Abstract
Background
Data on time-dependency of external ventricular drainage (EVD)- and lumbar drainage (LD)-associated meningoventriculitis (MV) are scarce and discussions on the subject are controversial; no data exist for infection rates (IR) relative to drainage-days. For this reason, we conducted an observational study to determine time-dependent IRs and to perform a risk factor analysis.
Patients and methods
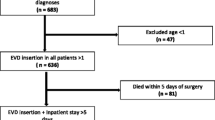
All patients (n = 210) requiring an EVD or LD during an 18-month period in 2007 and 2008 were enrolled and characterized. Data on type and duration of drainage, ICP measurement, number of drainage manipulations, hospital stay and time point of MV were analysed statistically.
Results
A total of 34 MV cases were reported with 17 for each kind of drainage accounting for an IR of 7.5 and 24.7 MV/1000 EVD- and LD-days, respectively. Of these, 28/34 MV (82%) occurred within the first 12 days, and IRs were highest between days 4 and 9. Longer drainage duration (>5 and >9 days, respectively) was correlated with a significant lower risk of MV (p = 0.03; p < 0.001). In this study, significant risk factors for MV were LD [vs. EVD, OR: 2.3 (1.1–4.7); p = 0.01], a previous MV [OR: 7.0 (2.1–23.3); p = 0.002], and neoplasm [OR: 11.6 (3.4–39); p = 0.001]. Simultaneous drainage, ICP and a previous drainage showed no influence on infection.
Conclusion
To the best of our knowledge, this study is the first to provide data on time dependency of EVD- and LD-associated MV-IR based on drainage-days. However, because of the limited scale of our study, it would be desirable to confirm these results in a more powerful larger study. In conclusion, we recommend that future efforts should be made to better identify preventable risk factors as well as to define time periods of higher risk for the difficult-to-diagnose MV infection as a first step in profiling high risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
External ventricular drainage (EVD)- and lumbar drainage (LD)-associated infections are a serious problem in up to 40% of patients, and early diagnosis is difficult [1, 2]. Currently, only a few studies have been published on the time dependence of EVD- and LD-associated meningoventriculitis (MV), and these are often controversially discussed [3–8]. The first infection rates (IRs) correlating the number of infection with the number of drainage-days (DD), have been published only recently [9]. Moreover, time-dependent MV-IRs are lacking. Further, risk factor analysis data of EVD- and LD-associated MV, taking into account such factors as total time of drainage, intracerebral pressure (ICP) and number of drainages, are also scarce [3, 4, 6].
A guideline recommending routine drainage exchange after five days has been implemented in some health centres; however, a comparison of MV incidences with and without routine exchange has revealed no differences [1, 10]. To date, the impact of previous drainage, total time of drainage, ICP at the time of drainage as well as number of drainages, has yet to be clearly defined [3–10]. For device-associated infections, IRs correlating infections with device days are the gold standard [9, 11, 12]. Therefore, we conducted a prospective study in which we correlated EVD- and LD-associated infection rates (IRs) with drainage duration in order to determine the time period associated with the highest risk. To extend our risk factor analysis, we also took into account previous and simultaneous periods of drainage as well as the ICP measurement at the time of drainage.
Patients and methods
All patients (n = 210) having an EVD or LD in the neurosurgery intensive care unit at a tertiary care centre (University Hospital, RWTH Aachen) during an 18-month period in 2007 and 2008 were enrolled. Admitting diagnoses of patients with drainage therapy were subarachnoid haemorrhage (SAH: 42%), intracerebral haemorrhage (ICB: 26%), traumatic brain injury (TBI) with SAH (9%), neoplasm (6%; comprising 9 primary brain tumours: 4 meningioma, 2 astrocytoma, 1 glioblastoma, 1 neurinoma, 1 neurocytoma, and 4 brain metastases), TBI with subdural/epidural haemorrhage (5%), SAH + ICB (4%), TBI without bleeding (3%) and others (5%). Data on type and duration of drainage, ICP measurement, number of drainage manipulations, hospital stay and occurrence of MV were recorded and analysed statistically (Students’ unpaired t-test, Wilcoxon rank sum test, squared Chi-test). The “Portex Epidural Catheter” and “Liquor Drainage Catheter Set” (both Smiths Medical, Kirchseeon, Germany) were used for EVD (n = 158) and LD (n = 79), respectively. Patients with Rickham reservoir were excluded. Neither antibiotic nor silver-impregnated catheters were used. A written protocol was followed for drainage placement without longer tunnelling. No prophylactic antibiotics were given and no catheter changes were performed prophylactically. Cerebrospinal fluid (CSF) analysis was routinely carried out 3 times a week and at additional times if MV was suspected. MV was defined according to a modified CDC definition: isolation of the pathogen (CSF) or a combination of (1) clinical symptoms, (2) at least one pathological laboratory test (CSF: e.g. decreased glucose level) and (3) initiation of antibiotics for suspected MV [12]. For skin commensals, diagnosis required isolation from at least two subsequent CSFs or the presence of clinical symptoms [9].
