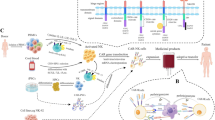
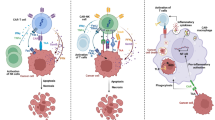
Abstract
Adoptive cell therapy with chimeric antigen receptor (CAR)-engineered T cells (CAR-Ts) has emerged as an innovative immunotherapy for hematological cancer treatment. However, the limited effect on solid tumors, complex processes, and excessive manufacturing costs remain as limitations of CAR-T therapy. Nanotechnology provides an alternative to the conventional CAR-T therapy. Owing to their unique physicochemical properties, nanoparticles can not only serve as a delivery platform for drugs but also target specific cells. Nanoparticle-based CAR therapy can be applied not only to T cells but also to CAR-natural killer and CAR-macrophage, compensating for some of their limitations. This review focuses on the introduction of nanoparticle-based advanced CAR immune cell therapy and future perspectives on immune cell reprogramming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The U.S. Food and Drug Administration (FDA) approved the first chimeric antigen receptor T cell (CAR-T) product, tisagenlecleucel of Novartis in 2017 As a cell therapy for acute lymphoblastic leukemia, a type of hematologic cancer, tisagenlecleucel is based on the enhanced adaptive immune response of engineered T cell [1]. Since then, five additional CAR-T products have been approved by the FDA for hematologic cancers, including diffuse large B cell lymphoma, mantle cell lymphoma, and multiple myeloma [2,3,4,5]. In addition, many new CAR-T products are undergoing clinical trials, and research is under way in laboratories.
CAR, which was first introduced in the mid-1980s, can dramatically enhance T-cell-mediated adaptive immune responses [6]. CAR-Ts can recognize certain tumor ligands and kill the targeted tumor cells specifically. Tumor cells often evade immune responses through a lack of expression of MHC class I molecules. MHC-independent CAR-Ts have significant advantages [6, 7]. CARs also have a higher antigen affinity than typical T cell receptors (TCRs) and form stable and functional immune synapses more quickly [8]. Costimulating molecules incorporated into CARs activate CAR-Ts more potently through the signaling pathway [9]. With these advantages, CAR-Ts have opened a new horizon in cancer immunotherapy, removing tumors in some patients who were unresponsive to chemo-, radiation, and other immunotherapies [10,11,12].
Nevertheless, CAR-T therapy has several limitations. Toxicity, such as systemic cytokine syndrome and neurotoxicity caused by excessive immune activity, is often present at a level that cannot be ignored [13]. Unlike hematologic cancers, there have been very few successful cases of treating solid tumors [11]. In addition, the high cost of production places an excessive burden on patients and pharmaceutical companies. All commercially available CAR-Ts use virus vectors to deliver CAR genes to T cells, which have high transfer efficiency but incur high costs and high regulatory demands in clinical settings, making it difficult for new CAR-Ts to be approved by the FDA [14].
Nanotechnology provides an alternative to conventional CAR-T therapy [15]. Nanoparticles (NPs) have long been studied for their benefits in cancer drug delivery owing to their size, high surface area, targeting ability, and ability to encapsulate a variety of drugs for controlled release [16, 17]. NPs have fully demonstrated their capabilities in many aspects of cancer immunotherapy, such as diagnosis through in vivo tumor imaging [18], reprogramming of the immune-suppressive tumor microenvironment (TME) [19, 20], and cancer vaccine delivery platforms [21]. The physicochemical properties of NPs, such as their size, surface charge, and surface ligands, can be modified by selecting appropriate materials. These modifications make NPs a possible tool for cancer immunotherapy by providing biocompatibility, toxicity control, and efficiency improvement [22,23,24,25]. NP-based CAR therapy can be applied not only to T cells but also to CAR natural killer (CAR-NK) and CAR macrophage (CAR-M) therapies, which compensates for some of their limitations (Table 1) [26, 27]. During the past five years, NP-based strategies have shown remarkable potential for CAR-T, CAR-NK, and CAR-M therapies. In this review, NP-based CAR therapies are introduced, and future prospects for CAR technology are discussed (Table 2).
