Abstract
Purpose
Antibiotic stewardship programs (ASP) optimize antibiotic usage and combat antibiotic resistance of bacteria. The objective of this study was to assess the impact of specific ASP interventions on antibiotic consumption in general pediatric wards.
Methods
We conducted a prospective study to compare a pre-intervention (Sept.–Dec. 2014) and post-intervention (Sept.–Dec. 2015) period. An ASP bundle was established including (1) infectious diseases (ID) ward rounds (prospective-audit-with-feedback), (2) ID consultation service, (3) internal guidelines on empiric antibiotic therapy. Medical records on four general pediatric wards were reviewed daily to analyze: (1) antibiotic consumption, (2) antibiotic dosage ranges according to local guidelines, and (3) guideline adherence for community-acquired pneumonia (CAP).
Results
Antibiotic prescribing for 273 patients (pre-intervention) was compared to 263 patients (post-intervention). Antibiotic prescription rate did not change (30.6 vs. 30.5%). However, overall days-of-therapy and length-of-therapy decreased by 10.5 and 7.7%, respectively. Use of cephalosporins and fluoroquinolones decreased by 35.5 and 59.9%, whereas the use of penicillins increased by 15.0%. An increase in dosage accuracy was noted (78.8 vs. 97.6%) and guideline adherence for CAP improved from 39.5 to 93.5%. Between the two study periods, no adverse effects regarding length of hospital stay and in-hospital mortality were observed.
Conclusions
Our data demonstrate that implementation of an ASP was associated with a profound improvement of rational antibiotic use and, therefore, patient safety. Considering the relatively short observation period, the long-term effects of our ASP bundle need to be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of drug-resistant pathogens is rising dramatically [1]. Regarding the lack of new antimicrobial drug classes, antibiotic resistance has become a major public health threat. Antibiotics are among the most commonly prescribed drugs in hospitalized children [2]. In pediatric patients, between 3.4 and 35% of antibiotic prescriptions were considered to be inappropriate [3, 4]. In light of a well-documented causal relationship between antibiotic overuse or misuse and emergence of resistant bacteria, action plans endorsed by different organisations like the World Health Organization (WHO), the Infectious Diseases Society of America (IDSA), and the German Society of Infectious Diseases (DGI) emphasize the importance of antibiotic stewardship programs (ASP) for monitoring and promoting the optimization of antimicrobial use to preserve our antibiotic armamentarium [5–7]. In adult patients, several studies have firmly established the positive impact of ASP on antibiotic density, expenditures, antimicrobial resistance, as well as antibiotic-related toxicity and complications such as Clostridium difficile–infections (CDI) [8, 9]. The main strategies of ASP include correct selection of the antimicrobial agent, dosing, and adequate lengths of antibiotic therapy. However, studies assessing the effects of ASP efforts in pediatrics are scarce [10–17]. Detailed analysis of antibiotic consumption beyond point prevalence studies from European pediatric hospitals [18, 19] is lacking.
In 2012, we established an ASP pilot program assessing the impact of ASP strategies [20]. This program resulted in estimated cost savings of more than 330,000 € per year and was still ongoing during the pre-intervention period. The main goal of the present study was to enhance previous achievements (i.e., decreased use of broad-spectrum antibiotics) and to extend the program by defining areas for further improvement. We carefully evaluated the effectiveness of our interventions in improving guideline adherence, antibiotic selection, dosing accuracy, and reduction of overall and specifically targeted antibiotics such as cephalosporins and fluoroquinolones (FQs).
Methods
Setting and study design
The study was conducted at the Dr. von Hauner Children’s Hospital, an academic tertiary care center at the Ludwig-Maximilians-University (LMU) Munich, Germany. Four general pediatric wards for all age–groups were included, with a total of 61 beds and approximately 3800 admissions per year. Pediatric surgery patients were not included.
Our goal was to assess the impact of an ASP using two 4-month periods of data collection: Sept 1, 2014–Dec 31, 2014 (pre-intervention period) and Sept 1, 2015–Dec 31, 2015 (post-intervention period). Drugs, including all antibiotics, are dispensed to each ward by the central pharmacy. No computerized physician order entry (CPOE) system, electronic medication record or unit-dose-system was available.
Antibiotic stewardship program (ASP)
As an extension to a previously reported pilot Antibiotic stewardship project [20], a more comprehensive ASP was implemented between January and August 2015. Core members of the ASP team were two pediatric infectious diseases (ID) specialists and two dedicated clinical pharmacists, trained in antibiotic stewardship and ID. The implemented interventions are described in detail below. Several main goals for the planned ASP were identified: (1) increased utilization of penicillins, (2) decreased use of second- and third-generation cephalosporins and FQs, (3) early de-escalation of broad empiric regimens guided by microbiological results, (4) dosing accuracy, (5) empiric antibiotic choice according to local guidelines, and (6) improving patient safety by optimizing antibiotic treatment and avoiding dosing errors.