Results
Total drainage time amounted to 2,954 DDs (EVD: 2,267; LD: 687) and duration was significantly longer for EVD (14.3 ± 9.3 days) than for LD (8.7 ± 7.6 days; p < 0.0001). A total of 34 MV cases were reported with 17 for each kind of drainage, accounting for IRs of 7.5 and 24.7 MV/1,000 EVD- and LD-days, respectively. Of these, 28/34 MV (82%) occurred within the first 12 days, and IRs were highest between days 4 and 9 (10 with each kind of drainage). Time dependence, daily and cumulative incidences and time-dependent IRs are shown in Fig. 1.
For EVD, duration of an individual drainage was significantly shorter in the infected compared to the non-infected patients (9 ± 6 days vs. 15 ± 9 days; p = 0.012). Longer drainage duration (>5 and > 9 days, respectively) was observed to produce a significantly lower risk of MV for EVD (p = 0.01; p < 0.001) and for all drainages (p = 0.03; p < 0.001).
Simultaneous drainage, ICP and a previous drainage were found not to increase the risk of MV. In contrast, significant risk factors were LD (vs. EVD), a previous MV (for LD and for all drainages), neoplasm (EVD) or ICB (LD, all drainages). Finally, the length of stay was significantly longer in MV patients (42 ± 30 vs. 28 ± 19 days; p = 0.001). For risk factor analysis see Table 1.
The 34 MV cases occurred in 28 patients with 4 patients (14%) having more than one episode with different pathogens. In 20 MV cases (59%) a single pathogen was isolated, in one MV case a polymicrobial spectrum was detected. Coagulase negative staphylococci (CoNS) dominated (9/20; 45%), followed by: S. aureus (4/20; 20%); E. coli (3/20; 15%); Enterobacter cloacae (2/20; 10%); and Acinetobacter calcoaceticus-complex, Candida albicans, Enterococcus faecalis and Klebsiella pneumoniae (all 1/20: 5%). Single isolation of skin commensals without symptoms were excluded as contaminants (n = 10) [9].
Discussion
Previous investigations have reported that longer drainage duration represents no increased risk of MV infection [3–8]. Additionally, we found that the risk of becoming infected with MV was significantly lower after day 9 of EVD. The shorter duration of EVD in infected compared to non-infected patients may be due to the removal or change of EVD in the case of MV. For other invasive devices, such as central line catheters, the IRs correlating the number of infections with the number of device days represent the best parameter for documenting the burden of infection [9, 12]. EVD-associated IRs were highest between days 4 and 9 of EVD (12.32 for days 3–6; 14.49 for days 7–9) and were lower before and afterwards. Interestingly, using incidences, some reported a decrease of MV after the first week [8, 9, 13], whereas others concluded that longer duration of drainage does indeed affect the chances of infection [4, 5, 14]. This may be partly explained by the practice of long-tunnelling, which seems to be associated with a time shift to a later occurrence of MV [15]. In line with our previous study [9], LD was found to be associated with a significantly higher risk of MV compared to EVD (IRs: 7.5/1,000 EVD days vs. 24.75/1,000 LD days; OR: 2.3; CI-95: 1.1–4.7; p = 0.01). Although the cause is currently not clear to us, it may be due to the higher risk of contamination associated with the internal position of the LD compared to the external position of the EVD. Despite this difference, time-dependency during the first 10 days seemed to be similar for EVD and LD. However, due to the limited number of EVDs and especially LDs, this finding is preliminary and therefore needs confirmation.
