Abstract
Purpose
Invasive Mycobacterium marinum disease (tenosynovitis and osteomyelitis) may be an increasingly common manifestation of M. marinum infection that presents unique diagnostic and therapeutic challenges. We conducted a retrospective case series and literature review of M. marinum infection to better understand the clinical spectrum of invasive versus cutaneous disease.
Methods
We reviewed electronic medical records for all M. marinum infections at Duke University Medical Center from January 1, 1996 to April 30, 2014. Published case series of M. marinum infection since 1990 reporting >5 cases were systematically ascertained and reviewed.
Results
Twenty-eight cases of M. marinum infection were identified from our institution. Twenty cases (87 %) involved aquatic exposure, and 26 (93 %) involved finger and/or hand lesions. Median time to diagnosis was 3.5 months. Nineteen (68 %) cases had invasive infection, and 9 (32 %) were cutaneous; invasive infection was more common with older age. Granulomatous inflammation and acid-fast bacilli were noted on pathologic examination in 11 (58 %) and 3 (16 %) cases, respectively. Primarily monotherapy was used in 2 (12 %) cases, dual therapy in 8 (47 %) cases, and three-drug therapy in 7 (41 %) cases; three-drug therapy was more common with invasive infection. Median duration of treatment was 5 months. Adjunctive surgery was performed for 18 (95 %) cases of invasive infection and 4 (44 %) of cutaneous infection. Twenty-one (75 %) cases improved, while 7 (25 %) were lost to follow-up.
Conclusions
Distinguishing between invasive and cutaneous M. marinum infection may have important consequences in terms of antibiotic choice and need for adjunctive surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycobacterium marinum is a nontuberculous mycobacterium that causes infections in humans ranging from simple cutaneous lesions to debilitating disseminated infections. M. marinum is an endemic fish pathogen found in a variety of aquatic settings, such as fish tanks, swimming pools, and natural bodies of water [1–3]. Transmission occurs through inoculation of the organism through a break in the skin in the presence of contaminated water or from direct contact with fish or shellfish [1–3]. The incubation period is usually <4 weeks, but can be up to 9 months long [4]. Infection results in ulcerating or nodular skin lesions that can progress to more invasive disease, including tenosynovitis, septic arthritis, or osteomyelitis, particularly if there is a delayed diagnosis or the patient is immunocompromised [2, 5]. Treatment usually involves a prolonged course of antimicrobial therapy with 2 active agents; however up to half of all cases may require surgery when persistent or deeper infection is present [2, 5, 6].
Infection is most often reported among fish fanciers and participants of waterborne activities; chlorination has reduced the number of swimming pool acquired cases [1, 2, 4]. While cases are rare, with an estimated annual incidence of 0.27 cases per 100,000 persons in the United States, outbreaks have been recently reported among patrons of seafood markets in New York City and at a fish farm in China [6–8].
The purpose of this study was to better understand the natural history and clinical spectrum of M. marinum infection. We identified 28 patients with M. marinum infection at the Duke University Medical Center (DUMC) during 1996–2014, and reviewed the epidemiology, treatment, and outcomes associated with infection. We also performed a comprehensive literature review of case series of M. marinum infection published since 1990 to evaluate trends in the presentation and management of M. marinum infection worldwide.
Materials and methods
Case series
We conducted a retrospective case series of all M. marinum infections among patients presenting to DUMC during January 1, 1996–April 30, 2014. Using DEDUCE (Duke Enterprise Data Unified Content Explorer), a web-based tool that permits access to electronic clinical data for all Duke Health System patients since 1996, cases were ascertained by searching for the keywords Mycobacterium marinum and tenosynovitis in combination with the International Classification of Disease, 9th revision (ICD-9) codes 031.1 (cutaneous diseases due to other mycobacteria), 031.8 (other specified mycobacterial diseases), and 031.9 (unspecified diseases due to mycobacteria) [9, 10]. Demographic, clinical, radiographic, microbiologic, pathologic, treatment, and outcome data were extracted from Duke’s electronic health record system. Confirmed cases were defined as the presence of M. marinum isolated in culture from any site, including both sterile (e.g., blood, tissue) and non-sterile (e.g., sputum, gastrointestinal tract) sites. Cases of presumptive M. marinum infection based upon an aquatic exposure or granulomatous inflammation from pathologic specimens without confirmatory cultures were excluded from our analysis.