Interventions
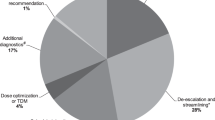
Core ASP strategies included activities earlier described in international and national guidelines: prospective-audit-with-feedback and bed-side academic detailing regarding every antibiotic regimen during weekly pediatric ID ward rounds by the ASP team. Chart reviews were conducted daily by the clinical pharmacist. In brief, indications and laboratory data were reviewed, and antibiotic choice, dosing, route of administration, additional diagnostic procedures, and length of therapy were discussed. Feedback was provided to the physician staff as well as to the nursing personnel on respective wards. A pre-existing ID consultation service was intensified, now operating on a 24/7 on-call basis with formal (written) ID consults documentation. Recommendations were given during pediatric ID ward rounds and written ID consults were documented in electronic patient charts. In general, there were five types of recommendations: (1) modification of antibiotic treatment, (2) additional diagnostics, (3) dose adjustment, (4) conversion from intravenous (i.v.) to oral (p.o.) formulations and (5) use of topical antiseptics instead of systemic antibiotic treatment. The implementation rate of provided recommendations was evaluated within the following 24 h.
Furthermore, we revised and implemented guidelines for the most frequent pediatric infectious diseases, including detailed recommendations regarding correct choice and precise dosing of antibiotics (see electronic Supplementary material Tables S1, S2). Dosing recommendations were consented by an internal multidisciplinary expert panel in accordance with national and international dosing guidelines. Internal guidelines were consented by all clinical divisions and were made internally available between June and Aug 2015 in written and electronic formats. A pocket-sized index card was given to all prescribers. The list of specific antibiotics requiring prior authorization by a senior ID physician remained unchanged throughout the study period (see electronic Supplementary material Table S3).
Data acquisition
Medical records of all patients were reviewed by a clinical pharmacist on a daily basis. A dataset from all patients receiving antibiotic therapy was established. This dataset included demographic parameters (age, weight, height, sex), antibiotic agent (single dose per administration, number of doses per day, route of administration, duration of antibiotic therapy), indication(s) for antibiotic therapy, laboratory parameters (leukocyte count, C-reactive protein, creatinine, liver function tests), and microbiological results as well as radiographic findings. Included was every antimicrobial substance with either i.v. or p.o. route of administration. Topical drugs, systemic antifungal or antiviral agents were excluded. Antibiotics were classified according to the internationally standardized WHO Anatomical Therapeutic Chemical (ATC) classification system (ATC group J01).
The study was conducted according to the ethical standards at LMU Munich. Formal ethical approval was obtained from the research ethics committee of the LMU Munich (ID 404-14). Data on daily admissions, case mix index (CMI), and in-hospital mortality during the study period were obtained from the hospital administration.
Analysis of antibiotic consumption data
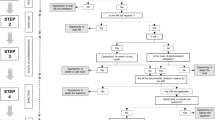
The impact of the ASP on antibiotic consumption was analyzed on antibiotic class and substance level. We assessed the (a) proportion of patients treated with antibiotics at any day during their hospital stay, (b) antibiotic density as days of antibiotic therapy per 1000 patient-days (DoT/1000 PD), (c) length of antibiotic therapy per 1000 patient-days (LoT/1000 PD), and d) doses administered per 1000 PD as described previously [11, 21]. DoT for a patient accounts for all antibiotics that this particular patient receives over a specific time. Thus, in case of prescription of three antibiotics for 4 days, each antibiotic contributes with individual DoT, in this example resulting in a total of 12 DoT. LoT calculates the actual length of the antibiotic treatment period for a patient, irrespective of how many antibiotics were prescribed per day. Thus, for a patient receiving three antibiotics for 4 days, the LoT is 4 [11]. LoT is always less than or equal to DoT. Our ASP did not focus on patients with chronic diseases; therefore, data of specific patient populations (i.e., cystic fibrosis, hemato-oncological diseases, pneumocystis prophylaxis, tuberculosis, and sickle cell anemia) were excluded from the study (Fig. 1). The denominator “patient-days” includes all hospital days for all patients admitted during the study period; hospital days of excluded patient groups were subtracted.
Analysis of antibiotic dosing
Prescribed dosages were compared to standard dosing as defined by a multidisciplinary expert panel in our hospital (see electronic Supplementary material Table S2) and categorized arbitrarily as follows: lower (i.e., <70% of standard dosing), higher (i.e., >130% of standard dosing) or within a range of ±30% of the recommended standard dose. Patients with dose adjustments due to special conditions (e.g., renal dysfunction, cystic fibrosis, or prophylactic therapies) were excluded from this analysis (Fig. 1).
