Abstract
Miliary tuberculosis (TB) is characterized by a multitude of small nodular opacities on chest radiography. Despite ultrasound of the chest gaining wider acceptance as a diagnostic tool of lung infections, sonographic changes of pulmonary miliary TB have not yet been reported. Here, we describe B-lines and comet-tail artifacts disseminated throughout multiple lung areas and a pattern of sub-pleural granularity as consistent changes seen in lung ultrasound of ten patients with pulmonary miliary TB diagnosed by chest radiography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) remains one of the deadliest infectious diseases, with disseminated and miliary TB resulting in a high case fatality rate. Miliary TB is more frequently seen in patients with HIV co-infection, especially if immunosuppression is advanced (CD4+ cell counts <200 cells/μL) [1]. Further risk factors for miliary TB are very young or advanced age, immunosuppressive therapy, malignancy, and malnutrition. Symptoms of miliary TB are similar to common constitutional TB symptoms (fever, weight loss, fatigue); pulmonary miliary lesions causing hypoxemia may entail dyspnea [2].
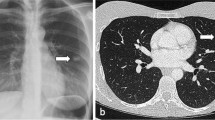
Miliary TB nodules are classically tiny (approximately 1–2 mm in diameter), discrete granulomatous lesions disseminated in lungs or other organs as a result of hematogenous seeding of tubercle bacilli. Commonly involved organs beside the lung include spleen, liver, bone marrow, kidneys, and adrenal glands. The characteristic finding of miliary TB on chest radiography is a multitude of small nodular opacities, which are considered pathognomonic (Fig. 1). Although lower lung zones are affected slightly more often, in most cases the nodular pattern is detected in all lung zones [3].
Detection of extra-pulmonary TB (EPTB) lesions by means of ultrasound is a widely accepted method in endemic settings [4]. EPTB in the spleen mostly presents with a “miliary” pattern of multiple hypoechoic micro-abscesses which are highly suggestive of disseminated TB; therefore, assessment for hypoechoic splenic lesions has been included in the Focused Assessment with Sonography for HIV-associated TB (FASH) ultrasound protocol [5].
In recent years, ultrasound of the chest has gained increasing interest and wider acceptance, e.g., for the diagnosis of consolidations suggesting pneumonic infiltrates [6]. Other ultrasound phenomena described are B-lines, which are vertical, hyperechoic reverberation artifacts projecting from the pleural line to the bottom of the screen indicating interstitial pathologies of the lung if number of B-lines is increased. When the vertical artifacts do not reach the far end of the image, usually the term “comet-tail artifacts” is used; these are also caused by interstitial lung pathology. A recent review in this journal focused on sonographic patterns associated with a variety of pulmonary pathology in HIV-infected patients, but sonographic changes of miliary TB have not yet been reported [7]. With this brief report, we aim at filling this gap and describing the sonographic changes visible in chest ultrasound in patients with miliary TB, and the possible diagnostic use of chest ultrasound in miliary TB is discussed.
Materials and methods
Ten patients diagnosed with miliary TB by chest radiograph in Khayelitsha District Hospital, Cape Town, South Africa, were evaluated. As part of clinical work-up, patients underwent a FASH ultrasound scan, which was extended to multiple scans of the main bilateral chest areas (anterior superior area, lateral area, posterior superior and posterior inferior area of the right and left chest, respectively). Images were obtained using a Vivid S5 (GE General Electrics Healthcare, USA) with a 3.5 MHz convex transducer. Images and findings were primarily evaluated at the point-of-care during image acquisition (LH); additionally video clips were stored and reviewed remotely (TH). The Health Research Ethics Committee of the University of Stellenbosch approved the publication of this brief report.
Results
Five of 10 patients were female, and the median age was 33 years (range 25–59). All but one patient were HIV-infected; median CD4 count was 70 cells/μL (range 8–103 cells/μL). While five patients were not receiving antiretroviral therapy (ART) at the time of diagnosis, the other four patients were on regimens according to national ART guidelines. In all patients, pulmonary miliary TB was diagnosed based on chest radiograph and TB was confirmed by Xpert MTB/RIF (in eight patients on sputum samples and in two patients on blood samples).
Video clips of sonographic scans from a minimum of four different lung zones were recorded for all patients. The typical sonographic artifact pattern observed in all patients comprised comet-tail artifacts and/or B-lines originating from the pleural/sub-pleural lung tissue and “radiating” to the distant areas of the image (Fig. 2a and supp video 1). In all patients, the changes were present bilaterally and in multiple lung zones underlining the disseminated character of the disease. Additionally, sub-pleural granular changes were visible in all patients, although not in every scan position. The sub-pleural echogenic granularity possibly reflects the miliary granulomatous lesions in the lung. The granularity was best seen during respiratory movement (Fig. 2b and supp video 2).
