Abstract
Background
Thrombocytopenia is a frequent finding among ill returned travellers and may be caused by a large number of different conditions, including infectious diseases specific or typical for tropical and subtropical regions. In order to assess the diagnostic significance of thrombocytopenia we investigated a large cohort of returned travellers.
Methods
This was a comparative study in which data collected on 19,473 returned travellers who consulted the outpatient travel clinic of the the University of Munich Hospital between 1999 and 2009 were analysed. Of these, 732 (3.8%) travellers were diagnosed with thrombocytopenia, and their data were compared with those of the remaining 18,741 travellers with normal platelet counts.
Results
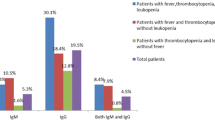
Thrombocytopenia was significantly more frequent among patients with malaria (63%), acute human immunodeficiency virus infection (48%), dengue fever/dengue haemorrhagic fever (DF/DHF; 47%), Epstein–Barr virus infectious mononucleosis (23%), paratyphoid/typhoid fever (14%), and rickettsiosis (12%). Malaria and DF/DHF caused 25% of all cases of thrombocytopenia (platelet count <140,000/μl) and 75% of all cases of severe thrombocytopenia (platelet count <30,000/μl). Sex, age, country of origin, duration and type of travel were not significantly correlated with thrombocytopenia. The most frequent travel destinations were Asia (42%), Africa (33%), and Latin America (14%). Travellers to Sub-Saharan Africa (high risk for malaria) and to South/South-east Asia (high risk for DF/DHF) had the highest relative risk for thrombocytopenia.
Conclusion
Platelet count among returned travellers is an essential screening parameter, as thrombocytopenia is highly correlated with important infectious diseases, particularly with malaria and DF/DHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombocytopenia (TP), generally defined as a platelet count of <150,000 thrombocytes per microlitre of blood (t/μl) [1], can be caused by a large number of congenital or acquired diseases. Platelet function and counts (normal 150,000–400,000 t/μl)] do not vary with age to any clinically significant degree. Patients with a platelet count of >60,000 t/μl usually do not present with clinical signs of haemorrhagic diathesis, such as petechiae, haematomas and mucocutaneous bleedings, whereas platelet concentrations between 30,000 and 60,000 t/μl are associated more frequently with effusions after minor trauma. Severe TP is generally defined as a platelet count of <30,000 t/μl and is associated with an increased risk of spontaneous bleeding [2].
Several infectious diseases induced by different protozoa, viruses, and bacteria are included in the large number of acquired causes of TP. Protozoal diseases such as babesiosis [3, 4], visceral leishmaniasis [5], toxoplasmosis [6, 7], African trypanosomiasis (sleeping sickness) [8, 9], American trypanosomiasis (Chagas disease) [8, 10] and especially malaria [11, 12] are known to cause TP, as are various viruses, such as dengue virus [13, 14], human immunodeficiency virus (HIV) [15–17], Epstein–Barr virus (EBV) [18, 19], hantavirus [20, 21], and cytomegalovirus [22]. TP is a also common manifestation of all bacterial tick-borne diseases [23], especially rickettsioses (including Q-fever and ehrlichiosis) [24–26], paratyphoid or typhoid fever [27, 28], borreliosis [29], leptospirosis [30, 31], congenital syphilis [32], brucellosis [33, 34] and infections due to Escherichia spp. (especially the haemolytic–uraemic syndrome caused by E. coli O157:H7 [35]).
As the majority of these infectious diseases are typical or specific to tropical and subtropical regions [1], travellers going to such destinations are particularly at an elevated risk of acquiring TP. Data on this subject are rare, and no systematic study on infection-induced TP among travellers has been reported to date.
The aim of this study was to identify cases of TP caused by infectious diseases among travellers seeking treatment or presenting for a medical check-up at the outpatient travel clinic of the Department of Infectious Diseases and Tropical Medicine (DITM) of the Ludwig-Maximilians University, Munich, following their return from tropical and non-tropical countries. We also evaluated risk factors for infection-induced TP among returned travellers by analysing their demographic, travel and clinical data and by comparing these data with those of returned travellers without TP.
