Abstract
Background
The aim of this study was to examine the safety and efficacy of de-escalating antimicrobial therapy in immunocompetent patients presenting with bacteraemia due to antibiotic-sensitive pathogens.
Methods
We screened 1,350 positive blood cultures identified in a single, 1,065-bed university hospital over 5 years, and retained 310 cases of bacteraemia due to antibiotic-sensitive pathogens, including (1) methicillin-sensitive staphylococci, (2) penicillin-sensitive streptococci, (3) β-lactam-sensitive (a) Escherichia coli, and (b) Klebsiella species. The efficacy of appropriate initial empirical antimicrobial therapy, the performance of de-escalated pathogen-directed therapy, and the safety and efficacy of de-escalated therapy were evaluated.
Results
Among 270 appropriately treated patients, 16 (6%) died, versus 6 (15%) among 40 who were inappropriately treated (p = 0.04). While 201 of 270 patients (74%) who received appropriate initial empirical therapy were candidates for de-escalation, the treatment was de-escalated in only 79 (39%). De-escalation was associated with (1) a trend toward a lower (a) death rate (1 vs. 5%) and (b) treatment failure (4 vs. 10%), and (2) (a) a 4-day longer median duration and (b) a $50 higher median cost of antimicrobial therapy (p < 0.001).
Conclusions
When the pathogen was sensitive to antimicrobial therapy and the initial empirical treatment was effective, de-escalation of antimicrobial therapy in immunocompetent patients with bacteraemia was safe and associated with acceptable outcomes. The rate of de-escalation of antimicrobial therapy was low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobials are a key component of a comprehensive management strategy against severe infections, including bacteraemia, a worldwide leading cause of deaths in intensive care units [1–3]. Choosing an appropriate optimal initial coverage of the pathogen that causes bacteraemia pending the results of cultures is a major factor associated with survival [4–8]. From this perspective, the recently updated Surviving Sepsis Campaign guidelines recommended a treatment strategy involving the use of a broad-spectrum, empirical antimicrobial regimen, followed by a de-escalated, pathogen-specific therapy [9]. De-escalation is strongly recommended in the context of an antimicrobial stewardship program in order to promote judicious antimicrobial use and to limit costs, adverse events, and the risk of developing antibiotic resistance [10].
Difficulties remain, however, with the practical implementation of de-escalation, especially when attempting to narrow the spectrum of the pathogen-directed antibiotic regimen, as one might hesitate to withdraw an effective initial prescription in favour of an antibiotic of unproven safety and efficacy. We designed this study to examine the outcomes of candidates for a de-escalation strategy who received an effective initial empirical antimicrobial treatment, followed by a narrowed, culture-directed regimen.
Patients and methods
This study was conducted in the Kyoto Prefectural School of Medicine and University Medical Centre with the approval (#E-213) of its institutional ethics committee, which waived the informed consent from the patients. All data were collected retrospectively from a database kept by a hospital-based infection control team, and by a review of medical records.
Between January 2004 and July 2009, the clinical laboratory of the Kyoto Prefectural University Medical Centre identified 1,350 patients with positive blood cultures, among whom 1,180 had confirmed bacteraemias, based on their overall clinical evaluation. In order to simplify the analysis of the relationship between antimicrobial therapy and patient outcomes, we selected four groups of pathogens, including (1) methicillin-sensitive staphylococci, (2) penicillin-sensitive streptococci, and (3) non-extended-spectrum β-lactamase-producing (a) Escherichia coli and (b) Klebsiella pneumoniae or oxytoca. Antibiotic sensitivity was tested according to the standards of the Clinical and Laboratory Standards Institute, using an agar dilution method. Patients presenting with (1) polymicrobial bacteraemia, (2) <1,000 neutrophils/m3/l or similarly severe immunosuppressive disorders, or (3) unknown empirical therapy were excluded, leaving 310 patients in this analysis (Fig. 1).
The initial empirical therapy, administered within 24 h after the initial collection of blood cultures, was classified as appropriate when it was (a) active against the causative pathogen based on the in vitro testing of sensitivity, and (b) administered intravenously, regardless of dose. Pathogen-directed antimicrobial therapy was the regimen administered after the sensitivity test results had been obtained. Recipients of an initially appropriate, broad-spectrum or combined empirical regimen were considered candidates for de-escalation after the results of the cultures. Treatment was considered de-escalated when the spectrum of antibiotics had narrowed, or when ≥2 initially prescribed antimicrobials were reduced to a single agent.