Ultrasound of the lung in miliary tuberculosis: a multiple vertical artifacts (up double arrow) (B-lines/“comet-tails”) “radiating” from the echogenic pleura (open left triangle) to the distant areas of the image (see also supp. video 1); b echogenic bright “granular” artifacts (up arrow) in the sub-pleural area of the lung (see also supp. video 2)
Discussion
The miliary pattern on posterior–anterior chest radiograph is considered to be the diagnostic hallmark of miliary TB. This classical pattern may, however, not be evident in all patients with miliary TB; sensitivity and specificity of chest radiograph are reported with 59–69 and 97–100 %, respectively [3]. High-resolution CT scans can considerably improve the diagnosis of miliary TB and may reveal pulmonary miliary patterns even when chest radiograph looks apparently normal [8]. Access to CT imaging is, however, limited in many settings where TB prevalence is high. Evaluating the performance of lung ultrasound as an alternative imaging modality, which is becoming increasingly available also in resource-limited areas, was therefore tempting.
Our case series observed that pulmonary miliary TB sonographically presents with bilateral vertical artifacts (B-lines and/or “comet-tail artifacts”) in multiple lung zones. These changes are in line with the pattern expected by interstitial lung alterations and are clearly different from the more localized, focal changes due to lobar pneumonia. The changes are similar to the disseminated vertical artifacts previously described in patients with Pneumocystis jirovecii pneumonia [9] and in other disseminated lung infections like cytomegaly virus pneumonitis [7]. The presence of sub-pleural granular changes may be more specific of miliary TB and was not seen in other patients scanned routinely in our hospital (personal communication Dr. L. Hunter). However, other pulmonary conditions, e.g., metastatic thyroid cancer, may cause similar ultrasound changes (personal communication Dr. T. Heller, Dr. M. Kumar). Due to the lack of prospective data, the frequency of the observed findings in the above-described conditions remains unknown.
In settings, where chest radiography is widely available, the additional benefit of lung ultrasound to investigate for miliary TB appears limited. In particular, with the availability of Xpert MTB/RIF, which was positive on sputum or blood in all our patients, the diagnostic work-up should focus on laboratory proof of TB. Nevertheless, in geographic areas, where access to radiology and laboratory infrastructure is not readily available, the described ultrasound changes may be helpful to suggest the diagnosis. Future prospective and well-designed studies are required to evaluate the applicability of lung ultrasound for HIV-infected and HIV-uninfected patients with sputum-positive and sputum-negative miliary TB in resource-limited settings and to evaluate the training required to confidently perform and interpret clinician-performed lung ultrasound.
In summary, we describe an interstitial pattern with B-lines and “comet-tail” artifacts disseminated in multiple lung areas and a pattern of sub-pleural granularity as the typical changes seen in lung ultrasound of patients with miliary TB. These observations may generate hypotheses for further investigations aiming at consolidation of evidence on the diagnostic value of chest ultrasound.
References
Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–30.
Fanning A. Tuberculosis: 6. Extrapulmonary disease. CMAJ. 1999;160:1597–603.
Kwong JS, Carignan S, Kang EY, Muller NL, FitzGerald JM. Miliary tuberculosis. Diagnostic accuracy of chest radiography. Chest. 1996;110:339–42.
van Hoving DJ, Lamprecht HH, Stander M, Vallabh K, Fredericks D, Louw P, et al. Adequacy of the emergency point-of-care ultrasound core curriculum for the local burden of disease in South Africa. Emerg Med J. 2013;30:312–5.
Heller T, Wallrauch C, Goblirsch S, Brunetti E. Focused assessment with sonography for HIV-associated tuberculosis (FASH): a short protocol and a pictorial review. Crit Ultrasound J. 2012;4:21.
Reissig A, Copetti R, Mathis G, Mempel C, Schuler A, Zechner P, et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. Chest. 2012;142:965–72.
Heuvelings CC, Belard S, Janssen S, Wallrauch C, Grobusch MP, Brunetti E, et al. Chest ultrasonography in patients with HIV: a case series and review of the literature. Infection. 2015. doi:10.1007/s15010-015-0780-z
Sharma SK, Mohan A, Pande JN, Prasad KL, Gupta AK, Khilnani GC. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM. 1995;88:29–37.
Japiassu A, Bozza F. Lung ultrasound can differentiate Pneumocystis jiroveci versus other etiologies among critically ill AIDS patients with pneumonia. International Symposium on Intensive care and Emergency medicine. P-086 20-23, Brussels, Belgium; 2012.
Acknowledgments
We thank all patients included in this case series. The authors did not receive any financial support for this study; no specific funding was received to carry out this work. SB is participant in the Charité Clinical Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Author contributions
LH, SJ, and TH conceived the idea for this manuscript. LH performed the scans and followed the patients clinically. TH reviewed the video clips and images remotely. SB, NvH, and all authors contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hunter, L., Bélard, S., Janssen, S. et al. Miliary tuberculosis: sonographic pattern in chest ultrasound. Infection 44, 243–246 (2016). https://doi.org/10.1007/s15010-015-0865-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-015-0865-8