2 Chimeric antigen receptor (CAR)
2.1 CAR structure
CARs are recombinant antigen receptors that redirect target cell surface antigens and the specificity and function of T cells and other immune cells [37]. CARs consist of an extracellular antigen-binding domain, transmembrane domain, and intracellular signaling domain (Fig. 1A). The signal is transmitted through the intracellular signaling domain of the CAR into T cells upon interaction between the single-chain variable fragment (scFv) and antigen. The extracellular antigen-binding domain is the scFv of a monoclonal antibody that recognizes an antigen [38]. The transmembrane domain consists of a hydrophobic α-helix that anchors CARs to the cellular membrane [39]. The intracellular signaling domain is the functional end of the receptor and is typically composed of a CD3ζ chain containing three immunoreceptor tyrosine-based activation motifs (ITAMs) [40]. Similar to conventional TCRs, CAR-Ts are activated when their ITAMs are phosphorylated [6].
A Schematic illustration of CAR and CAR-T and B Schematic diagram of conventional process of CAR-T therapy using viral vectors (created with BioRender.com)
CAR generation is typically divided according to the structure and composition of the intracellular signaling domain. The first-generation CAR has a single CD3ζ as an intracellular signaling domain, which results in low cytotoxicity and proliferation [40]. Second-generation CAR enhances the cytotoxicity, proliferation, and persistence of CAR-Ts by adding a costimulatory domain (e.g., CD27, CD28, CD134, or CD137) to the intracellular signaling domain [41]. Third-generation CARs are composed of two or more costimulatory domains and have stronger cytokine production and killing abilities [42].
2.2 Process of conventional CAR-T therapy
Genetic modification of autologous T cells from patients for conventional CAR engineering can be implemented using viral vectors (Fig. 1B) [41, 43]. As of 2022, all six FDA-approved CAR-T products use viral vectors, such as lentivirus and retrovirus, for CAR gene transduction [44,45,46, 108, 109]. Mononuclear cells collected from patients through leukapheresis are subjected to T cell isolation using T-cell-specific antibody-coated magnetic beads [47, 48]. For separated T cells to become CAR Ts, they must undergo activation and gene modification. T cells are activated by anti-CD3/CD28 monoclonal antibodies (mAbs) in the form of soluble, magnetic bead-coated, plate-coated, or cell-based antigen-presenting cells (APCs) as endogenous antigen-presenting cells are restricted to ex vivo T cell activation by good manufacturing practice [47, 49, 50]. CAR-expressed T cells must undergo an expansion process using bioreactors, prodigy systems, or artificial APCs to ensure the amount is sufficient before being applied to patients [51]. This complex process increases production cost and duration and limits the widespread use of CAR Ts in cancer treatment.
3 Nanoparticle-based CAR-T therapy
3.1 Ex vivo engineering for CAR-T therapy
To solve the challenges caused by the transduction of CAR genes by viral vectors, researchers have begun to focus on nonviral gene delivery systems, such as electroporation (EP), sonication, and NPs [14, 46]. Viral vectors and non-viral vectors are the same in terms of delivering genes into cells, but have various differences (Table 3). Although transfection efficiency is lower than that of viral vectors, nonviral vectors have strengths in terms of manufacturing costs and processes and have low tumorigenicity and immunogenicity. However, physical methods such as EP, microinjection, and sonication have obvious limitations in that they can damage cells. Owing to their advantages, NPs have attracted attention as gene delivery platforms for nonviral vectors [52]. The relatively low transfection efficiency of nonviral vectors can be overcome through various properties of the nanosystem [53]. A high surface-area-to-volume ratio and small size (nanometer scale) facilitate interaction with the cell membrane and penetration into the target [52, 54]. In addition, NPs provide barriers protecting the loaded genes from immediate endosomal degradation that occurs after cell internalization, keeping genes intact [53]. The biggest feature of NPs is their designability. NPs can be designed with a variety of materials, and their surface can be modified in any way desired. NPs that have been proven to be effective and stable through various assays can be mass-produced, which leads to lower costs in the production process [55].
Polymers are materials used in a large proportion of biomedical industry owing to their biodegradability, easy synthesis and modification, diversity of functions, and high possibility of mass production [56]. Cationic polymers at physiological pH, as gene carriers, can form nanoscale polyplexes through electrical interactions with negatively charged genetic materials. Polyethyleneimine (PEI), poly(L-lysine), and poly(2-dimethylamino)ethyl methacrylate are representative polymers widely used as materials for polymer-based nanoparticles (PNPs) [56, 57]. As they are electrically cationic, they are internalized by electrostatic binding to the anionic cell membrane when they meet the target cells. In many cases, they escape the endosomes through the proton sponge effect [58].