Cases were classified as cutaneous if only involving cutaneous or subcutaneous tissue, or invasive if tenosynovitis, septic arthritis, or osteomyelitis was present radiographically or noted during surgical exploration. Aquatic exposure was categorized as exposure to fish tanks, handling fish or other seafood, and boating and/or fishing-related activities. Immunocompromised status included patients with HIV, organ transplantation, chronic corticosteroid use or other immunosuppressive medication use, or other immunosuppressive conditions (i.e., inflammatory bowel disease, rheumatoid arthritis). Time to diagnosis was defined as the time from symptom onset to diagnosis of M. marinum infection. Initial antibiotic regimen was defined as the first treatment regimen prescribed, while backbone antibiotic regimen was defined as the regimen used for the majority of the treatment period. Surgery was defined as any invasive surgical procedure performed in the operating room, excluding simple office biopsy and aspiration procedures. Treatment outcomes were defined as improved if there was no evidence of disease after cessation of therapy or ongoing improvement during therapy without documentation of cessation of therapy or subsequent documentation after the diagnosis of M. marinum that does not mention persistence or recurrence of disease, improved with morbidity if case was improved but without return of function of the affected limb, failed if there was recurrence of disease after cessation of therapy or persistence of disease despite therapy, or lost to follow-up if there was no documentation of treatment or outcome without any subsequent medical records.
Frequency counts and percentages were used to describe categorical variables, and means/medians were used to describe continuous variables. Comparisons among groups employed Fisher’s exact test for categorical variables, and Student’s t test and Mann–Whitney U test for continuous variables. P values of <0.05 were considered statistically significant. R version 3.1.1 (Vienna, Austria) was used for statistical analysis.
Literature review
Using the PubMed database, we searched for articles with the keywords Mycobacterium marinum and mycobacterium with tenosynovitis, filtered for articles published in the English language and involving humans after January 1, 1990. We included all original case series reporting treatment outcomes of >5 patients with M. marinum infection that were available in English. All articles were reviewed to ensure no redundancy of cases and adequate information was available for inclusion in the review. Number and percentages of cases with invasive disease, aquatic exposure, surgery performed, and treatment outcome, as well as mean and median time to diagnosis and antibiotic duration (calculated in months using 30 days per month), were recorded and compiled into a table. If individual case level data were available, those variables were re-calculated to ensure accuracy of data; if only aggregate level data was available, the article’s reported values were listed.
Results
Case series
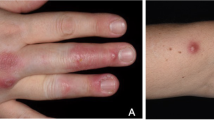
Twenty-eight cases of M. marinum infection were identified, with 19 (68 %) invasive infections and 9 (32 %) cutaneous infections (Table 1). The incidence of M. marinum infection over time in 6-year intervals is demonstrated in the Fig. 1. Among the cases of invasive infection, 18 involved tenosynovitis and 1 involved septic arthritis. Twenty (87 %) cases reported aquatic exposure; exposure to fish tanks was associated with cutaneous infection (P = 0.002), while exposure to boating and/or fishing was associated with invasive infection (P = 0.007). Twenty-six (93 %) cases had infection of the fingers and/or hands, and 4 (14 %) involved the arm. The median time to diagnosis was 3.5 months (IQR 2–5.3, range 1–24 months).
Mycobacterium marinum infection was diagnosed through a surgical procedure for 18 (95 %) invasive cases and three (33 %) cutaneous cases, while infection was diagnosed through an office biopsy or aspiration for one (5 %) invasive case and 6 (67 %) cutaneous cases (Table 2). Pathologic examination of tissue was positive for granulomatous inflammation and acid-fast bacilli (AFB) staining for 11 (58 %) and three (16 %) cases, respectively. Seven (37 %) cases of M. marinum infection involved tissue without granulomatous inflammation or positive AFB staining; pathologic examination of tissue from these cases was described to contain acute and chronic inflammation, synovitis, necrosis, and/or hyperplasia.
Single drug therapy for the backbone antibiotic regimen was uncommon; 8 (47 %) cases used dual therapy and 7 (41 %) cases used three-drug therapy (Table 3). Three-drug therapy was more common for invasive infections. Eight (47 %) cases required a change in the antibiotic regimen during treatment; three cases involved poor progress with the initial antibiotic regimen, two cases involved antibiotic side effects, one case involved both poor progress and antibiotic side effects, one case involved narrowing the initial antibiotic regimen after improvement, and one case had a change in the antibiotic regimen for an unknown reason. Median treatment duration was 5 months (IQR 3–9.8, range 2–12 months). The most common antibiotic agents used were ethambutol, rifampin, clarithromycin, azithromycin, and moxifloxacin.