Patients and methods
Study population and inclusion criteria
From January 1999 through December 2009, 42,863 patients with symptoms suggestive of travel-related infections or individuals seeking a medical check-up presented at the DITM. Two criteria for inclusion in our study were defined: (1) the concentration of thrombocytes had been assessed (28,169 individuals fulfilled this criterion: 65.7%) and (2) the destination and the duration of travel was known (22,560: 52.6%). Overall, 19,473 (45.4%) travellers fulfilled both criteria and were enrolled in the study (study population).
Specimen collection and assessment of platelet count
Blood was collected in EDTA (ethylenediaminetetraacetic acid) tubes, and the concentration of thrombocytes in the collected blood was immediately assessed on a Sysmex KX-21 N blood analyser (Sysmex, Hamburg-Norderstedt, Germany) without prior storage.
Definition of TP
A case of TP was defined as a returned traveller with a platelet count at admission or first presentation of less (or more) than 140,000 t/μl according to in-house standards. Those travellers with a concentration of <60,000 t/μl (or 30,000 t/μl) were defined as cases of pronounced (or severe) TP. The data of travellers diagnosed with TP were compared with those of travellers with normal platelet counts.
Analysed variables
Demographic data were analysed for the whole study population of 19,473 travellers. Analysis of travel and clinical data were performed only among of 16,795 travellers (586 travellers with TP, 16,209 travellers without TP) of German origin to avoid confounding by country of origin. The diagnosis of infectious diseases among these travellers was confirmed by laboratory testing and was not based solely on clinical suspicion.
Statistical analysis
Approximative tests (χ2 tests) and t tests as the parametric test were conducted using Stata software ver. 9.0. (Stata Corp, College Station, USA) and EpiInfo ver. 3.3.2. (Centers for Disease Control and Prevention, Atlanta, GA). Significant differences were defined as p values of <0.05 or as those not overlapping the 95 percent confidence intervals (95% CI) of proportions.
Results
Demographic data
Among the 19,473 returned travellers comprising the study population, 732 (3.8%) were determined to be travellers with TP and 18,741 (96.2%) to be travellers without TP. Males travellers accounted for 9,707 (49.8%) of the study population, but the proportion of men (70.8%) was significantly (p < 0.01) higher among the returned travellers with TP than among those without TP (49.0%). The age of all travellers ranged from 0.5 to 97 years, with the majority (14,990, 77.0%) falling in the age group 20–49 years. The mean age of travellers with TP (40.7 years; 95% CI 39.6–41.8 years) was significantly higher than of travellers without TP (37.6 years; 95% CI 37.4–37.8 years). Among travellers with TP, the proportion of travellers in age group 20–49 years (67.6%) was significantly (p < 0.01) lower than that among travellers without TP (75.3%); in contrast, among travellers with TP, the proportion of travellers in age group 50–97 years (28.0%) was significantly (p < 0.01 each) higher than that among travellers without TP (19.9%).
Among travellers with TP, the proportion of travellers of German (80.1%) and Eastern European (1.2%) origin was significantly lower (p < 0.01 and 0.03, respectively), and the proportion of travellers with African origin (11.6%) was significantly higher (p < 0.01), than those among travellers without TP (86.5, 2.5 and 3.6%, respectively) (Table 1).
Travel data
The most frequent travel destinations of the 16,795 returned travellers (with TP: 586, 3.5%; without TP: 16,209, 96.5%) of German origin were Asia (7,071, 42.1%), Africa (5,529, 32.9%) and Latin America (3,134: 18.7%), followed by Western Europe (393, 2.3%), Eastern Europe (352, 2.1%), Oceania (199, 1.2%), and North America (117, 0.7%). Among the travellers with TP, the proportion of those returning from Asia was significantly (p = 0.03) higher, and those returning from Latin America was significantly (p = 0.04) lower, than those of travellers without TP.
About one-third of each group of travellers (those with TP and those without) had been abroad for 1–14 days, 15–30 days and more than 30 days, respectively. Adventure travel (47.3%) was the most frequent type of travel, followed by all-inclusive (20.1%) and business travel (15.4%). No significant association was found between the duration or type of travel and presence of TP (Table 2).