The decisions to obtain blood cultures and initiate antimicrobial therapy were left to the physician(s) in charge of the patient’s care, in optional and occasional consultation with infection control specialists. In our institution, physicians must declare their intent, though they are not prohibited to use carbapenems, fourth-generation cephalosporin, agents effective against methicillin-resistant staphylococci, or antifungals.
The patients were followed by the hospital-based infection control team until the discontinuation of antimicrobial therapy for bacteraemia, or until their death. It is our usual practice to keep the patients in our hospital until their treatment for bacteraemia has been completed.
Data collection and classification
The demographic and clinical data collected included age, sex, body weight, acute physiology and chronic health evaluation II (APACHE II) score, McCabe class at the time of blood culture collection, origin of bacteraemia, and the patient’s surgical history. The overall duration of antimicrobial therapy, and the costs of empirical and pathogen-directed treatment of bacteraemia were recorded. We calculated the cost of the antimicrobials using the official drug price list from the year 2010 published by the Ministry of Health, Labour and Welfare of Japan. All-cause and infection-related mortality were measured (1) 14 days after the blood culture collection, and (2) at the end of the follow-up by the infection control team. A positive blood culture after the initiation of treatment containing the original pathogen was classified as persistent, and not counted as a new infection. Bacteraemia due to the same microbiologically documented pathogen that developed after the discontinuation of treatment was classified as recurrent. Persistent or recurrent bacteraemia was classified as treatment failure.
Statistical analysis
Continuous data are expressed as medians (ranges), and were compared using the Mann–Whitney U-test. Categorical data are expressed as counts and percentages, and were compared using the chi-square test. A p value of <0.05 was considered statistically significant.
Results
Among 1,350 patients with positive blood cultures, we identified 1,180 who suffered from clinically confirmed bacteraemia (Fig. 1). After the exclusion of (1) 772 patients whose blood cultures grew pathogens other than those included in the four pre-specified groups, (2) 58 patients with <1,000 neutrophils/m3/l or major immunosuppressive disorders, (3) 38 patients presenting with polymicrobial bacteraemias, and (4) 2 patients whose empirical therapy could not be ascertained, 310 patients fulfilled the criteria for inclusion in this analysis (Fig. 1).
Appropriateness of initial antimicrobial therapy and outcomes
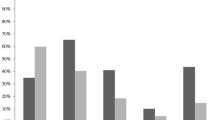
The initial empirical therapy was appropriate in 270 (87%) and inappropriate in 40 (13%) patients. The empirical and pathogen-directed antimicrobial regimens administered to the 310 patients are shown in Table 1. Among the 270 patients who were treated appropriately, 16 died (6%), versus 6 patients (15%) among the 40 who had inappropriate initial antimicrobial treatment (p = 0.04). Similarly, 10 patients (4%) died of infection-related causes in the appropriately treated group versus 5 (12%) among patients who were treated inappropriately (p = 0.04). As 1 patient in the inappropriately treated group died at 19 days, the 14-day all-cause and infection-related mortalities in that group were 12 and 10%, respectively. The rate of continuous bacteraemia during and recurrence after the course of antimicrobial therapy tended to be lower (p = 0.06) in the appropriately (n = 18 patients; 7%) than in the inappropriately (n = 6 patients; 15%) treated group.
Among the 270 patients, 201 patients (a) received empirical broad-spectrum or combined therapy, and (b) survived >3 days after collection of the blood culture, and so were candidates for de-escalation.
Outcomes of de-escalation
Among the 201 candidates, antimicrobial therapy was de-escalated in 79 (39%) and remained unchanged or was escalated in 122 (61%) patients. The baseline characteristics, including age, sex distribution, mean APACHE II score, non-fatal McCabe class, and proportion of patients recovering from a recent surgical intervention, were similar in both groups (Table 2). The empirical administration of combined antimicrobials or of single fourth-generation cephalosporins or carbapenems was also similar in both groups (Table 2). Urinary tract and central venous catheters were the most common sources of bacteraemia in both groups. The abdomen, including the biliary tract, liver and peritoneum, were significantly less frequently (p < 0.01) detected in the de-escalated than in the escalated group (Table 2). Finally, bacteraemia caused by methicillin-sensitive Staphylococcus aureus was significantly more prevalent in the de-escalation group (p < 0.001).
