evaluation of certain diagnostic aspects concerning antimicrobial treatment of EOS and LOS in German NICUs. 80 of 231 contacted NICUs (34.6%) provided complete datasets with more than 70% of all questions answered. This is in concordance with the experiences of other research groups concerning physician specialist response rates to web-based surveys using a personalized invitation email strategy [26]. Since 65.4% of all 231 German level 1 or level 2 NICUs did not participate at all or sent incomplete datasets (n = 21), we cannot exclude that a higher response rate would have an impact on our results.
Only one-third out of the NICUs who participated belongs to university hospitals or teaching hospitals cooperating with a university clinic. In Germany, regional neonatal referral centers are allowed to treat VLBWI as long as they fulfill certain criteria of the German Federal Joint Committee (GBA) for level 1 or 2 NICUs [27].
Ampicillin and gentamicin are still the first choice for empirical treatment of suspected EOS in most participating NICUs, as recommended by current guidelines [28,29,30] including the German AWMF guideline from 2018 [24]. Local resistance patterns may lead to alterations of this schedule, in particular regarding a higher proportion of ampicillin-resistant E. coli or vaginal colonization of the mother with MRGN [31].
The results of this survey show that empirical treatment regimens for the treatment of LOS are highly heterogeneous. This observation has been made by many studies from other countries, too [14,15,16, 18, 32]. A European survey detected more than 20 different regimens [33], a study form the Netherlands described up to 24 different antibiotics used in NICUs [16], and a French survey including 44 NICUs depicted 444 dosage regimens for 41 antibiotics [18]. A survey conducted in neonatal units in the UK [17] revealed 24 different combinations of dose, timing of dose and timing of monitoring for gentamicin, and 17 different regimens for vancomycin, respectively. National guidelines may positively influence the rational use of antibiotics in NICUs as long as the attending neonatologists adopt these guidelines [9, 11, 33].
Two aspects depicted in our survey concerning the empirical treatment of LOS warrant further discussion. First, empirical use of vancomycin in infants with suspected LOS—before any methicillin-resistant Gram-positive pathogen is detected in blood cultures or from the tip of a removed central venous catheter—is still routine in 48% of all participating NICUs.
This practice of untargeted vancomycin use should be reconsidered in light of recent publications which show a very low attributable mortality in infants with methicillin-resistant CoNS blood stream infections [34]. The situation is more ambivalent in case of S. aureus bloodstream infection (BSI). Thaden et al. [35] recently reviewed 3.339 infants with S. aureus BSI, comprising data from 348 NICUs managed by the Pediatrix Medical Group. Inadequate empirical antibiotic therapy was administered in 725 (22%) cases. Among infants infected with MRSA, inadequate empirical antibiotic therapy was associated with increased 30-day mortality (odds ratio: 2.03; 95% confidence interval: 1.08–3.82).
Consequently, the previously known MRSA colonization of an infant (or its parent/sibling) supports the decision to use vancomycin empirically. This is one among other arguments to screen for S. aureus colonization in infants treated in NICUs [21, 36, 37]. NICUs should define the indications for vancomycin together with dosing regimens and drug monitoring issues in an internal standard [38].
Second, a relevant proportion of all participating German NICUs still use third generation cephalosporins in infants with suspected LOS. The recent AWMF guideline [24] recommends avoiding the use of 3rd generation cephalosporins as empiric therapy in neonates with LOS (except in infants with meningitis) since it may cause adverse effects concerning individual outcomes [8] and increase the selective pressure for MRGN [39]. Infants with Gram-negative BSI and septic shock show a worse outcome in case of initial inadequate empirical antibiotic therapy [7, 8]. In this regard, it could be reasonable to use meropenem in infants with LOS previously colonized with carbapenem-sensitive MRGN or in all infants with septic shock. Indications for meropenem should be defined in an internal standard [9, 11], and a critical reevaluation after 3 days of treatment should be accomplished in all infants with LOS [40].