Yu et al. [29] reported in vitro CAR-T engineering using self-assembled nanoparticles (SNPs) with less toxicity and a higher CAR expression. SNPs were prepared by self-assembly using plasmid DNA (pDNA), polyethylene glycol (PEG) and the cationic polymers polyamidoamine (PAMAM) and PEI, and pDNA was successfully encapsulated (Fig. 2A). The SNPG1/800 (adamantane-grafted polyamidoamine (Ad-PAMAM) dendrimer: G1, CD-PEI: 800 Da) showing the highest T cell transfection efficiency was screened by controlling the generation of PAMAM and the molecular weight of PEI. As a result, the EGFRvIII CAR gene delivered by SNP in Jurkat cells showed much less toxicity than PEI and showed tenfold higher luciferase activity than Lipofectamine 2000 transfection reagent (Fig. 2B). Unlike the control group, Jurkat CAR + cells showed specific recognition of EGFRvIII-positive tumor cells (Fig. 2C). These results demonstrated that jurkat T cells can be effectively engineered using SNP to transiently express CARs that recognize EGFRvIII and exhibit specific reactivity to target tumor cells.

(Reproduced from a previous report [29] with permission from Dove Medical Press Limited)
A Schematic diagram of preparation and formulation screening of SNPs by comparing T cell transfection efficiency depending on the type of Ad-PAMAM and CD-PEI, and anticancer effect of anti-EGFRvIII CAR-T. B Transfection efficiency of Lipofectamine 2000 (Lipo2000), PEI800, or SNPG1/800 via luciferase activity (*p < 0.05). C Confocal microscopy images of interaction between EGFRvIII-positive HuH7 cells (green) and Jurkat T cells (red) transfected with or without pEGFRvIII-CAR@SNPsG1/800. Scale bars, 20 μm.
In 2018, the FDA approved a lipid nanoparticle (LNP)-based drug for RNA interference (RNAi) to treat polyneuropathy caused by transthyretin amyloidosis, which opened the era of LNP-RNA [59, 60]. In general, LNPs are composed of ionizable lipids, cholesterol, helper lipids, PEG lipids, and nucleic acids [61]. At acidic pH, positively charged ionizable lipids can condense negatively charged nucleic acids into LNPs and contribute to endosomal escape by fusion with the endosome membrane. Toxicity is minimized because of their electrically neutral properties at physiological pH [62, 63]. Components other than ionizable lipids are involved in the stability of LNP, formation of the bilayer structure, and reduction of aggregation [61].
mRNA has several advantages over DNA in the selection of nucleic acid substances for transfection. Translation occurs directly in the cytoplasm without passage through the nuclear membrane or transcription [64]. In addition, mRNA is unlikely to be integrated into the host genome and enables rapid and transient expression of the target protein [65]. Considering these advantages, the LNP-mRNA-based strategy has potential for effective CAR-T therapy.
Billingsley et al. [30] reported that LNPs can deliver mRNA to primary human T cells with low toxicity compared with EP. Twenty-four different ionizable lipids were combined with cholesterol, phospholipid, and lipid-anchored PEG and mixed using a microfluidic device with CAR mRNA to form LNP (Fig. 3A). The library of 24 ionizable lipids consists of a combination of three alkyl chains and eight polyamine cores; consequently, the combination of C14 (1,2-Epocytetradecane) and the polyamine was determined to be optimal. To determine usability in the CAR-T manufacturing process, the anti-CD19 CAR gene was delivered to primary T cells to evaluate transfection efficiency, viability, and cancer cell killing in vitro. Ionizable LNPs expressed CAR with an efficiency similar to that of EP in primary T cells, whereas they had a significant advantage in terms of toxicity to cells when compared with viability (Fig. 3B). The ability to kill tumor cells in vitro also did not lag behind other CAR-Ts engineered using conventional methods (Fig. 3C). These results indicate the ability of LNP to deliver mRNA to primary human T cells and the potential of mRNA-based CAR-T therapy.

(Reproduced from a previous report [30] with permission from the American Chemical Society)
A Schematic diagram of LNP preparation via microfluidic device. B Viability of primary T cells treated with crude LNP, pure LNP, and EP group. n = 3 biological replicates, *p < 0.05 in paired t-test to EP. C Comparison of tumor cell killing ability in vitro of CAR-Ts engineered by EP, LNP (C14-4), lentiviral vector, and control. n = 3 wells. *p < 0.05, **p < 0.01 in the paired t-test to control.