The risk factor analysis revealed that simultaneous or previous drainage, as well as simultaneous ICP measurement, did not influence MV. The findings for EVD are in accordance with previous studies [1, 2, 13], although Lo et al. reported previous EVDs, the cumulative number of EVDs and a previous MV infection as risk factors [7]. Interestingly, a previous MV infection acted as a risk factor only for LD. This finding may be due to replacement of an infected EVD, usually by LD; hence, our number of EVD patients was thereby simultaneously reduced. Moreover, also unlike Lo, et al., we did not identify the female sex as a risk factor [7]. However, we did find that patients suffering from neoplasm (for EVD) or from ICB (for LD) had a significantly higher risk for MV, in line with a previous study [9]. However, we found that patients with MV had a significantly longer hospital stay (42 vs. 28 days; p = 0.001) as also shown in other studies [4, 9].
To the best of our knowledge, this study represents the first to provide data correlating EVD- as well as LD-associated MV-IRs calculated with the duration of drainage. MV was increased by (1) LD in comparison to EVD (OR: 2.3; p = 0.01), (2) a previous MV (for LD, OR: 6.04; p = 0.017), and (3) the underlying disease. Comparison of MV-IR with drainage time revealed the highest IRs to be between days 4 and 9 for both EVD and LD. Later on, the risk of MV seemed to be lowered (EVD: >9 days: OR: 0.18; p = 0.001). Moreover, simultaneous or previous drainage, as well as ICP sensor or number of manipulations, did not increase the risk of MV in our patient group, thus suggesting some characteristics for identifying patients at highest risk. However, because of the limited scale of our study, it would be desirable to confirm these results in a more powerful larger study. In conclusion, we recommend that future efforts should be made to better identify preventable risk factors, as well as to define time periods of greater susceptibility for the difficult-to-diagnose MV infection as a first step in profiling high risk patients.
References
Mayhall CG, Archer NH, Lamb VB, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. N Engl J Med. 1984;310:553–9.
Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr. Ventriculostomy-related infections: a critical review. Neurosurgery. 2002;51:170–81.
Pfisterer W, Mühlbauer M, Czech T, Reinprecht A. Early diagnosis of external ventricular drainage infection: results of a prospective study J. Neurol Neurosurg Psychiatry. 2003;74:929–32.
Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001;33:2028–33.
Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir. 2008;150:209–14.
Rebuck JA, Murry KR, Rhoney DH, Michael DB, Coplin WM. Infection related to intracranial pressure monitors in adults: analysis of risk factors and antibiotic prophylaxis. J Neurol Neurosurg Psychiatry. 2000;69:381–4.
Lo CH, Spelman D, Bailey M, Cooper DJ, Rosenfeld JV, Brecknell JE. External ventricular drain infections are independent of drain duration: an argument against elective revision. J Neurosurg. 2007;106:378–83.
Park P, Garton HJL, Kocan MJ, Thompson BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. 2004;55:594–601.
Scheithauer S, Bürgel U, Ryang YM, Haase G, Schiefer J, Koch S, et al. Prospective surveillance of drain associated meningitis/ventriculitis in a neurosurgery and neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2009;80:1381–5.
Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: result of a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2002;73:759–61.
Edwards JR, Peterson KD, Andrus ML, Dudeck MA, Pollock DA, Horan TC. NHSN Facilities: National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007;35:290–301.
Nationales Referenzzentrum (NRZ) für die Surveillance von nosokomialen Infektionen. Definition nosokomialer Infektionen (CDC-Definitionen). Berlin 2008, 6. Auflage, 19–20. http://www.nrz-hygiene.de/surveillance/its.htm.
Holloway KL, Barnes T, Choi MSS, Ward JD, Young HF, Marmarou A. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;5:419–24.
Schade RP, Schinkel J, Visser LG, Van Dijk JM, Voormolen JH, Kuijper EJ. Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J Neurosurg. 2005;102:229–34.
Khanna RK, Rosenblum ML, Rock JP, Malik GM. Prolonged external ventricular drainage with percutaneous long-tunnel ventriculostomies. J Neurosurg. 1995;83:791–4.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Results were partly presented at the 19. European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Helsinki, Finland; 16–19 May 2009.
Rights and permissions
About this article
Cite this article
Scheithauer, S., Bürgel, U., Bickenbach, J. et al. External ventricular and lumbar drainage-associated meningoventriculitis: prospective analysis of time-dependent infection rates and risk factor analysis. Infection 38, 205–209 (2010). https://doi.org/10.1007/s15010-010-0006-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-010-0006-3