De-escalation of treatment and outcomes
All-cause mortality in the group of patients whose therapies were de-escalated was 1% versus 5% in the non-de-escalated group, a nonsignificant difference (Table 3). Likewise, the 0% infection-related mortality rate in the de-escalated group versus the 3% rate in the non-de-escalated group was a nonsignificant difference (Table 3). A single patient died on day 13 in the de-escalated group, and 6 patients in the non-de-escalated group died on days 5, 6, 6, 8, 11 and 12, respectively. Treatment was unsuccessful in 4% of patients whose regimens were de-escalated, versus 10% in the non-de-escalated group, a nonsignificant difference (Table 3). The combined rate of deaths and treatment failures was 15% in the non-de-escalated versus 5% in the de-escalated group (p = 0.05).
Antimicrobial therapy was administered for a median of 12 days in patients whose treatment regimens were de-escalated, versus 8 days in the non-de-escalated group (p < 0.001; Table 3). The result was similar when we limited the analysis to the survivors.
The overall costs of antimicrobial therapy were significantly higher in the de-escalated than in the non-de-escalated group (p < 0.001; Table 3). After excluding the nonsurvivors from the analysis, the overall costs remained significantly higher in the de-escalated than in the non-de-escalated group (p < 0.001; data not shown). The daily costs of antimicrobial therapy were nonsignificantly lower in the de-escalated group.
Similar results were observed when we limited the analysis to the 42 patients presenting with methicillin-sensitive S. aureus bacteraemia, for whom a longer duration of therapy is recommended. In this subgroup, the median duration of antimicrobial therapy was 13 days in 28 patients whose antimicrobial regimens were de-escalated, versus 9 days in 14 patients whose antimicrobial regimens were not de-escalated (p < 0.001). All-cause mortality was 1% in both groups, and no treatment failure was observed.
Discussion
Our analysis showed that, in patients suffering from bacteraemia due to antibiotic-sensitive pathogens, (1) the empirical choice of an effective initial antimicrobial is a critical determinant of survival at 28 days, (2) de-escalation was feasible in 74% of patients successfully treated empirically, although the treatment was actually de-escalated in only 39% of candidates, and (3) compared with no de-escalation, de-escalation was associated with a trend toward lower rates of death or treatment failure, though the total duration and costs of antimicrobial therapy were greater.
Bacteraemia is among the infectious complications associated with the highest mortality and morbidity rates and costs, particularly in patients admitted to the intensive care unit [1, 2]. The choice of effective antimicrobials is a key strategic point in the management of bacteraemic patients [4–8]. Since the identification of the microorganism and the completion of the culture and sensitivity tests take ≥48 h, antimicrobial therapy must be initiated empirically before the results are known. Several studies have suggested that the empirical choice of an ineffective antimicrobial or a delay in the initiation of therapy are associated with adverse outcomes [4–8]. In a recent meta-analysis, an inappropriate choice of empirical therapy for patients presenting with bloodstream infections significantly increased the odds of dying [11]. Our observations suggest that this also applies to bacteraemias caused by antibiotic-sensitive microorganisms.
One other important factor after the initial administration of empirical antimicrobial therapy is the judicious narrowing of the spectrum, while retaining the effectiveness of the treatment against the identified pathogen(s). The long-term administration of a broad-spectrum antibiotic, or of multiple agents, is associated with negative consequences, including the selection of antibiotic-resistant pathogens, antibiotic-associated diarrhoea, and high costs [9, 12–14]. Thus, it is advised—whenever possible—to de-escalate the initial treatment to a single, narrow-spectrum antibiotic based on the results of the cultures [15, 16].
In a prospective study of ventilator-associated pneumonia, the mortality rate was significantly lower in 22% of patients whose antimicrobial treatments could be de-escalated than in 43% patients whose therapies were escalated, or 24% in whom the therapies were left unchanged [17]. The treatment was not de-escalated when the causative pathogen was antibiotic resistant, a factor that has a significant impact on the clinical outcome [6]. In another study of ventilator-associated pneumonia, treatment was de-escalated in 27% of patients, and this was associated with similar relapse rates to non-de-escalation [18]. However, it is not clear how the outcomes of patients whose treatments were not (though they could have been) de-escalated might have been changed. In our study, patients were “de-escalation candidates” when they met specific criteria, which allowed us to assess the impact of de-escalation of treatment.
In this immunocompetent population, and after an effective initial empirical therapy, de-escalation was safe and associated with a trend toward lower mortality and treatment failure rates. One might hypothesize, however, that physicians were reluctant to de-escalate the treatment regimen in patients who did not respond readily to the initial antimicrobial(s), and were more likely to de-escalate in patients with a clinical status that improved rapidly in response to the initial therapy. Additional randomized studies are needed to evaluate the effect of de-escalation on outcome by stratifying the data according to the severity of the underlying disease, or to the initial response to therapy.