3.2 In vivo engineering of CAR-T therapy
Current methods of ex vivo CAR-T generation are labor-intensive and require considerable cost and time owing to the complexity of the process. As explained above, autologous T cells extracted from patients undergo a series of processes, such as isolation, genetic modification, and expansion. Patients who fail to withstand the excessive cost and long time of these processes cannot even undergo treatment and die [47, 51, 66, 67, 110]. Thus, it was necessary to find an “in vivo engineering” system that would make the T-cell engineering process simpler. With this concept, it is no longer necessary to extract and isolate T cells from patients, and proliferation of powerfully genetically modified T cells can occur spontaneously in the bodies of patients.
To generate CAR-Ts in vivo, a platform capable of safely transferring genetic material to the target T cells is required. The targeting strategy is considered in addition to the characteristics required for ex vivo engineering. The surfaces of NPs are commonly coated with monoclonal antibodies specific to T cell surface ligands [110]. When NPs loaded with the genetic material are injected, internalization of the NPs occurs through the interaction of the surface antigen of the target T cells and the surface antibody of the NPs while circulating in the living body, so that the T cells can express CARs [68]. The generated CAR-Ts encounter the target cancer cells and are activated, causing rapid cell division and cytotoxicity to the target tumor.
To the best of our knowledge, the first attempt to program CAR-Ts in vivo was reported in 2017. Smith et al. [31] devised a polymer-based nanocarrier to deliver CAR-encoded DNA to T cells in vivo. Unlike when transferring genes to highly pure isolated T cells outside the living body, gene-loaded nanocarriers must be able to target T cells. Furthermore, if the genetic material is DNA, it should be possible to deliver it to the nucleus. To achieve effective pDNA delivery into T cells, the surface of the NP formed by a cationic polymer, poly-(β-amino ester) (PBAE), was coated with a polyglutamic acid (PGA)-conjugated CD3e f(ab′)2 antibody (Fig. 4A). In the meantime, PBAE was conjugated with peptides composed of microtubule-associated sequences (MTASs) and nuclear localization signals (NLSs), which contribute to the transfer of anti-CD19 CAR pDNA to the nucleus using a microtubule transport mechanism in the cell. In this study, bolus injections of NPs induced rapid and efficient programmed peripheral T cells to recognize leukemia cells (approximately 6% CD3+ on day 6 and approximately 20% CD3+ on day 12; Fig. 4B). The bioimaging results for the distribution of the NPs demonstrate the validity of the targeting capability through CD3 antibody. Most of them without CD3 antibody moved to the liver, but the others with CD3 antibody were accumulated at significant levels in the spleen, lymph node, and bone marrow. In addition, it was confirmed that CD3-targeting NPs were binded more than 12 times compared to non-targeting NP in the peripheral T cell. As a result of injecting the 194-1BBz(+ iPB7 transposase) nanocarrier into the mouse leukemia model, tumors were eradicated in 7 out of 10 mice, and the average survival rate improved by 58 days (Fig. 4C). Furthermore, PNPs made from PBAE and PGA-conjugated antibodies are available as mRNA carriers for in vivo CAR expression [69]. This strategy exhibited disease regression at levels similar to those of ex vivo-engineered lymphocytes.

(Reproduced from a previous report [31] with permission from Nature Publishing Group)
A Schematic diagram of fabrication of anti-CD3e f(ab′)2-coated PBAE NP carrying CAR plasmid DNA for in vivo CAR generation. B Flow cytometry of peripheral T cells after injection of NP encapsulating 194-1BBz_2A_GFP genes. C Survival of animals following the various groups of CAR-T therapy.
In vivo CAR-T therapy can be applied not only to cancer, but also to such diseases as cardiac fibrosis [70]. Cardiac fibroblasts activated by myocardial damage secrete excessive extracellular matrix, leading to cardiac fibrosis, and the disease has a significant adverse effect on the state and function of the myocardium, but the level of treatment has been inadequate. Although CAR-Ts targeting activated fibroblasts have been devised and their effectiveness has been demonstrated, continuous in vivo expression of fibroblast-specific CARs has been a problem because it can interfere with the normal functions of fibroblasts. In this regard, temporary expression of CAR in vivo using the mRNA-LNP system is a promising strategy. Aghajanian et al. reported the treatment of cardiac injury through CD5 targeting LNPs encapsulated with fibroblast activation protein (FAP)-specific CAR-encoding mRNA (Fig. 5A) [32]. The expression of luciferase in the spleen only appeared in the group injected with CD5/LNP-Luc is one of the results supporting the effectiveness of this targeting strategy. In contrast to nontargeted (IgG/LNP-FAPCAR) and targeted LNP with GFP (CD5/LNP-GFP), consistent populations (17.5%–24.7%) of FAPCAR + T cells were identified only in mice that received CD5/LNP-FAPCAR (Fig. 5B). In addition, splenocyte flow cytometry revealed that the expression of FAR in other immune cells was not significant. FAP-CAR T cells prevented the progression of fibrosis and restored heart function by removing FAP from the activated fibroblasts (Fig. 5C). This study is of great significance in that CAR-T engineering in vivo is not limited to cancer immunotherapy but has potential as a treatment for various diseases.