One important question for clinical practice is whether the causative pathogen of LOS in an individual child is related to bacteria, which previously only colonized the skin or nares (S. aureus; including MSSA and MRSA), the throat (S. aureus, Streptococci, Gram-negatives such as Enterobacteriaceae and non-fermenters), or the gastrointestinal tract (Enterobacteriaceae and non-fermenters, Enterococci) [41,42,43]. In case of a significant correlation, microbiologic colonization screening is importing for guiding individual treatment decisions [8, 22]. A recent systematic review and meta-analysis [43] reevaluated the relationship between colonization with Gram-negative pathogens and subsequent blood culture-positive LOS in neonates; the findings of this analysis are limited by the small number and high heterogeneity of the included studies. Eventually, 157 of 1984 colonized neonates (7.9%) developed Gram-negative -BSI compared with 85 of 3583 (2.4%) non-colonized neonates. Interestingly, 94% of the participating NICUs regularly adjust antimicrobial treatment of LOS to recent results of colonization screening [21]. It is unknown so far how this decision refers to the local epidemiology of Gram-negative pathogens detected in LOS in the participating institutions and how this consensus affects the overall utilization of meropenem and the selection of carbapenem-resistant pathogens.
Recent publications of the KRINKO [44] and the 2018 German AWMF Guideline [24] recommend to sample at least 1 ml of blood for an aerobic blood culture in infants with suspected LOS. According to our results, neonatologists are still reluctant to sample at least 1 ml although the volume of the sample is the most important means to increase blood culture sensitivity [45]. The German AWMF Guideline [24] suggests considering an additional anaerobic blood culture in special clinical situations such as an infant with severe intraabdominal infection. However, there are still no commercial blood culture bottles available to detect anaerobic pathogens in a blood volume of 1–3 ml.
The laboratory and cultural investigation of cerebrospinal fluid (lumbar puncture, LP) is the only way to confirm or exclude involvement of the central nervous system in infants with LOS and positive blood cultures. The German AWMF Guideline [24] outlines that infants with blood culture positive LOS have an increased risk of meningitis and that meningitis may be present even in infants with LOS and negative blood cultures. We excluded CoNS in this question since CoNS mainly act as relevant CNS pathogens in nosocomial meningitis related to foreign materials such as ventriculo-peritoneal shunts or Rickham reservoirs, and after neurosurgical interventions. In particular in patients with blood culture positive LOS due to Gram-negative pathogens, meningitis has to be excluded since its detection significantly impacts the choice of antibiotics, the duration of treatment and details of subsequent management such as CNS magnet-resonance imaging [46]. Neonatologists who regularly perform an LP in all infants with blood culture-positive LOS (except CoNS) probably document the involvement of the central nervous system in systemic infection more often than others who do not do LPs. We do not have a final explanation for the statistical association between NICUs who state, that a lumbar tap is obligate in an infant with LOS and a positive blood culture (except CoNS), and the empirical use of third generation cephalosporins in infants with suspected LOS.
Which is the most appropriate dosing [47] and drug monitoring of aminoglycosides and vancomycin in premature infants [17, 48] is still a very complex and controversial issue. However, a comprehensive discussion is beyond the scope of this article. Nonetheless, our survey identifies some potential areas of improvement. Gentamicin trough levels should be measured in all infants with LOS. In infants, who receive gentamicin once daily (or less than all 24 h), it is assumed that gentamicin is regularly eliminated at trough levels below 1 µg/ml [49, 50]; some studies and the German AWMF guideline refer to a targeted trough level of 2 µg/ml [24, 51].
In case of Vancomycin, the trough level (taken just before the third dose) does not only demonstrate regular elimination of the drug but also correlates with the area-under the curve (AUC), which is important for effective therapy. As recommended in the German AWMF guideline [24], the targeted vancomycin trough level in neonates with normal renal function is 5–10 µg/ml [52, 53]. Higher trough levels, as up to 15–20 µg/ml for adults, are difficult to reach in infants with normal renal function [54] and require much higher dosing, which may be associated with nephrotoxicity and ototoxicity in particular after prolonged treatment [55]. We can only speculate that in NICUs targeting Vancomycin through levels < 5 µg/ml this background information is not available.
Conclusion
This up-to-date survey elucidates EOS and LOS treatment and sampling of blood cultures and CSF as currently practiced in 80 German highest acuity level 1 and level 2 NICUs (response rate 34.6%). The survey describes potential areas of improvement concerning the use of vancomycin and third generation cephalosporins in infants with LOS, the minimal volume of blood sampling for aerobic culture, the consideration of lumbar tap in any child with blood culture positive LOS, and details of gentamicin and vancomycin drug monitoring.
References
Troger B, Gopel W, Faust K, et al. Risk for late-onset blood-culture proven sepsis in very-low-birth weight infants born small for gestational age: a large multicenter study from the German Neonatal Network. Pediatr Infect Dis J. 2014;33:238–43.