Clinical data
Among the 16,209 travellers of German origin, the symptoms fever, headache, and arthralgia were significantly more (p < 0.01 each) associated with TP, and diarrhoea was significantly less associated (p < 0.01). Nausea, skin disorders, dyspnoea, and other symptoms (cardiac, neurological, urological, genital, or psychiatric disorders) were not significantly associated with TP.
A significant correlation (p < 0.01 each) was found between TP and malaria, dengue fever/dengue haemorrhagic fever (DF/DHF), acute HIV infection, EBV infectious mononucleosis (EBV-IM), rickettsiosis, and also for para-/typhoid fever (p = 0.01). Among the 119 patients with malaria, 63.0% were diagnosed with TP. The proportion of travellers with TP among patients with other infectious diseases was: 46.8% of patients with DF/DHF, 48.0% of those with acute HIV infection, 22.7% of those with EBV-IM, 10.5% of those with rickettsiosis, and 14.3% of those with para-/typhoid fever. No other infectious disease was detected to be significantly correlated with TP in this study (Table 2).
Destinations and infectious diseases
Among all 586 travellers of German origin with TP who returned from abroad, the proportions of patients with the most frequently infectious diseases were: 12.8% (75 cases) with malaria, 11.3% (66) with DF/DHF, 2.0% (12) with acute HIV infection, 1.7% (10) with EBV-IM, 1.0% (6) with rickettsiosis, and 0.5% (3) with para-/typhoid fever.
Among the 163 travellers with TP returning from Sub-Saharan Africa, there was a significantly (p < 0.01) higher proportion (50/163, 30.7%) of patients with malaria and a significantly (p < 0.01) lower proportion (1/163, 0.6%) of patients with DF/DHF. Among the 260 travellers with TP returning from South/South-east Asia, there was a significantly (p < 0.01) higher proportion (55/260, 21.2%) of patients with DF/DHF and a significantly (p < 0.01) lower proportion (20/260, 7.7%) with malaria. Of the six returned travellers with TP diagnosed with rickettsiosis, four (66.7%) travellers were returning from South Africa, and one each from Turkey and Malta. Of the three travellers with TP diagnosed with para-/typhoid fever, all were travellers returning from India. Among travellers with TP, no significant correlation was found between acute HIV infections or EBV-IM and destination. Most of cases of TP were detected among travellers returning from Thailand (88 cases: 1 case diagnosed with malaria; 23 cases diagnosed with DF/DHF), India (57 cases: 2, 10), Indonesia (37 cases: 6, 8), and South Africa (24 cases: 1, 0).
Pronounced and severe TP
Among the 586 travellers with TP of German origin, there were 36 (6.1%) cases of pronounced TP and 12 (2.0%) cases of severe TP, including one single case diagnosed with 16,000 t/μl caused by falciparum malaria and resulting in severe haemorrhagic diathesis with disseminated intravascular coagulation.
The proportion of pronounced TP was significantly elevated in the presence of the variables malaria (p < 0.01) and DF/DHF (p = 0.02). Of 36 cases of pronounced TP, there were 17 (47.2%) cases of malaria, eight (22.2%) cases of DF/DHF, and one (2.8%) case of acute HIV infection. For the remaining 10 (27.8%) cases of pronounced TP, no infectious disease was detected as potential cause.
Of the 12 cases diagnosed with severe TP, there were seven (58.3%) cases of malaria (four cases of Plasmodium falciparum infection, two cases of P. vivax infection, one case of P. ovale infection) and two (16.7%) cases of DHF with petechiae but without serious haemorrhagic diathesis. For the remaining three (25.0%) cases diagnosed with severe TP, no infectious disease was detected as potential cause.