(Reproduced from a previous report [32] with permission from American Association for the Advancement of Science)
A Schematic illustration of T cell engineering via FAP CAR-mRNA/LNP against activated fibroblast. B Percentage of FAPCAR positive T cells isolated from mice 48 h after injection of 10 μg of mRNA-LNPs. C Histologic analysis of coronal cardiac sections of animals and quantification of fibrosis. Picrosirius red staining indicates collagen (pink). Inset shows magnification of the LV myocardium. Scale bar, 100 μm.
4 NP-based CAR-NK therapy
Despite continuous advances, the limitations described above have prompted the application of CAR technology to other immune cells. NK cells play a role similar to that of cytotoxic T cells in the innate immune response. Although there are obvious differences between NK cells and cytotoxic T cells, their functions are similar in that they recognize and destroy target cells. In addition, the differences between NK cells and cytotoxic T cells make the immune responses of the two cells complementary to each other [71]. The unique characteristics of NK cells, which differ from those of T cells, serve as an advantage in the application of CAR technology [28, 72].
First, the target cell recognition method for NK cells is MHC class I independent. Rather, killer Ig-like receptors (KIR) in NK cells can inhibit the activation of NK cells through interaction with MHC class I molecules. This mechanism reduces the aggression of NK cells against host cells that normally express MHC and allows them to maintain their aggression against tumor cells with reduced MHC expression [28, 72, 73]. Furthermore, there is a significant degree of freedom in selecting the cell source to produce CAR-NK compared with CAR-T [28]. In the CAR-T case, if homogeneous T cells are used, there is a risk of developing graft-versus-host disease (GVHD) owing to T cells exhibiting alloreactivity for such reasons as human leukocyte antigen (HLA) mismatch [28, 74,75,76]. The target recognition of T cells, based on determining whether the antigen originates from itself or not, provides the possibility of attacking cells expressing homogeneous nonself HLA types. To prevent this immunological risk, autologous T cells of patients should be used as a source of CAR-T engineering; however, many patients do not have sufficient T cell populations. There continue to be reports of deaths without attempting treatment owing to the inability to wait for this process, which requires considerable time, or because the conditions are not met [48, 77,78,79]. However, NK cells, which can only discriminate MHC expression, are free from GVHD pathogenesis after transplantation [80]. This is supported by the fact that GVHD progression was not observed in 11 patients who received HLA-mismatched anti-CD19 CAR-NK treatment for non-Hodgkin’s lymphoma and chronic lymphocytic leukemia [81]. Therefore, it is possible to produce “off-the-shelf” CAR-NK cells that can be used immediately when needed by patients with NK cells extracted from a number of possible sources, including cell lines, peripheral blood-derived NK cells, umbilical cord blood-derived NK cells, and stem-cell-derived NK cells, without the need to produce patient-specific products [28, 72, 82, 83].
Second, in terms of toxicity, CAR-NK treatment is safer than CAR-T. One challenge that CAR-T treatment must overcome is the uncontrollable toxicity that sometimes occurs, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome. This toxicity results from the excessive release of inflammatory cytokines, such as IL-1, IL-6, IL-10, and TNF from CAR-T immunity [13, 84, 85]. However, cytokines that mediate NK cell immunity differ from these inflammatory cytokines in their profiles, including IFN-γ and GM-CSF [84, 85]. In a previous study that revealed that CAR-NK cells were free from GVHD, there was no CRS or neurotoxicity [81]. Moreover, even if an unexpected “extratarget tumor” phenomenon occurs, the problem can be naturally solved by the rapid depletion of CAR-NK cells owing to the short life cycle of NK cells, such as two weeks. However, the therapeutic effect appears only temporarily, and thus continuous injection of CAR-NK cells is necessary [28, 83, 86].