Stichtenoth G, Demmert M, Bohnhorst B, et al. Major contributors to hospital mortality in very-low-birth-weight infants: data of the birth year 2010 cohort of the German Neonatal Network. Klin Padiatr. 2012;224:276–81.
Alshaikh B, Yusuf K, Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol. 2013;33:558–64.
Reichert F, Piening B, Geffers C, Gastmeier P, Buhrer C, Schwab F. Pathogen-specific clustering of nosocomial blood stream infections in very preterm infants. Pediatrics. 2016;137:e 20152860.
Leistner R, Piening B, Gastmeier P, Geffers C, Schwab F. Nosocomial infections in very low birthweight infants in Germany: current data from the national surveillance system NEO-KISS. Klin Padiatr. 2013;225:75–80.
Cantey JB, Ronchi A, Sanchez PJ. Spreading the benefits of infection prevention in the neonatal intensive care unit. JAMA Pediatr. 2015;169:1089–91.
Kermorvant-Duchemin E, Laborie S, Rabilloud M, Lapillonne A, Claris O. Outcome and prognostic factors in neonates with septic shock. Pediatr Crit Care Med. 2008;9:186–91.
Patel SJ, Green N, Clock SA, et al. Gram-Negative Bacilli in infants hospitalized in the neonatal intensive care unit. J Pediatric Infect Dis Soc. 2017;6:227–30.
Cantey JB. Optimizing the use of antibacterial agents in the neonatal period. Paediatr Drugs. 2016;18:109–22.
Cantey JB, Milstone AM. Bloodstream infections: epidemiology and resistance. Clin Perinatol. 2015;42:1–16 (vii).
Cantey JB, Patel SJ. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am. 2014;28:247–61.
Cantey JB, Wozniak PS, Pruszynski JE, Sanchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16:1178–84.
Cantey JB, Wozniak PS, Sanchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J. 2015;34:267–72.
Lutsar I, Chazallon C, Carducci FI, et al. Current management of late onset neonatal bacterial sepsis in five European countries. Eur J Pediatr. 2014;173:997–1004.
Fernando AM, Heath PT, Menson EN. Antimicrobial policies in the neonatal units of the United Kingdom and Republic of Ireland. J Antimicrob Chemother. 2008;61:743–5.
Liem TB, Krediet TG, Fleer A, Egberts TC, Rademaker CM. Variation in antibiotic use in neonatal intensive care units in the Netherlands. J Antimicrob Chemother. 2010;65:1270–5.
Kadambari S, Heath PT, Sharland M, Lewis S, Nichols A, Turner MA. Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. J Antimicrob Chemother. 2011;66:2647–50.
Leroux S, Zhao W, Betremieux P, Pladys P, Saliba E, Jacqz-Aigrain E. Therapeutic guidelines for prescribing antibiotics in neonates should be evidence-based: a French national survey. Arch Dis Child. 2015;100:394–8.
Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut B; Ergänzende E. zur “Prävention nosokomialer Infektionen bei neonatologischen Intensivpflegepatienten mit einem Geburtsgewicht unter 1.500 g” (2007). Epidemiol Bulletin des Robert Koch-Instituts, Berlin. 2012;(16. January 2012/Nr. 2):13–15.
Christoph J, Dame C, Eckmanns T, et al. Risikocharakterisierung intensivmedizinisch behandelter Früh- und Neugeborener und Daten zur Ist-Situation in deutschen neonatologischen Intensivpflegestationen 2013 - Fachliche Erläuterungen zu folgender Empfehlung: Praktische Umsetzung sowie krankenhaushygienische und infektionspräventive Konsequenzen des mikrobiellen Kolonisationsscreenings bei intensivmedizinisch behandelten Früh- und Neugeborenen Ergänzende Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut, Berlin zur Implementierung der Empfehlungen zur Prävention nosokomialer Infektionen bei neonatologischen Intensivpflegepatienten mit einem Geburtsgewicht unter 1.500 g aus dem Jahr 2007 und 2012 (Epidemiologisches Bulletin 42/2013). Epidemiol Bulletin des Robert Koch-Instituts, Berlin 2013;Supplement zu Ausgabe 42(21. Oktober 2013).
Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut B. Praktische Umsetzung sowie krankenhaushygienische und infektionspräventive Konsequenzen des mikrobiellen Kolonisationsscreenings bei intensivmedizinisch behandelten Früh- und Neugeborenen -Ergänzende Empfehlung der KRINKO beim Robert Koch-Institut, Berlin, zur Implementierung der Empfehlungen zur Prävention nosokomialer Infektionen bei neonatologischen Intensivpflegepatienten mit einem Geburtsgewicht unter 1.500 g aus dem Jahr 2007 und 2012. Epidemiol Bulletin des Robert Koch-Instituts, Berlin. 2013;Nr. 42(21. Oktober 2013):421–433.
Smith A, Saiman L, Zhou J, Della-Latta P, Jia H, Graham PL. Concordance of gastrointestinal tract colonization and subsequent bloodstream infections with Gram-negative Bacilli in very low birth weight infants in the neonatal intensive care unit. Pediatr Infect Dis J. 2010;29:831–5.
Haertel C, Simon A, Geffers C, et al. Nosokomiale Infektionen bei Frühgeborenen—Umsetzung der KRINKO-Empfehlungen im Deutschen Frühgeborenennetzwerk. Monatsschr Kinderheilkd. 2013;161:27–33.
Deutsche Gesellschaft für Neonatologie und Pädiatrische Intensivmedizin. Deutsche Gesellschaft für Pädiatrische Infektiologie, Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG), Arbeitsgemeinschaft der wissenschaftlichen Fachgesellschaften (AWMF). Bakterielle Infektionen bei Neugeborenen. AWMF Register No 024—008 2018; 30.04.2018.
Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut. Definition der Multiresistenz gegenüber Antibiotika bei gramnegativen Stäbchen im Hinblick auf Maßnahmen zur Vermeidung der Weiterverbreitung. Epidemiol Bull des Robert Koch-Instituts Berlin. 2011;12:337–9.
Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol 2015;1532.
Gemeinsamer Bundesausschuss (GbA). Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Qualitätssicherungs-Richtlinie Früh- und Reifgeborene/QFR-RL: (§ 7 Nachweisverfahren und Anlage 2 Anforderung zum Pflegepersonal) 2016;15. Dezember 2016. http://www.english.g-ba.de/.
Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–15.
Mukherjee A, Davidson L, Anguvaa L, Duffy DA, Kennea N. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed. 2015;100:F248–9.
Mukherjee A, Ramalingaiah B, Kennea N, Duffy DA. Management of neonatal early onset sepsis (CG149): compliance of neonatal units in the UK with NICE recommendations. Arch Dis Child Fetal Neonatal Ed. 2015;100:F185.
Denkel LA, Schwab F, Kola A, et al. The mother as most important risk factor for colonization of very low birth weight (VLBW) infants with extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E). J Antimicrob Chemother. 2014;69:2230–7.
Patel SJ, Oshodi A, Prasad P, et al. Antibiotic use in neonatal intensive care units and adherence with centers for disease control and prevention 12 step campaign to prevent antimicrobial resistance. Pediatr Infect Dis J. 2009;28:1047–51.
Spyridis N, Syridou G, Goossens H, et al. Variation in paediatric hospital antibiotic guidelines in Europe. Arch Dis Child. 2016;2016:72–6.
Ericson JE, Thaden J, Cross HR, et al. No survival benefit with empirical vancomycin therapy for coagulase-negative staphylococcal bloodstream infections in infants. Pediatr Infect Dis J. 2015;34:371–5.
Thaden JT, Ericson JE, Cross H, et al. Survival benefit of empirical therapy for Staphylococcus aureus bloodstream infections in infants. Pediatr Infect Dis J. 2015;34:1175–9.
Popoola VO, Colantuoni E, Suwantarat N, et al. Active surveillance cultures and decolonization to reduce staphylococcus aureus infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2016;37:381–7.
Wisgrill L, Zizka J, Unterasinger L, et al. Active surveillance cultures and targeted decolonization are associated with reduced methicillin-susceptible Staphylococcus aureus infections in VLBW infants. Neonatology. 2017;112:267–73.
Holzmann-Pazgal G, Khan AM, Northrup TF, Domonoske C, Eichenwald EC. Decreasing vancomycin utilization in a neonatal intensive care unit. Am J Infect Control. 2015;43:1255–7.
Le J, Nguyen T, Okamoto M, McKamy S, Lieberman JM. Impact of empiric antibiotic use on development of infections caused by extended-spectrum beta-lactamase bacteria in a neonatal intensive care unit. Pediatr Infect Dis J. 2008;27:314–8.
Stocker M, Ferrao E, Banya W, Cheong J, Macrae D, Furck A. Antibiotic surveillance on a paediatric intensive care unit: easy attainable strategy at low costs and resources. BMC Pediatr 2012;12196.