The de-escalation strategy was not associated with lower costs of antimicrobial therapy. This was mainly due to a median 4-day longer duration of treatment compared with the non-de-escalated group. Recent studies have suggested that <7 days of therapy are sufficient for the treatment of bacteraemia [19, 20]. Another study has shown that ~6 days of intravenous antimicrobial therapy followed by oral therapy would suffice for complicated skin and skin structure infections [21]. Shortening the duration of antimicrobials after the initiation of a culture-directed regimen might lower the overall costs of antimicrobial therapy.
Our observation of a <40% rate of de-escalation among legitimate candidates for this treatment strategy is a concern. Previous studies have shown that the unavailability of conclusive cultures to guide treatment [22, 23] or bacteraemia caused by multiresistant strains should not be treated with de-escalation [24]. Our study, however, enrolled patients who were candidates for de-escalation by excluding these characteristics. A recent study has pointed to the difficulties involved in de-escalating treatment in “real-world” practice, including for non-life-threatening urinary tract infections due to antibiotic-sensitive E. coli [25]. Nonadherence to the practice guidelines that have been issued regarding antimicrobial therapy is not uncommon when treating bacteraemia [26] or pneumonia [27], though it must be overcome with a view to improving clinical outcomes. Emphasis at the bedside by disseminating evidence-based knowledge and by motivating healthcare providers may be a solution [28]. From this perspective, the better clinical outcomes associated with the de-escalation of antimicrobial therapy in our analysis may encourage caregivers to adopt and adhere to this strategy.
The retrospective design of our study is a methodological limitation that is difficult to overcome due to the obvious ethical issues that have to be considered when studying the management of a life-threatening illness. The timing of the initial suspicion and the decision to obtain the first blood culture may greatly influence the appropriate timing of treatment and the outcome of bacteraemia. We could not control this variable, since the data collection started after the prescription of antimicrobials. Likewise, we could not control the frequency and importance of interventions by infectious disease specialists, a variable factor that has a significant impact on the prescription of antimicrobials [5, 22, 29]. Second, the suspicion of “bacteraemia”, the decision to obtain blood cultures, or the choice and doses of antimicrobials depended mostly on the primary care physicians; it was not guided by a protocol or by recommendations made by infectious disease specialists. This might be reflected in the use of first- or second-generation cephalosporins, a narrow spectrum of antimicrobials often used as empirical therapy. Third, the pathogens we studied were limited to four categories of antibiotic-sensitive Gram-negative and -positive microorganisms, which are implicated in the majority of bacteraemia [1–3]. Therefore, our data cannot be extrapolated to other pathogens, particularly antibiotic-resistant species, which might require a different therapeutic strategy. Finally, the source of bacteraemia, particularly abdominal [20], might affect the performance of de-escalation. Further studies are needed to assess the effects of de-escalation for each source in order to optimize the selection of candidates for de-escalation.
In conclusion, our study showed that the de-escalation of antimicrobial therapy for bacteraemia was safe when the pathogen was antibiotic sensitive and the initial empirical treatment was effective, and confirmed the importance of starting empirical therapy early, preferably within <24 h, to increase the chance of survival. Additional, prospective studies should be planned to confirm the safety and efficacy of the de-escalation of antimicrobial therapy against other clinically important drug-resistant strains [8], including extended-spectrum β-lactamase-producing enterobacteriaceae, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus [30].
References
Pittet D, Tarara D, Wenzel RP, et al. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–601.
Richards MJ, Edwards JR, Culver DH, Gaynes RP, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5.
Harbarth S, Ferrière K, Hugonnet S, Ricou B, Suter P, Pittet D, et al. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch Surg. 2002;137:1353–9.
Leibovici L, Drucker M, Konigsberger H, Samra Z, Harrari S, Ashkenazi S, Pitlik SD, et al. Septic shock in bacteremic patients: risk factors, features and prognosis. Scand J Infect Dis. 1997;29:71–5.
Byl B, Clevenbergh P, Jacobs F, Struelens MJ, Zech F, Kentos A, Thys JP, et al. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis. 1999;29:60–6.
Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55.
Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–35.
Sostarich AM, Zolldann D, Haefner H, Luetticken R, Schulze-Roebecke R, Lemmen SW. Impact of multiresistance of gram-negative bacteria in bloodstream infection on mortality rates and length of stay. Infection. 2008;36:31–5.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM, Infectious Diseases Society of America, Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;15(44):159–77.
Kuti EL, Patel AA, Coleman CI, et al. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care. 2008;23:91–100.