Third, CAR-NK cells have other routes that can be activated in addition to CAR. KIR, which functions as an important inhibitory regulator of NK cell-mediated cytotoxic immune responses, basically implements “self-tolerance” to stop attacks by MHC class I normal cells. However, when the expression of MHC class I molecules decreases as an immune avoidance method in infected or tumor cells, inhibitory signals through the immunoreceptor tyrosine-based inhibitory motif, an intracellular domain of KIR, are not transmitted, enabling NK cells to maintain their cytotoxicity [87,88,89]. CD16 expressed on NK cell surfaces mediates antibody-dependent cytotoxicity (ADCC) in NK cells. CD16 (also called FcγRIII) has a low affinity for Ig G1 or G3, which can be combined with the Fc region of the antibody bound to target antigens to activate ADCC of NK [90]. Bi- or tri-specific killer engines, made using a combination of scFVs of antibodies, can improve CD16-mediated ADCC in the NK cells [91]. In conclusion, for CAR-NK cells, anticancer effects can be expected as a route separate from CAR, so it is possible to retain cytotoxicity to target tumors with insufficient expression of CAR-specific antigen.
With the introduction of nanotechnology, CAR-T treatment has made many advances, and it can also be applied to CAR-NK treatment. Transferring CAR genes to immune cells to create CAR immune cells is the first step in CAR therapy. However, the transfection efficacy of viral vectors in NK cells is much lower than that in T cells; hence, alternative transfection methods should be considered [92, 93]. Although the transfection efficacy could be increased by attempting to transfer genes using electroporation, the stress applied to the cell itself cannot be ignored as described above.
In recent studies, there have been attempts to increase the transfection efficacy through nanoparticles and realize CAR-NK with improved effect [88]. Several characteristics of NPs can be strategically used to increase transfection efficiency. The first is to increase the interaction with the NK cell by controlling the surface and size of the NPs. The addition of targeting moiety, such as anti-CD16 or anti-NK1.1, to the surface of NPs may increase the interaction between NK cells and NPs. Also, the size of NPs affects the endocytosis of NK cells. It is known that the smaller the diameter of NPs, the more advantageous it is to internalize to NK cells through clathrin-mediated endocytosis [94]. The second is that NP can increase endosomal escape. LNPs made of lipids sensitive to the pH environment can mediate endosomal escape of cargo through fusion with the endosome membrane [62, 63]. These strategies have actually been attempted in CAR-T engineering. In addition, since NPs have a significant degree of freedom in selecting cargo, it is also possible to try using surface-active peptides such as melittin [95].
McKinlay et al. developed the charge-altering releasable transporter (CART) as an effective mRNA delivery system [96]. The CART is designed as an oligomer (carbonate-b-α-amino ester) that is cationic at low pH but undergoes rearrangement at pH 7.4. Owing to these properties, oligomers can form complexes with polyanionic molecules, such as mRNA, at low pH levels, protect them, and deliver them intracellularly. Subsequently, proteins are effectively expressed by the translation of the released mRNA, and the oligomers are biologically degraded. Using the CART system, Wilk et al. achieved efficient NK cell transfection and the generation of cytotoxic primary anti-CD19 CAR NK cells (Fig. 6A) [33]. BDK- O7:N7:A13 was selected from several candidate groups through high-efficiency lipid library screening. Compared with lipofectamine (< 1%), a commercially available transfection reagent, CART BDK-O7:N7:A13 more successfully delivered antihuman CD19-41BB-CD3ζ CAR mRNAs to isolated resting human NK cells (> 10%) (Fig. 6B). NK cells expressing antihuman CD19 CAR via CART transfection showed superiority in CD107a, TNF-α, IFN-γ, and indicators of activation level. Antihuman hCAR-transfected NK cells showed enhanced killing of the target cells (human CD19 + Nalm6), whereas the negative control antimurine mCAR did not (Fig. 6C). Considering these results, the CART system may be an attractive technology as it can increase the efficiency of transfection through chemical modifications or lipid library alteration [97, 98].

(Reproduced from a previous report [33] with permission from the American Society of Hematology)
A Schematic diagram of CART/mRNA polyplex preparation. B Percentage of antihuman CD19-41BB-CD3ζ CAR expression by isolated primary resting human NK cells. C Percentage of dead target cells (CD19 positive Nalm6: filled circles, CD19 knockout Nalm6: open circles) after 20:1 (transfected NK:target) ratio coculture for 6 h before flow cytometric analysis.