Adherence to treatment guidelines for community-acquired pneumonia (CAP)
As a specific clinical question, guideline adherence was evaluated regarding empiric treatment of community-acquired pneumonia (CAP). All patients hospitalized for a suspected diagnosis of pneumonia and older than six months of age were eligible for this analysis. Exclusion criteria were chronic respiratory disorders (e.g., asthma bronchiale or cystic fibrosis), immunosuppression, severe central nervous system comorbidities with risk of aspiration, respiratory failure requiring intubation, or previous admission to our hospital within the last 4 weeks. Pneumonia was diagnosed by the attending physician based on current guidelines and definitions of CAP [22]. This information was retrieved from medical records.
Statistical methods
For comparison of patient’s characteristics during the two study periods, Chi square test was used for categorical variables, Fisher’s exact test for in-hospital mortality, and Mann–Whitney U test for continuous variables. Antibiotic use density in the two periods was compared assuming Poisson distribution. Analysis and figures were performed using Microsoft Excel® 2010 and IBM SPSS Statistics® 23.
Results
Study population and descriptive data
In the pre-intervention period, a total of 273 patients receiving antibiotic therapy were enrolled. During the post-intervention period, 263 patients received antibiotics during their stay on general pediatric wards. Overall, no statistically significant differences were observed between the two patient populations (Table 1). The 273 and 263 patients resulted in 308 and 298 hospital admissions, respectively, as some patients were admitted more than once during the study period (Fig. 1).
Interventions
The ASP team gave 167 recommendations during regular ID ward rounds during the four-month post-intervention period. Modifications of antibiotic choice (81/167, 48.5%) were the most common interventions, followed by advice of additional diagnostics (49/167, 29.3%), dose adjustment (20/167, 12.0%), use of topical antiseptics instead of systemic antibiotic treatment (10/167, 6.0%) and i.v.-to-p.o.-conversion (7/167, 4.2%). Among the modifications of antibiotic treatment, discontinuation represented the majority of recommendations (37/81, 45.7%), followed by de-escalation (12/81, 14.8%) and recommendations of alternative antibiotic therapy (12/81, 14.8%). The remaining modifications were: Escalation, prolongation and starting of antibiotic therapy (in total 20/81, 24.7%). Overall, compliance with recommendations was 95.8%.
Antibiotic usage, days of therapy (DoT), length of therapy (LoT)
During the pre-intervention period, 30.6% (308 of 1007) of hospitalized children received at least one antibiotic. After implementation of our ASP bundle approach, this percentage did not change significantly (298 of 976, 30.5%, p = 1).
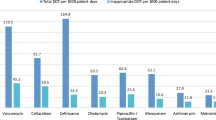
However, overall antibiotic doses administered per 1000 PD decreased by 4.9% from 1233 to 1172 (see electronic Supplementary material Table S4). Antibiotic treatment days decreased by 10.5% (p < 0.001) from 483.6 (pre-intervention) to 432.9 (post-intervention) days of therapy per 1000 patient-days (DoT/1000 PD) with a significant effect regarding cephalosporin consumption (−35.5%, p < 0.001) (Table 2). The use of second-generation cephalosporins decreased by 36.3% (p < 0.001), while the use of third-generation cephalosporins decreased by 22.3% (p < 0.05). Use of FQ was reduced from 32 to 13 DoT/1000 PD (−59.9%, p < 0.001) and metronidazole use showed a similar result, declining from 27 to 13 DoT/1000 PD (−51.1%, p < 0.001). As intended, narrow-spectrum penicillins (phenoxymethylpenicillin and benzylpenicillin) and aminopenicillins (ampicillin and amoxicillin) were more frequently prescribed during the post-intervention period [+22.5% (p = 0.54) and +20.8% (p = 0.08), respectively]. However, this effect needs to be classified as a “trend” as it did not reach statistical significance. The use of aminopenicillins with beta-lactamase inhibitors (ampicillin/sulbactam and amoxicillin/clavulanic acid) increased by 78.8% (p < 0.001). There was no impact on broad-spectrum antibiotic piperacillin/tazobactam use (−0.5%, p = 0.95). The overall consumption of carbapenems was low. However, there was an increase during the post-intervention period (16.6 to 30.1 DoT/1000 PD, +80.8%, p < 0.001). Specific substances for treatment of infections caused by methicillin-resistant Staphylococcus aureus (MRSA) (i.e., vancomycin, teicoplanin, and linezolid) increased by 12% from 23.5 to 26.7 DoT/1000 PD. Prescriptions of antibiotics with activity against Pseudomonas aeruginosa (piperacillin/tazobactam, carbapenems, aminoglycosides, ceftazidime, and FQ) decreased by 8.8% from 164.5 to 151.3 DoT/1000 PD. On a substance level, the consumption data indicate that cefuroxime, piperacillin/tazobactam, and metronidazole were among the most commonly used antibiotics in the pre-intervention period. The ASP interventions in particular reduced cefuroxime (108 to 68 DoT/1000 PD, −37.0%), cefotaxime (24 to 19 DoT/1000 PD, −20.8%) and ciprofloxacin (20 to 10 DoT/1000 PD, −50.0%). In contrast, ampicillin (25 to 35 DoT/1000 PD, +36.7%), amoxicillin/clavulanic acid (10 to 18 DoT/1000 PD, +72.3%), and ampicillin/sulbactam (7 to 13 DoT/1000 PD, +88.9%) were more frequently prescribed in post-intervention period (Table 2; Fig. 2).