Among 119 patients with malaria, 17 (14.3%) cases of pronounced TP (including 7 cases of severe TP), 58 (48.7%) cases of not pronounced TP, and 44 (37.0%) travellers without TP were detected. Among 141 patients with DF/DHF, eight (5.7%) cases of pronounced TP (including 2 cases of severe TP), 58 (41.1%) cases of not pronounced TP, and 75 (53.2%) travellers without TP were detected. Among 25 patients with acute HIV infection, one (4.0%) case of pronounced TP, 11 (44.0%) cases of not pronounced TP, and 13 (52.0%) travellers without TP were detected.
Discussion
This is the largest comparative study on infection-induced TP among travellers returning from tropical and non-tropical countries reported to date. In this study, we analysed the demographic, travel and clinical data of 732 returned travellers with TP and compared these data with those of 18,741 returned travellers without TP. We also assessed risk factors for acquiring TP among travellers, and we evaluated several significant associations between potential risk factors (independent variables: demographic, travel and clinical data) and TP (dependent variable). However, most of these associations were confounded by malaria and DF/DHF. Of 19,473 travellers returning from abroad and presenting at the outpatient travel clinic of the DITM, 3.8% were diagnosed with TP. Approximately 35% of all cases with TP were caused by infectious diseases, particularly malaria (13%) and DF/DHF (11%). No potentially causative infectious or non-infectious agent was detected in the remaining 65% of patients with TP.
The variables sex, age, and African origin appeared to be risk factors, whereas German and Eastern European origin appeared to be protective factors for TP. These findings are due to the fact that more males, more travellers of age >50 years, and more travellers of African origin, but fewer travellers of German and Eastern European origin, travel to Sub-Saharan Africa. In this study, sex, age and origin were not assessed to be significant risk or protective factors for TP because correlations were confounded by travelling to malaria endemic regions.
TP was significantly correlated with the symptoms fever, headache and arthralgia. These correlations were highly confounded by infectious diseases, such as malaria, DF/DHF, acute HIV infection, EBV-IM, rickettsiosis, paratyphoid and typhoid fever. Most of these diseases typically develop fever, headaches and arthralgia.
The risk to acquire TP was reduced among patients with diarrhoea. One possible explanation is dehydration, which may lead to an elevated concentration of platelets. Diarrhoea was a less frequent symptom among patients with malaria (in 9%) and DF/DHF (in 20%), whereas it was the most common symptom in the total study population (34%).
The highest prevalence of TP (63%), pronounced TP (14%) and severe TP (6%) was found among patients with malaria, whereas no differences between falciparum, tertian or quartan malaria could be found. In two other studies, the prevalence of TP was estimated to be higher for infections with P. falciparum (73–90%) than for infections with P. vivax (70%), whereas the prevalence of severe TP was estimated in the same range (9 and 2%, respectively) as in our study [11, 12].
Among patients with DF/DHF, the prevalence of TP (47%), pronounced TP (6%) and severe TP (1.4%) was comparable with that of these conditions among patients with acute HIV infection (48, 4, 0%, respectively). As shown in other studies, about one-half of individuals infected with one of these viruses develop TP, whereas severe TP is rare [13, 15–17].
TP was diagnosed in 23% of patients with EBV-IM, whereas no case of severe TP was detected in this study. This result corresponds to those published earlier showing that severe TP is a rare complication of acute EBV infection [18, 19].
The prevalence of TP among patients with rickettsioses has been described to vary from <10% up to >90% [24–26] because many different species of the family Rickettsiaceae cause rickettsioses. In our study, this prevalence was calculated to be 12%. The prevalence of TP among patients with para-/typhoid fever has been described to be <50% [27, 28]; in comparison, it was calculated to be 14% in our study.
Travelling in Sub-Saharan Africa, especially in Central and West Africa, is a clear risk factor for acquiring malaria, whereas travelling in South and South-east Asia, especially in Thailand, are risk factors for DF/DHF. The great majority of patients with rickettsiosis had travelled in South Africa, whereas all patients with para-/typhoid fever had travelled in India. Taking into account the number of air passengers flying to a certain destination, the highest risk for TP was assessed for travellers in high endemic regions for malaria, namely, Central, West and East Africa, followed by travellers in high endemic regions for DF/DHF, i.e. South-east and South Asia. Compared with the risk for acquiring TP during travelling in tropical or subtropical regions, the relative risk was almost threefold higher for travellers in Sub-Saharan Africa and twofold higher for travellers in South/South East Asia.