Twenty-two (79 %) cases required surgical intervention. Patients with invasive infection were more likely to have surgery and had more surgeries per case than patients with cutaneous infection. Other than debridement and tenosynovectomy/synovectomy procedures, three patients had carpal tunnel release procedures and one patient had a neurectomy and tendon reconstruction surgery. The median number of surgical procedures performed for invasive infection was one (IQR 1–3, range 0–7). All cases with known outcomes, which includes 21 (75 %) cases, were improved; one case required a partial amputation of the finger attributed to methicillin-resistant staphylococcal superinfection. Seven (25 %) cases were lost to follow-up without known treatment outcomes.
Literature review
Thirteen articles met inclusion criteria after further review. Table 4 summarizes these case series, categorized into three groups: cutaneous infection, invasive infection, and both cutaneous and invasive infection. Four articles were case series of cutaneous infection; the primary author was a dermatologist for three articles and an internal medicine physician for the other article. Two articles were case series of invasive infection; the primary author was an orthopedic surgeon for one article and a plastic surgeon for the other article. Seven articles were case series of both cutaneous and invasive infection; the primary author was an infectious disease physician for three articles, dermatologist for two articles, and a microbiologist and general internal medicine physician for the other two articles.
Discussion
In other case series, M. marinum infection was associated with a reported aquatic exposure in 24–100 % of cases [1, 5, 11–21]. A total of 87 % of cases in our study had an aquatic exposure, with boating or fishing being the most commonly described exposure. Interestingly, boating or fishing exposure was associated with invasive disease, while fish tank exposure was associated with cutaneous disease. This may be related to the mechanism of injury; boating and fishing injuries may involve deep puncture wounds from fish spines, fish hooks, or other equipment, while fish tank injuries may involve more superficial exposures such as minor scrapes while cleaning or maintaining fish tanks. Other case series also report lower rates of invasive infection in the setting of presumably superficial fish tank exposures, but additional studies are needed to investigate this observation further [1, 5, 16].
The vast majority (93 %) of cases in our study had M. marinum infection involving the fingers and/or hands, with wrist involvement reported in 32 % of cases and arm involvement reported in 14 % of cases. This agrees with Aubry et al. [5] who reported 95 % of skin lesions involving the upper hand. Our study had a high rate of invasive infection, with 68 % of cases classified as tenosynovitis or septic arthritis. The invasive infection rates from other case series which include both invasive and cutaneous infection are lower, ranging from 11 to 43 % [1, 5, 12–14, 21]. This may be due to an inherent bias among our cases, as DUMC is a tertiary referral center which would likely manage more complicated invasive M. marinum infections than simple cutaneous infections which could be managed in the community without having to refer to DUMC.
The median time to diagnosis for all cases of M. marinum infection in our study was 3.5 months. Although not statistically significant, invasive infections were diagnosed a median of 4 months after symptom onset versus 1.5 months for cutaneous infections. Also, steroid injections were more common among invasive infections, with 29 % of invasive infections receiving an injection prior to diagnosis. These findings highlight a significant diagnostic delay among patients with invasive infection, with frequent initial misdiagnosis and utilization of treatment (i.e., steroid injections) that likely exacerbates the infection. Practitioners who frequently see patients with chronic tenosynovitis need to be aware of the indolent presentation of M. marinum infection and consider it early in the diagnostic evaluation [22].
Not surprisingly, cases which were diagnosed through surgical procedures were more commonly classified as invasive infections while cases which were diagnosed through an office biopsy or aspiration were more commonly classified as cutaneous infections. Similarly, advanced imaging was used more frequently in the diagnostic work-up of invasive infections. Simple cutaneous lesions can primarily be managed in the outpatient setting with no need for magnetic resonance imaging, but deeper and more debilitating infections rely upon surgical exploration and additional imaging to determine the extent of disease.