Assessing LoT instead of DoT, similar results were observed: Overall LoT decreased significantly by 7.7% (377.4 to 348.3 LoT/1000 PD, 95% CI 1.1–13.9%, p = 0.02).
Dosing accuracy
In the pre-intervention period, out of 378 antibiotic treatment courses, only 78.8% were within ±30% of our pre-defined target dosage, while 97.6% out of 376 antibiotic treatment courses were in this range during the post-intervention period (p < 0.0001) (Fig. 3). Regarding all published dosage ranges, in the pre-intervention period, 14 courses (3.7%) were not justified by any recommendation in the literature. 13 courses were below all available dose recommendations: Cefuroxime (6), ceftazidime (3), cefpodoxime (1), meropenem (1), clarithromycin (1), and ampicillin/sulbactam (1). In one case of metronidazole use, the dose was above all available recommended dose ranges. In the post-intervention period, only one therapy of metronidazole was below all available dose recommendations.
Adherence to treatment guidelines for community acquired pneumonia (CAP)
During the pre-intervention period, 38 patients were hospitalized for uncomplicated CAP. Nineteen (50.0%) of these patients were empirically treated with cephalosporins [second-generation cephalosporins (16), third-generation cephalosporins (3)] and 4 patients (10.5%) were treated with piperacillin/tazobactam. Aminopenicillins were prescribed for 15 (39.5%) patients: Ampicillin (14), amoxicillin (1). In total, six patients (15.8%) received an antibiotic combination therapy with additional macrolides. In the post-intervention period, out of 32 patients with CAP, 30 (93.8%) were treated with aminopenicillins (23 ampicillin, 7 amoxicillin) and two patients (6.2%) received second-generation cephalosporins. A combination with macrolides was prescribed for one patient (Fig. 4). During the pre-intervention period, 23/38 (60.5%) patients were treated with a maximum of 7 days, while in the post-intervention period, 30/32 (93.8%) received antibiotics for 7 days or less.
Length of stay, in-hospital mortality and case mix index (CMI)
The average length of stay remained stable: median 7.0 days in the pre-intervention period (range 1–93 days), and media 6.0 days in the post-intervention period (range 2–123 days), (Table 1). There was no significant difference with regard to in-hospital mortality during pre- and post-intervention period (1 death out of 273 patients and 1 death out of 263 patients, respectively (0.37% vs. 0.38%, p = 1)). The CMI increased slightly during the post-intervention period, but the difference was not statistically significant (data not shown).
Discussion
Despite global efforts to reduce antimicrobial prescribing, antibiotic consumption continues to increase in pediatric and adult hospitals [23]. ASPs offer evidence-based tools to control antibiotic prescribing in different hospital settings. However, only few studies have carefully analyzed antibiotic use in pediatric hospitals [10–19, 24].
We performed a thorough analysis of all systemic antibiotics used in general pediatric wards of a large university children’s hospital before and after the implementation of an ASP bundle. Our ASP interventions included different core ASP strategies described earlier [6, 25, 26] all of these being exceptionally well accepted with a compliance rate of more than 95%. To be able to compare our results with other studies, we assessed and calculated all process measures (compliance rates with ASP recommendations, antibiotic use, DoT/1000 PD, LoT/1000 PD, Doses/1000 PD, and antimicrobial appropriateness, i.e., guideline adherence), as well as outcome measures (length of stay and in-hospital mortality) as reported previously [27].