As malaria and DF/DHF were the main causes for infection-induced TP, the typical risk factors for TP among travellers were assessed to be the same as for these two infections. About 60% of adult travellers who presented with fever and TP in the outpatient travel clinic after returning from West Africa or Central Africa were diagnosed with malaria. More than 60% of adult travellers who presented with fever, arthralgia and TP after returning from South or South East Asia were diagnosed with DF/DHF.
There are a number of limitations to our study. First, travellers diagnosed with TP might have received more extensive diagnostic workups from the healthcare providers than those without TP. Secondly, no data on the prevalence of TP before travelling were available for assessment; consequently, the absolute risk for acquiring TP during travel could not be assessed.
Conclusion
Platelet count among returned travellers is an inexpensive and easy-to-perform laboratory test and essential for the diagnosis of TP, the occurrence of which, in turn, is highly correlated with major infectious diseases that are specific for or typical of tropical and subtropical regions, such as malaria, DF/DHF, acute HIV infection, EBV-IM, rickettsiosis and paratyphoid and typhoid fever. The most frequent infectious diseases were malaria and DF/DHF, which caused about 25% of all cases of TP, about 70% of all cases of pronounced TP and about 75% of all cases of severe TP. These results may provide valuable information to those clinicians working with patients with TP who have returned from tropical and subtropical regions.
References
Fleming AF, de Silva PS. Haematological diseases in the tropics. In: Manson’s tropical diseases. Elsevier WB Saunders, 2003;174.
George JN. Platelets. Lancet. 2000;355:1531–9.
Dolan TT. Thrombocytopenia in experimental babesiosis. Trans R Soc Trop Med Hyg. 1974;68:274.
Kettner F, Reyers F, Miller D. Thrombocytopenia in canine babesiosis and its clinical usefulness. J S Afr Vet Assoc. 2003;74:63–8.
Costa CH, Werneck GL, Costa DL, Holanda TA, Aguiar GB, Carvalho AS, Cavalcanti JC, Santos LS. Is severe visceral leishmaniasis a systemic inflammatory response syndrome? A case control study. Rev Soc Bras Med Trop. 2010;43:386–92.
Gürkan E, Başlamişli F, Güvenç B, Bozkurt B, Unsal C. Immune thrombocytopenic purpura associated with Brucella and Toxoplasma infections. Am J Hematol. 2003;74:52–4.
Tuon F, Higashino HR, Amato VS, Nicodemo AC. Acute immune-mediated thrombocytopenic purpura related to Toxoplasma gondii infection. Int J Infect Dis. 2008;12:671–2.
Robins-Browne RM, Schneider J, Metz J. Thrombocytopenia in trypanosomiasis. Am J Trop Med Hyg. 1975;24:226–31.
Davis CE. Thrombocytopenia: a uniform complication of African trypanosomiasis. Acta Trop. 1982;39:123–33.
Tribulatti MV, Mucci J, Van Rooijen N, Leguizamón MS, Campetella O. The trans-sialidase from Trypanosoma cruzi induces thrombocytopenia during acute Chagas’ disease by reducing the platelet sialic acid contents. Infect Immun. 2005;73:201–7.
Jadhav UM, Patkar VS, Kadam NN. Thrombocytopenia in malaria—correlation with type and severity of malaria. J Assoc Physicians India. 2004;52:615–8.
Shaik QH, Ahmad SM, Abbasi A, Malik SA, Sahito AA, Muni SM. Thrombocytpenia in malaria. J Coll Physicians Surg Pak. 2009;19:708–10.
Schexneider KI, Reedy EA. Thrombocytopenia in dengue fever. Curr Hematol Rep. 2005;4:145–8.
Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop Med Int Health. 2006;11:1238–55.
Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 2002;16:73–6.
Sundell IB, Koka PS. Thrombocytopenia in HIV infection: impairment of platelet formation and loss correlates with increased c-Mpl and ligand thrombopoietin expression. Curr HIV Res. 2006;4:107–16.