PEI-coated magnetic NPs (MF-NPs) have also been designed for use in CAR-NK therapy in a multifunctional manner [34]. The cationic polymer (PEI) and magnetic core (Zn/Fe) form core–shell structured NPs through adhesive molecules called polydopamine. The PEI shell provides an electrostatic attraction for anti-EGFR CAR pDNA to bind, transfecting NK cells, and the magnetic core is conceived for in vivo tracking and imaging through magnetic resonance (MR) (Fig. 7A). Via endocytosis, MF-NP/anti-EGFR CAR pDNA was internalized in NK-92MI cells, and exhibited a significant level of in vitrotransfection efficiency (≈60%). This led to a high level of antitumor effect without any specific toxicity to NK cells (Fig. 7B). Stability and efficiency were improved compared with those of the viral vector or EP. The biological behavior of CAR-NK cells in vivo can be observed not only through MR but also through a fluorescent imaging device owing to the near-infrared radiation (NIR) fluorescent dye (cyanine 7) conjugated to the PEI shell (Fig. 7C and D). The application of this multifunctional NP can contribute to simplifying and efficiently transforming the process of CAR-NK therapy.

(Reproduced from a previous report [34] with permission from Elsevier)
A Schematic illustration of multifunctional nanoparticle (MF-NP) and its bioapplications. B Quantitative analysis data of EGFR-CAR expression on NK-92MI surface and cancer cell killing capability of EGFR-CAR expressing NK-92MI cells according to the concentration of MF-NPs. in vivo fluorescence, optical imaging C and MR imaging D of mice transplanted with MF-NP–labeled NK-92MI cells.
5 Nanoparticle-based CAR-M therapy
Macrophages are the most abundant innate immune cells in solid tumors and constitute up to 50% of the cell mass in the tumor microenvironment (TME) of solid tumors [111]. Since macrophages are phagocytic innate cells and professional APCs, they serve as a bridge between innate and adaptive immunity [99]. Macrophages prime and activate T cells through antigen presentation after phagocytosis by tumor cells [100]. In general, macrophages can be divided into classically activated M1 macrophages and alternatively activated M2 macrophages according to their activation state and function [101]. M1 macrophages are pro-inflammatory, which secrete pro-inflammatory cytokines and chemokines to help eliminate tumor cells, whereas M2 macrophages are anti-inflammatory, which induce immune suppression and promote tumor progression [102].
CAR NK cell therapy has several advantages over CAR-T therapy, but most of the limitations of CAR-T cell therapy can be applied to CAR-NK cell therapy, including target antigen selection, migration to tumor sites, and immunosuppressive TME. In addition, NK cells are not immune cells that are mainly present in the TME and have a short half-life of less than 10 days [103]. This property can be advantageous when severe toxicity occurs; however, repeated administration may be required for a sustained therapeutic response. These limitations made it difficult for NK cells to be selected as an alternative to overcome the limitations of T cells for solid tumors, which eventually led to the emergence of CAR-M [104]. CAR-M therapy refers to the delivery of specific CAR genes to macrophages, with the ability to bind to the tumor cell surface through specific antigen identification and subsequently activate macrophage activity against tumor cells [105]. CAR-M also share several characteristics and limitations with CAR-T but has two unique advantages over CAR-T: immune cell trafficking and infiltration in (immunosuppressive) TME.
CAR-M is generally produced ex vivo by using viral vectors. This approach is a complex, time-consuming, and expensive process, limiting its clinical application, and there are concerns about the oncogenic potential of viral vectors [106]. In contrast, NP-based strategies have the advantage of using a nonviral vector to reduce the experimental burden and avoid tumorigenicity. Kang et al. reported the in vivo programming of CAR-M1 macrophages through nanocomplex-mediated gene delivery (Fig. 8A) [35]. In this study, mannose-conjugated polyethylenimine (MPEI) was used for macrophage-targeted delivery through macrophage-overexpressed mannose receptors [107, 112]. The prepared nanocomplex (MPEI/pCAR-IFN-γ) was formed by electrostatic interactions between the positively charged MPEI and negatively charged CAR-IFN-γ pDNA. The nanocomplex effectively transfected M2 bone-marrow-derived macrophages (BMDMs) to express CAR (with an average transfection efficiency of approximately 14%) and showed a 20-fold increase in IFN-γ expression compared with the control group. Transfected macrophages showed CAR-mediated antigen-specific phagocytosis, and it was confirmed that the M2-to-M1 phenotype shift occurred in vitro and in vitvo owing to IFN-γ release in an autocrine and paracrine manner. In addition, it was confirmed that 82.0% ± 27.0% of CAR expression in the tumor during intratumor injection and 63.4% ± 14.4% after intraperitoneal injection were macrophages, confirming that effective macrophage targeting was achieved (Fig. 8B). MPEI/pCAR-IFN-γ increased the CD8+ T cell immune response, showing the highest CD8/Treg ratio compared with the control group and the best tumor growth inhibitory effect (Fig. 8C and D). This approach has the potential to overcome several challenges of current CAR-T therapy, including the complex procedure of CAR immune cell fabrication and unsatisfactory clinical outcomes of solid tumor therapy.