To better account for real antibiotic consumption in pediatrics, the antibiotic density is calculated as days of antibiotic therapy per 1000 patient-days (DoT/1000 PD). This parameter corresponds to the defined daily dose (DDD) or recommended daily dose (RDD) per 1000 PD used in adults ASP programs. Total antibiotic density was low, 483.6 (pre-intervention) and 432.9 DoT/1000 PD (post-intervention)), compared to Newland et al. (883 and 787 DoT/1000 PD) [11]. The difference in baseline antibiotic density is probably partly explained by the fact that Newland et al. assessed an entire tertiary care children’s hospital, including hemato-oncological as well as neonatal and pediatric intensive care units, while we focused on general pediatric wards. We also previously established an antibiotic stewardship pilot program picking the “low-hanging fruits” [28] and reduced the overall antibiotic costs by about 50% [20].
Recently, a large point prevalence study showed that 39.5% of all children in one German Children’s Hospital received antibiotics on a given day [19]. In our setting, the percentage of hospitalized children receiving at least one antibiotic was slightly lower and remained stable during the study periods (30.6 and 30.5%, respectively). These findings are in concordance with previously published data, where rates of patients treated with antibiotics remained unchanged with/without ASP strategies [15]. In this particular study, rates were higher (44 and 43%, respectively), probably because patients from intensive care units were included.
The proportion of children receiving at least one antibiotic remained stable in our study, while the overall antibiotic DoT/1000 PD decreased by 10.5% after introduction of the ASP. These results are in line with Newland et al. who analyzed the effect of prospective-audit-with-feedback using time series analysis. There was a decline in overall antibiotic DoT/1000 PD by 10.9% reported [11]. Comparable effects were shown by Agwu et al. (−14% DoT/1000 PD) after the implementation of an internet-based ASP in a pediatric tertiary care center [24]. Di Pentima et al. demonstrated that prospective-audit-with-feedback and reinforcement of prior authorization led to a decrease of targeted antibiotic doses of as much as 21%. However, this study did not provide DoT and evaluated only individual doses [15].
LoT as a metric for antibiotic usage was first introduced in an adult study by Polk et al. in 2011 to provide additional information complementing DoT evaluations [21]. A combination therapy with two antibiotics doubles the DoT. However, a broad-spectrum antibiotic may combine the activity of two narrow-spectrum antibiotics and prescription of this drug would reduce DoT by 50% as only one substance is used instead of two. Therefore, it is important to report also the absolute length of antibiotic therapy. In our study, the overall antibiotic usage expressed as LoT (377.4 pre- and 348.3 LoT/1000 PD post-intervention) is significantly lower than published data from Newland et al. (567 pre- and 523 LoT/1000 PD post-intervention) and Agwu et al. (485.4 pre- and 417.6 LoT/1000 PD post-intervention) [11, 24]. However, as mentioned above, both studies measured antibiotic usage in tertiary care children’s hospital, including intensive care units and hemato-oncology wards. Even though our reduction in LoT was significant (−7.7%) and similar to the impact described by Newland et al. (−7.8%), it was not as large as reported by Agwu et al. (−13.9%). This might be related to our lower LoT baseline, which might be influenced by our focus on general pediatric wards. By demonstrating a significant decrease of both metrics, DoT and LoT, we have achieved a reduction of the absolute number of antibiotics, as well as the actual lengths of antibiotic therapies.
Cefuroxime and ceftriaxone are among the most prescribed antibiotics in German adult hospitals [1]. In line with these epidemiological data, cefuroxime was the most used antibiotic in the pre-intervention period, while ceftriaxone with its wider antimicrobial spectrum was prescribed less often in our general pediatric wards. It is well known that cephalosporins, in particular third-generation cephalosporins and FQs, are associated with a significant increase in the incidence of CDI and are key selection drivers for hospital- and community-acquired MRSA, vancomycin-resistant Enterococcus (VRE), and extended-spectrum β-lactamase producing gram-negative bacteria (ESBL) [9, 29, 30]. Therefore, we specifically targeted third-generation cephalosporins and FQs. We were able to reduce the consumption of these substances by 22.3 and 59.9%, respectively (in total −38.1%). As intended they were mostly replaced by narrow- and intermediate-spectrum penicillins (+38.4% DoT/1000 PD). Di Pentima achieved a comparable decrease of targeted third-generation cephalosporin and FQ use, as well as a reduction of overall antibiotic consumption in a pediatric setting [15]. In adults, Borde et al. showed a similar impact on cephalosporins (−33% RDD/1000 PD) and FQs (−31% RDD/1000 PD) [31].
Meropenem prescription rates in our center (3.4 in pre- and 6.9% in post-intervention period of total antibiotic DoT) were not different from the data reported for Western European patients (4.2%) [18]. However, we observed an—albeit small (i.e., +13.4 DoT/1000 PD)—increase in meropenem use in the post-intervention period. This might potentially be caused by a rise in the number of patients colonized with multi-resistant Gram-negative bacteria (MRGN), as a higher number of refugees were treated in our hospital during the post-intervention period. In addition, the increase of meropenem use might be linked to our recommendation to use meropenem empirically in suspected septic shock. While this serious condition has a low incidence, inexperienced physicians may have used meropenem more broadly than intended (e.g., for sepsis without shock). Therefore, addressing the issue of inappropriate use of carbapenems will be a focus of future ID interventions in our setting, including educational measures towards more accurate clinical identification of patients with severe sepsis or septic shock.