Marks KM, Clarke RM, Bussel JB, Talal AH, Glesby MJ. Risk factors for thrombocytopenia in HIV-infected persons in the era of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;52:595–9.
Steeper TA, Horwitz CA, Moore SB, Henle W, Henle G, Ellis R, Flynn PJ. Severe thrombocytopenia in Epstein–Barr virus-induced mononucleosis. West J Med. 1989;150:170–3.
Likic R, Kuzmanic D. Severe thrombocytopenia as a complication of acute Epstein–Barr virus infection. Wien Klin Wochenschr. 2004;116:47–50.
Rasche FM, Uhel B, Krüger DH, Karges W, Czock D, Hampl W, Keller F, Meisel H, von Müller L. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg Infect Dis. 2004;10:1420–5.
Denecke B, Bigalke B, Haap M, Overkamp D, Lehnert H, Haas CS. Hantavirus infection: a neglected diagnosis in thrombocytopenia and fever? Mayo Clin Proc. 2010;85:1016–20.
Fisgin T, Yarali N, Duru F, Kara A. CMV-induced immune thrombocytopenia and excessive hematogones mimicking an acute B-precursor lymphoblastic leukemia. Leuk Res. 2003;27:193–6.
Pantanowitz L. Mechanisms of thrombocytopenia in tick-borne diseases. The Internet J Infect Dis. Available at: http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijid/vol2n2/tick.xml (2003). Accessed 12 June 2011.
Pancholi P, Kolbert CP, Mitchell PD, Reed KD Jr, Dumler JS, Bakken JS, Telford SR 3rd, Persing DH. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–12.
Mert A, Ozaras R, Tabak F, Bilir M, Ozturk R. Mediterranean spotted fever: a review of fifteen cases. J Dermatol. 2006;33:103–7.
Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler SJ, Nicholson WL, Walker DH, Eremeeva ME, Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: rocky mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27.
Faierman D, Ross FA, Seckler SG. Typhoid fever complicated by hepatitis, nephritis, and thrombocytopenia. JAMA. 1972;221:60–1.
Hustinx WN, Nio Y, Speelman P. Abdominal typhus and paratyphoid fever in 2 academic hospitals: 1984–1990. (in Dutch) Ned Tijdschr Geneeskd. 1993;137:291–4.
Alugupulli KR, Michelson AD, Joris I, Schwan TG, Hodivala-Dilke K, Hynes RO, Leong JM. Spirochete–platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood. 2003;102:2843–50.
Nicodemo AC, Negro del G, Amato Neto V. Thrombocytopenia and leptospirosis. Rev Inst Med Trop São Paulo. 1990;32:252–9.
Wagenaar JF, Goris MC, Partiningrum DL, Isbandrio B, Hartskeerl RA, Brandjes DP, Meijers JC, Gasern MH, van Gorp EC. Coagulation disorders in patients with severe leptospirosis are associated with severe bleeding and mortality. Trop Med Int Health. 2010;15:152–9.
Juhlin L. Early congenital syphilis and thrombocytopenia. Acta Derm Venereol. 1968;48:166–9.
Di Mario A, Sica S, Zini G, Salutari P, Leone G. Microangiopathic hemolytic anemia and severe thrombocytopenia in Brucella infection. Ann Hematol. 1995;70:59–60.
Altuntas F, Eser B, Sari I, Yildiz O, Cetin M, Unal A. Severe thrombotic microangiopathy associated with brucellosis: successful treatment with plasmapheresis. Clin Appl Thromb Hemost. 2005;11:105–8.
Guo YL, Liu DQ, Bian Z, Zhang CY, Zen K. Down-regulation of platelet surface CD47 expression in Escherichia coli O157:H7 infection-induced thrombocytopenia. PLoS One. 2009;4:e7131.
Acknowledgements
The authors thank all patients in this study for their cooperation.
Conflict of interest
None of the authors had any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herbinger, KH., Schunk, M., Nothdurft, H.D. et al. Comparative study on infection-induced thrombocytopenia among returned travellers. Infection 40, 373–379 (2012). https://doi.org/10.1007/s15010-012-0242-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-012-0242-9