Pathologic examination of tissue was relatively insensitive, with 37 % of cases demonstrating no evidence of granulomatous inflammation or positive AFB staining among culture-positive M. marinum infections. Pathology examination has poor specificity because other infectious processes (i.e., other non-tuberculous mycobacteria, tuberculosis, Nocardia, Cryptococcus, black mold infections) can also cause granulomatous inflammation or positive AFB staining. While Abbas et al. [15] reported that granulomas were present in 100 % of their cohort, Edelstein [20] reported similar results to ours with 63 % of cases demonstrating granulomas and 8 % of cases having positive AFB staining among biopsy specimens. Thus, obtaining adequate surgical cultures is critical due to the poor specificity of the procedure.
The most recent treatment recommendations from the 2007 joint American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) guidelines recommend 2 active agents (clarithromycin/azithromycin, ethambutol, or rifampin) for 3–4 months with adjunctive surgical debridement for invasive infections [2, 6]. However, a recent Chinese study demonstrated that monotherapy with clarithromycin can be an effective regimen for simple cutaneous infection, and some case series of cutaneous infection have demonstrated high treatment success rates with single-drug treatment [8, 13, 15]. In our study, 88 % of cases were treated with ≥2 drugs for the backbone antibiotic regimen; three-drug therapy was employed for 58 % of invasive infections. Aside from 2 case series from the United States, antibiotic treatment with ≥2 agents was only reported in 11–63 % of other cases series [1, 5, 11–21]. Almost half of all cases in our study required a change in the initial antibiotic regimen, commonly due to side effects or slow progress, which is similar to the 35 % modification rate reported by Aubry et al. [5]. The median total duration of therapy in our study was 5 months, with a median of 5.5 months for invasive infection and 4 months for cutaneous infection which was not significantly different. Other case series have reported mean treatment durations ranging from 2.7 to 8.3 months, with shorter durations reported among case series of cutaneous M. marinum infection [1, 5, 11–21]. In our study, ethambutol was the most frequently used antibiotic, followed by rifampin, clarithromycin, and azithromycin in accordance with ATS/IDSA guidelines [2, 6]. The high rate of multidrug therapy and longer duration of treatment in our study probably reflect the higher rate of invasive infections among our cohort.
Surgery was required for 79 % of cases in our study; 95 % of invasive infections required surgery, while 44 % of cutaneous infections required surgery. Other case series of invasive and cutaneous M. marinum infection reported rates of surgical intervention ranging from 0 to 88 %, while other case series of invasive infection reported 100 % of cases undergoing surgery and other case series of cutaneous infection reported 0–3 % of cases undergoing surgery [1, 5, 12–17, 19, 20]. Multiple surgeries were often required for cases of invasive infection in our study, with a median of one surgical procedure (range 0–7) performed per case of invasive infection. Cheung et al. [11] reported that 50 % of cases of invasive infection required more than one debridement. This underscores that additional debridements or surgical interventions are often required to eradicate invasive infection; patients with invasive infection should be counseled at the time of diagnosis about the possible need for multiple surgeries.
Finally, treatment outcomes were excellent among both invasive and cutaneous infection in our study, with rates of improvement reported with 79 and 67 % of cases, respectively. However, no treatment failures were reported among our 28 cases of M. marinum infection; all other cases were lost to follow-up. Other case series report similar treatment outcomes with improvement reported in 68–100 % of cases [1, 5, 11–21].
Limitations of this study include poor follow-up of some cases of M. marinum infection. A significant percentage of patients were referred to DUMC only for surgical intervention; thus the antibiotic regimen and treatment outcomes were not documented within Duke’s electronic health record system. Also, to ensure high-quality data, we excluded presumptive M. marinum infections that were diagnosed based upon an aquatic exposure or granulomatous inflammation from pathologic specimens; as the sensitivity of cultures for nontuberculous mycobacteria is poor, particularly for M. marinum which optimally grows at a cooler temperature, we may have missed some cases of M. marinum infection in our study [3].
Our literature review included a high percentage of case series from Asia, with 5 case series from Taiwan, Hong Kong, and Singapore, and 2 cases series from Lebanon and Israel. There were only 3 case series from North America, 2 from Europe, and 1 from Australia. These regional and cultural differences can bias our analysis. Also, comparisons among case series of M. marinum infection in the literature are challenging, as case series with only cutaneous infection are usually written by dermatologists and often include simpler cases that can be treated with shorter courses of antibiotic therapy, while cases series of invasive infection are written by surgeons and focus on surgical techniques and functional outcomes rather than cure rates. Thus it is important to look closely at the authors and study goals of articles when reviewing M. marinum literature.