In published literature, the use of CPOE and the presence of ward-based pharmacists have been most successful in reducing the rate of medication errors (ME) in hospitalized patients [16, 32]. Published data demonstrate that the rate of dosage-related errors is much more substantial in children than in adults with underdosing being the most common ME in pediatrics [16, 33, 34]. Ekins-Daukes et al. confirmed that a high percentage (19.2%) of antibiotics prescribed in primary care setting showed lower doses than recommended in the Summary of Product Characteristic by the manufacturer [35]. These low doses may have caused the significant increase in the total number of antibiotic courses prescribed due to incomplete resolution of infection. Besides the risk of ineffective drug levels and treatment failure, low doses increase the risk for selection of resistant bacteria [36, 37]. Similar to previously published results, underdosing was common (16.9%) and overdosing was observed in only 4.3% of treatment courses during our pre-intervention period. However, the rate of correct dosing significantly increased from 78.8 to 97.6% after implementation of guidelines with precise dosing recommendations and a particular focus on dosing accuracy during regular ID ward rounds. Aseeri et al. achieved similar effects after disseminating standardized dosing tables resulting in a reduction of dosing errors from 34.3 to 5.1% [38]. Our dosing recommendations were based on the published literature and represent a consensus of an internal multidisciplinary expert panel. Accurate dosing was somewhat arbitrarily defined as ±30% of our internal recommendations. Therefore, we analyzed all under- or overdosed antibiotic treatment courses and compared dosing with the recommendations in the literature. We found that 3.7% antibiotic courses in the pre-intervention period and only one antibiotic course (0.27%) in the post-intervention period were not within the published dosing ranges. However, none of these antibiotic treatment courses resulted in significant toxicity for the patient.
For children over six months of age admitted with uncomplicated CAP, national and international guidelines recommend empiric therapy with aminopenicillins (i.e., amoxicillin or ampicillin) [22]. Our ASP strategies led to a remarkable and significant increase in guideline adherence. These findings are in accordance with published data where an increase of ampicillin use of 34% and a reduction of cephalosporin use of 72% were found after implementation of CAP guidelines [12]. Ambroggio et al. increased appropriate first-line antibiotic prescribing for children with CAP from 30 to 100% in hospital resident teams through implementing internal guidelines and education on CAP treatment [39]. Smith et al. analyzed the impact of an ASP team and CAP guideline and demonstrated an improvement of their initially low rate of ampicillin prescribing 2–44% and a decrease of ceftriaxone usage (59–28%) [40].
Our approach has some limitations: The exclusion of patients with chronic diseases comprises a potential patient bias. However, this seems to be unlikely as the numbers of patients that were excluded were similar between the pre- and post-intervention periods (Fig. 1). In addition, this is a single-center study, investigating the effect of ASP strategies only on general pediatric wards. As patient characteristics vary widely between different subspecialties, we decided to focus on general pediatric wards since these are more homogenous and represent the majority of in-patients. Similar studies are currently ongoing in our hospital on high-prescribing units like hemato-oncology, neonatology, pediatric surgery, and intensive care. The endpoints assessed were limited to previously recommended outcome measures [27], as well as crude estimators of safety outcomes (length of stay and in-hospital mortality). Our study did not address changes in reinfection rate, changes in bacterial resistance rates, or the incidence of CDI in children. A recent Cochrane review showed that the effect of ASP interventions on microbiological endpoints is usually delayed [41]. Considering the relatively short four-month observation period, the risk of losing the observed positive impact cannot be excluded, as previously shown for adults (Standiford et al. [42]). The long-term effects of our ASP bundle need to be further investigated. However, this study demonstrates that an ASP bundle approach can successfully reduce the overall antibiotic use and the use of broad-spectrum antibiotics. In addition, our ASP interventions increased antibiotic dosing accuracy and guideline adherence for the treatment of CAP, which both are a major issue of patient safety.