Philippe E, Weiss M, Shultz JM, Yeomans F, Ehrenkranz NJ, et al. Emergence of highly antibiotic-resistant Pseudomonas aeruginosa in relation to duration of empirical antipseudomonal antibiotic treatment. Clin Perform Qual Health Care. 1999;7:83–7.
Patterson JE. Antibiotic utilization: is there an effect on antimicrobial resistance? Chest. 2001;119:426S–30S.
Willemsen I, Bogaers-Hofman D, Winters M, Kluytmans J, et al. Correlation between antibiotic use and resistance in a hospital: temporary and ward-specific observations. Infection. 2009;37:432–7.
Niederman MS. De-escalation therapy in ventilator-associated pneumonia. Curr Opin Crit Car. 2006;12:452–7.
Lisboa T, Rello J, et al. De-escalation in lower respiratory tract infections. Curr Opin Pulm Med. 2006;12:364–8.
Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, Rodino FJ, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006;129:1210–8.
Eachempati SR, Hydo LJ, Shou J, Barie PS, et al. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients? J Trauma. 2009;66:1343–8.
Corona A, Wilson AP, Grassi M, Singer M, et al. Prospective audit of bacteraemia management in a university hospital ICU using a general strategy of short-course monotherapy. J Antimicrob Chemother. 2004;54:809–17.
Corona A, Wilson AP, Grassi M, Singer M, et al. Short-course monotherapy strategy for treating bacteremia in the critically ill. Minerva Anestesiol. 2006;72:841–57.
Vick-Fragoso R, Hernández-Oliva G, Cruz-Alcázar J, Amábile-Cuevas CF, Arvis P, Reimnitz P, Bogner JR, et al. STIC study group: efficacy and safety of sequential intravenous/oral moxifloxacin vs intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection. 2009;37:407–17.
De Waele JJ, Ravyts M, Depuydt P, Blot SI, Decruyenaere J, Vogelaers D, et al. De-escalation after empirical meropenem treatment in the intensive care unit: fiction or reality? J Crit Care. 2010;25:641–6.
Rello J, Vidaur L, Sandiumenge A, Rodríguez A, Gualis B, Boque C, Diaz E, et al. De-escalation therapy in ventilator-associated pneumonia. Crit Care Med. 2004;32:2183–90.
Giantsou E, Liratzopoulos N, Efraimidou E, Panopoulou M, Alepopoulou E, Kartali-Ktenidou S, Manolas K, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33:1533–40.
Donaldson AD, Barkham T, et al. De-escalation for amoxicillin-susceptible Escherichia coli: easier said than done. J Hosp Infect. 2010;74:304–5.
Minton J, Clayton J, Sandoe J, McGann H, Wilcox M, et al. Improving early management of bloodstream infection: a quality improvement project. BMJ. 2008;336(7641):440–3.
Barlow G, Nathwani D, Myers E, Sullivan F, Stevens N, Duff R, Davey P, et al. Identifying barriers to the rapid administration of appropriate antibiotics in community-acquired pneumonia. J Antimicrob Chemother. 2008;61:442–51.
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65.
Deresinski S. Principles of antibiotic therapy in severe infections: optimizing the therapeutic approach by use of laboratory and clinical data. Clin Infect Dis. 2007;45(Suppl 3):S177–83.
Pea F, Viale P, et al. Could de-escalation of antibiotic therapy be feasible even for documented methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia? J Trauma. 2009;67:893–4.
Acknowledgments
Nobuaki Shime, M.D., Ph.D., contributed to the design of the study, data collection and analysis, and to the preparation of the manuscript. Sakiko Satake, M.D., contributed to the data collection and analysis. Naohisa Fujita, M.D., Ph.D., contributed to the preparation of the manuscript. This study was partially supported by a grant-in-aid for scientific research (#18591988 for NS) from the Ministry of Education, Science, Sports and Culture of Japan, and was not commercially funded. The authors thank Rodolphe Ruffy, MD, an English-speaking physician, who reviewed the manuscript for style and language. His services were paid for by unrestricted institutional funds.
Conflict of interest
The authors have no potential conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed at the University Medical Centre of Kyoto University Prefectural School of Medicine.
Rights and permissions
About this article
Cite this article
Shime, N., Satake, S. & Fujita, N. De-escalation of antimicrobials in the treatment of bacteraemia due to antibiotic-sensitive pathogens in immunocompetent patients. Infection 39, 319–325 (2011). https://doi.org/10.1007/s15010-011-0116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-011-0116-6