Simon A, Tenenbaum T. Surveillance of multidrug-resistant Gram-negative pathogens in high-risk neonates-does it make a difference? Pediatr Infect Dis J. 2013;32:407–9.
Seidel J, Haller S, Eckmanns T, Harder T. Routine screening for colonization by Gram-negative bacteria in neonates at intensive care units for the prediction of sepsis: systematic review and meta-analysis. J Hosp Infect. 2018 (in press).
Folgori L, Tersigni C, Hsia Y, et al. The relationship between Gram-negative colonization and bloodstream infections in neonates: a systematic review and meta-analysis. Clin Microbiol Infect. 2018;24:251–7.
Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch Institut Berlin. Prävention von Gefäßkatheter-assoziierten Infektionen bei Früh- und Neugeborenen—Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robeert Koch Instituut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61:608–26.
Dien Bard J, McElvania TeKippe E. Diagnosis of bloodstream infections in children. J Clin Microbiol. 2016;54:1418–24.
Berger A, Rohrmeister K, Haiden N, Assadian O, Kretzer V, Kohlhauser C. Serratia marcescens in the neonatal intensive care unit: re-emphasis of the potentially devastating sequelae. Wien Klin Wochenschr. 2002;114:1017–22.
Pineda LC, Watt KM. New antibiotic dosing in infants. Clin Perinatol. 2015;42:167–76 (ix–x).
Pauwels S, Allegaert K. Therapeutic drug monitoring in neonates. Arch Dis Child. 2016;101:377–81.
Fonzo-Christe C, Guignard B, Zaugg C, et al. Impact of clinical decision support guidelines on therapeutic drug monitoring of gentamicin in newborns. Ther Drug Monit. 2014;36:656–62.
Fuchs A, Guidi M, Giannoni E, et al. Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br J Clin Pharmacol. 2014;78:1090–101.
Antolik TL, Cunningham KJ, Alabsi S, Reimer RA. Empirical gentamicin dosing based on serum creatinine levels in premature and term neonates. Am J Health Syst Pharm. 2017;74:466–72.
Frymoyer A, Hersh AL, El-Komy MH, et al. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother. 2014;58:6454–61.
Cole TS, Riordan A. Vancomycin dosing in children: what is the question? Arch Dis Child. 2013;98:994–7.
Ringenberg T, Robinson C, Meyers R, et al. Achievement of therapeutic vancomycin trough serum concentrations with empiric dosing in neonatal intensive care unit patients. Pediatr Infect Dis J. 2015;34:742–7.
Lestner JM, Hill LF, Heath PT, Sharland M. Vancomycin toxicity in neonates: a review of the evidence. Curr Opin Infect Dis. 2016;29:237–47.
Acknowledgements
Our thanks go to all participating neonatologists and to the members of the KRINKO working group “Neonatal intensive care”: Dr. Jürgen Christoph, Prof. Dr. Christof Dame, Prof. Dr. Christine Geffers, Prof. Dr. Christian Gille, Prof. Dr. Irene Krämer, Dr. Matthias Marschal, Prof. Dr. Andreas Müller, and Prof. Dr. Mardjan Arvand and Vanda Marujo (Robert Koch Institute). We thankfully acknowledge the help of Gudrun Wagenpfeil concerning statistical analysis.
Funding
The German Society for Pediatric Infectious Diseases (DGPI) actively promoted the distribution of the survey (website and E-Mail invitation) and provided the technical prerequisites (Survey Monkey™).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Arne Simon is the second chair of the German Commission for Hospital Hygiene and Infection Prevention (KRINKO) affiliated at the Robert Koch Institute, Berlin, and coordinates the KRINKO working group “Neonatal Intensive Care”. Michael Zemlin is the coordinator of the AWMF Recommendation “Neonatale Sepsis” (AWMF Registration Number 024/008) lead-managed by the German Society of Pediatric and Neonatal Intensive Care (GNPI). Christoph Haertel is a member of the KRINKO working group “Neonatal Intensive Care” and co-authored the AWMF Recommendation “Neonatale Sepsis” (AWMF Registration Number 024/008).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Litz, J.E., Goedicke-Fritz, S., Härtel, C. et al. Management of early- and late-onset sepsis: results from a survey in 80 German NICUs. Infection 47, 557–564 (2019). https://doi.org/10.1007/s15010-018-1263-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1263-9