Abbreviations
- ASP:
-
Antibiotic stewardship program
- ATC:
-
Anatomical therapeutic chemical
- CAP:
-
Community-acquired pneumonia
- CDI:
-
Clostridium difficile–infection
- CMI:
-
Case mix index
- CPOE:
-
Computerized physician order entry
- DDD:
-
Defined daily dose
- DGI:
-
German Society of Infectious Diseases
- DoT:
-
Days of antibiotic therapy
- ESBL:
-
Extended spectrum β-lactamase producing Gram-negative bacteria
- FQ:
-
Fluoroquinolone
- ID:
-
Infectious diseases
- IDSA:
-
Infectious Diseases Society of America
- i.v.:
-
Intravenous
- LMU:
-
Ludwig-Maximilians-University
- LoT:
-
Length of antibiotic therapy
- ME:
-
Medication errors
- MRGN:
-
Multi-resistant Gram-negative bacteria
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- PD:
-
Patient-days
- p.o.:
-
Oral
- RDD:
-
Recommended daily dose
- VRE:
-
Vancomycin-resistant Enterococcus
- WHO:
-
World Health Organization
References
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit PEG eV. GERMAP 2015–Bericht über den Antibiotikaverbrauch und die Verbreitung von Antibiotikaresistenzen in der Human- und Veterinärmedizin in Deutschland. 2016. http://www.p-e-g.org/econtext/germap/. Accessed 04 Jan 2017.
Gerber JS, Newland JG, Coffin SE, Hall M, Thurm C, Prasad PA, et al. Variability in antibiotic use at children’s hospitals. Pediatrics. 2010;126:1067–73. doi:10.1542/peds.2010-1275.
Bolon MK, Arnold AD, Feldman HA, Rehkopf DH, Strong EF, Goldmann DA, et al. Evaluating vancomycin use at a pediatric hospital: new approaches and insights. Infect Control Hosp Epidemiol. 2005;26:47–55. doi:10.1086/502486.
Levy ER, Swami S, Dubois SG, Wendt R, Banerjee R. Rates and appropriateness of antimicrobial prescribing at an academic children’s hospital, 2007–2010. Infect Control Hosp Epidemiol. 2012;33:346–53. doi:10.1086/664761.
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–77. doi:10.1093/cid/ciw118.
de With K, Allerberger F, Amann S, Apfalter P, Brodt HR, Eckmanns T, et al. Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Infection. 2016;44:395–439. doi:10.1007/s15010-016-0885-z.
World Health Organization, WHO. Global Action Plan on Antimicrobial Resistance. 2015. http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf. Accessed 04 Jan 2017.
Schmitt S, McQuillen DP, Nahass R, Martinelli L, Rubin M, Schwebke K, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis. 2014;58:22–8. doi:10.1093/cid/cit610.
Dancer SJ, Kirkpatrick P, Corcoran DS, Christison F, Farmer D, Robertson C. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum beta-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2013;41:137–42. doi:10.1016/j.ijantimicag.2012.10.013.
McCulloh RJ, Queen MA, Lee B, Yu D, Stach L, Goldman J, et al. Clinical impact of an antimicrobial stewardship program on pediatric hospitalist practice, a 5-year retrospective analysis. Hosp Pediatr. 2015;5:520–7. doi:10.1542/hpeds.2014-0250.
Newland JG, Stach LM, De Lurgio SA, Hedican E, Yu D, Herigon JC, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatr Infect Dis Soc. 2012;1:179–86. doi:10.1093/jpids/pis054.
Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129:e597–604. doi:10.1542/peds.2011-1533.
Lee KR, Bagga B, Arnold SR. Reduction of Broad-Spectrum Antimicrobial Use in a Tertiary Children’s Hospital Post Antimicrobial Stewardship Program Guideline Implementation. Pediatr Crit Care Med. 2016;17:187–93. doi:10.1097/PCC.0000000000000615.
Hersh AL, De Lurgio SA, Thurm C, Lee BR, Weissman SJ, Courter JD, et al. Antimicrobial stewardship programs in freestanding children’s hospitals. Pediatrics. 2015;135:33–9. doi:10.1542/peds.2014-2579.
Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children’s hospital. Pediatrics. 2011;128:1062–70. doi:10.1542/peds.2010-3589.
Di Pentima MC, Chan S, Eppes SC, Klein JD. Antimicrobial prescription errors in hospitalized children: role of antimicrobial stewardship program in detection and intervention. Clin Pediatr (Phila). 2009;48:505–12. doi:10.1177/0009922808330774.
Nguyen-Ha PT, Howrie D, Crowley K, Vetterly CG, McGhee W, Berry D, et al. A quality assessment of a collaborative model of a pediatric antimicrobial stewardship program. Pediatrics. 2016;137:e20150316. doi:10.1542/peds.2015-0316.
Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, Group Ap. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71:1106–17. doi:10.1093/jac/dkv418.
Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H, et al. The antibiotic resistance and prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. 2013;32:e242–53. doi:10.1097/INF.0b013e318286c612.