Conclusions
Incidence of M. marinum infection appears to be increasing. In our study, cases of M. marinum infection had more aquatic exposures, received more multidrug therapy with longer duration of treatment, and more frequently required surgery when compared to other case series. Distinguishing between invasive and cutaneous infection is important as the management approach to antibiotics and surgery may differ. It is difficult to draw conclusions about an optimal treatment strategy due to our high treatment success rate and the frequent modifications made to antibiotic regimens, but it is clear that longer courses of multidrug therapy were frequently employed with improved treatment outcomes in our study.
References
Lewis FMT, Marsh BJ. Fordham von Reyn C. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390–7.
Griffith DE, Aksamit T, Brown-Elliott BA, Cantazaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial disease. Am J Respir Crit Care Med. 2007;175:367–416.
Stamm LM, Brown EJ. Mycobacterium marinum: the generalization and specialization of a pathogenic mycobacterium. Microbes Infect. 2004;6:1418–28.
Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of literature. Clin Infect Dis. 2000;31:439–43.
Aubry A, Chosidow O, Caumes E, Robert J, Cambau E. Sixty-three cases of Mycobacterium marinum infection. Arch Intern Med. 2002;162:1746–52.
Harris DM, Keating MR. Mycobacterium marinum: current recommended pharmacologic therapy. J Hand Surg Am. 2009;34:1734–5.
The New York City Department of Health and Mental Hygiene. Health department warns patrons of seafood markets in Chinatowns about skin infection from handling live or raw fish or seafood. 2014. http://www.nyc.gov/html/doh/html/pr2014/pr006-14.shtml. Accessed 17 Oct 2014.
Feng Y, Xu H, Wang H, Zhang C, Zong W, Wu Q. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian. Chin Diagn Microbiol Infect Dis. 2011;71:267–72.
Horvath MM, Rusincovitch SA, Brinson S, Shang HC, Evans S, Ferranti JM. Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE). J Biomed Inform. 2014;pii:S1532-0464(14)00153-1.
Centers for Medicare and Medicaid Service. ICD-9 Code Lookup. 2014. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed 16 Oct 2014.
Cheung JPY, Fung B, Ip WY, Chow SP. Mycobacterium marinum infection of the hand and wrist. J Orthop Surg. 2012;20:214–8.
Wu TS, Chiu CH, Yang CH, Leu HS, Huang CT, Chen YC, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
Eberst E, Dereure O, Guillot B, Trento C, Terru D, van de Perre P, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:e15–6.
Chen HY, Chen CY, Huang CT, Ruan SY, Chou CH, Lai CC, et al. Skin and soft-tissue infection caused by non-tuberculous mycobacteria in Taiwan, 1997–2008. Epidemiol Infect. 2011;139:121–9.
Abbas O, Marrouch N, Kattar MM, Zeynoun S, Kibbi AG, Rached RA, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33–42.
Dodiuk-Gar R, Dyachenko P, Ziv M, Shani-Adir A, Oren Y, Mendelovici S, et al. Nontuberculous mycobacterial infectious of the skin: a retrospective study of 25 cases. J Am Ac Dermatol. 2007;57:413–20.
Ho MH, Ho CK, Chong LY. Atypical mycobacterial cutaneous infections in Hong Kong: 10-year retrospective study. Hong Kong Med J. 2006;12:21–6.
Hess CJ, Wolock BS, Murphy MS. Mycobacterium marinum infections of the upper extremity. Plast Reconstruct Surg. 2005;115:55e–9e.
Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343–7.
Edelstein H. Mycobacterium marinum skin infections. Arch Intern Med. 1994;154:1359–64.
Iredell J, Whitby M, Blacklock Z. Mycobacterium marinum infection: epidemiology and presentation in Queensland 1971–1990. Med J Aust. 1992;157:596–8.
Hsiao CH, Cheng A, Huang YT, Liao CH, Hsueh PR. Clinical and pathological characteristics of mycobacterial tenosynovitis and arthritis. Infection. 2013;41:457–64.
Conflict of interest
The authors have no potential conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, M.G., Stout, J.E. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection 43, 655–662 (2015). https://doi.org/10.1007/s15010-015-0776-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-015-0776-8