Huebner J, Rack-Hoch AL, Pecar A, Schmid I, Klein C, Borde JP. Pilot project of a pediatric antibiotic stewardship initiative at the Hauner children’s hospital. Klin Padiatr. 2013;225:223–9. doi:10.1055/s-0033-1349063.
Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis. 2011;53:1100–10. doi:10.1093/cid/cir672.
Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. doi:10.1093/cid/cir531.
Pakyz AL, Gurgle HE, Ibrahim OM, Oinonen MJ, Polk RE. Trends in antibacterial use in hospitalized pediatric patients in United States academic health centers. Infect Control Hosp Epidemiol. 2009;30:600–3. doi:10.1086/597545.
Agwu AL, Lee CK, Jain SK, Murray KL, Topolski J, Miller RE, et al. A World Wide Web-based antimicrobial stewardship program improves efficiency, communication, and user satisfaction and reduces cost in a tertiary care pediatric medical center. Clin Infect Dis. 2008;47:747–53. doi:10.1086/591133.
Metjian TA, Prasad PA, Kogon A, Coffin SE, Zaoutis TE. Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital. Pediatr Infect Dis J. 2008;27:106–11. doi:10.1097/INF.0b013e318158603a.
Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J Pediatr Infect Dis Soc. 2015;4:e127–35. doi:10.1093/jpids/piu141.
Newland JG, Banerjee R, Gerber JS, Hersh AL, Steinke L, Weissman SJ. Antimicrobial stewardship in pediatric care: strategies and future directions. Pharmacotherapy. 2012;32:735–43. doi:10.1002/j.1875-9114.2012.01155.x.
Goff DA, Bauer KA, Reed EE, Stevenson KB, Taylor JJ, West JE. Is the “low-hanging fruit” worth picking for antimicrobial stewardship programs? Clin Infect Dis. 2012;55:587–92. doi:10.1093/cid/cis494.
McKinnell JA, Kunz DF, Moser SA, Vangala S, Tseng CH, Shapiro M, et al. Patient-level analysis of incident vancomycin-resistant enterococci colonization and antibiotic days of therapy. Epidemiol Infect. 2016;144:1748–55. doi:10.1017/S0950268815003118.
Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1748–54. doi:10.1093/jac/dku046.
Borde JP, Litterst S, Ruhnke M, Feik R, Hubner J, deWith K, et al. Implementing an intensified antibiotic stewardship programme targeting cephalosporin and fluoroquinolone use in a 200-bed community hospital in Germany. Infection. 2015;43:45–50. doi:10.1007/s15010-014-0693-2.
Upperman JS, Staley P, Friend K, Neches W, Kazimer D, Benes J, et al. The impact of hospitalwide computerized physician order entry on medical errors in a pediatric hospital. J Pediatr Surg. 2005;40:57–9. doi:10.1016/j.jpedsurg.2004.09.024.
Engum SA, Breckler FD. An evaluation of medication errors-the pediatric surgical service experience. J Pediatr Surg. 2008;43:348–52. doi:10.1016/j.jpedsurg.2007.10.042.
Lesar TS. Errors in the use of medication dosage equations. Arch Pediatr Adolesc Med. 1998;152:340–4.
Ekins-Daukes S, McLay JS, Taylor MW, Simpson CR, Helms PJ. Antibiotic prescribing for children. Too much and too little? Retrospective observational study in primary care. Br J Clin Pharmacol. 2003;56:92–5.
Guillemot D, Carbon C, Balkau B, Geslin P, Lecoeur H, Vauzelle-Kervroedan F, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365–70.
Schrag SJ, Pena C, Fernandez J, Sanchez J, Gomez V, Perez E, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001;286:49–56.
Aseeri MA. The impact of a pediatric antibiotic standard dosing table on dosing errors. J Pediatr Pharmacol Ther. 2013;18:220–6. doi:10.5863/1551-6776-18.3.220.
Ambroggio L, Thomson J, Murtagh Kurowski E, Courter J, Statile A, Graham C, et al. Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics. 2013;131:e1623–31. doi:10.1542/peds.2012-2635.
Smith MJ, Kong M, Cambon A, Woods CR. Effectiveness of antimicrobial guidelines for community-acquired pneumonia in children. Pediatrics. 2012;129:e1326–33. doi:10.1542/peds.2011-2412.
Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:CD003543. doi:10.1002/14651858.CD003543.pub3.
Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol. 2012;33:338–45. doi:10.1086/664909.
Acknowledgements
This work was supported by AOK Bayern and the interprofessional PhD-program Clinical Pharmacy, LMU Munich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kreitmeyr, K., von Both, U., Pecar, A. et al. Pediatric antibiotic stewardship: successful interventions to reduce broad-spectrum antibiotic use on general pediatric wards. Infection 45, 493–504 (2017). https://doi.org/10.1007/s15010-017-1009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-1009-0