(Reproduced from a previous report [35] with permission from Wiley‐VCH)
A Schematic diagram of MPEI/pDNA(CAR-IFN-γ) nanocomplex preparation and antitumoral mechanisms of CAR-M1 macrophages. B Representative fluorescence images (left) of major organs and tumor 48 h after an intra-tumoral injection of vehicle (negative control) or MPEI/pCAR-IFN-γ. Flow cytometry data (right) of CAR + cell percentages in the tumor tissues (n = 3). C Mean tumor growth profiles of Neuro-2a tumor-bearing mice after various treatments (n = 15). D Survival rate of Neuro-2a-bearing mice after various treatments (n = 6).
Ye et al. reported effective CAR expression in primary macrophages through LNP-based mRNA delivery (Fig. 9A) [36]. Through transient CAR expression using LNP-mediated mRNA delivery, an alternative method that can reduce adverse effects (such as host genome integration, on-target, and off-tumor effects) was used [30]. In this study, a top-performing mRNA modification (N1-methylpseudouridine, N1mψ) that showed the highest expression level (53.1%) among mRNA types and an optimized mRNA delivery system (9322-O16B LNP) for macrophages were screened. The LNP (9322-O16B LNP/N1mψ-mRNA) prepared using this method showed a high eGFP-positive cell population of 51.1% for primary BMDM (Mφ) transfection and 60.7% for M1 macrophages (Fig. 9B). Compared with primary BMDM treated with empty LNP, the CAR-Mφ and CAR M1 groups showed significant cytotoxicity against FLuc+ human B lymphoma, for which the luminescence levels were reduced by 32.54% and 22.50%, respectively (Fig. 9C). This study highlights the great potential of adoptive cell therapy for B lymphoma using CAR-macrophages in vitrothrough LNP formulation and mRNA modification.

(Reproduced from a previous report [36] with permission from the American Chemical Society)
A Schematic illustration of CAR mRNA delivery to murine primary macrophages and killing assay of FLuc+ human B lymphoma. B Flow cytometry data of transfection efficiency of m1ψ-eGFP mRNA in Mφ and M1 treated in different formulations of 9322-O16B. C Killing assay of FLuc+ B lymphoma following different treatments (Mφ, p < 0.05*; M1, p < 0.05*). Data are presented as mean ± SD (n = 3).
6 Summary and future perspectives
CAR-T therapy has shown great success in the treatment of hematological cancers. Nevertheless, CAR-T therapy has limitations, such as its limited efficacy for solid tumors, complicated processes, and excessive manufacturing costs. The introduction of nanotechnology can overcome the limitations of conventional CAR-T cell therapy. Owing to their unique properties, NPs can not only serve as a delivery platform for drugs but also target specific cells. NP-based CAR therapy can be applied not only to T cells but also to CAR-NK and CAR-M, compensating for their limitations. In this review, three strategies were introduced in which NPs have been applied to advances in CAR therapy: (i) NP-based CAR-T therapy, (ii) NP-based CAR-NK therapy, and (iii) NP-based CAR-M therapy.
Ex vivo engineering using viral vectors leads to tumorigenicity risks, complicated procedures, and high costs. NP-based CAR-T therapy has been proposed as an alternative to viral vectors in ex vivo engineering. Ad-PAMAM, PEI, PBAE and ionizable LNP successfully produced CAR-T cells in vivo or ex vivo by transferring pDNA or mRNA using an NP system and showed lower toxicity, similar treatment effects, and lower cost than conventional CAR-Ts [29, 30]. These results are expected to increase access to CAR-T generation, which is currently only possible in highly specialized centers.
The in vivo engineering strategy of CAR-T therapy is designed to compensate for the limitations of ex vivo engineering systems, such as labor intensity and the significant cost and time required because of the complexity of the process. With this concept, it is no longer necessary to extract and isolate T cells from patients, and the proliferation of strongly genetically modified T cells can occur spontaneously in the body of the patient. To generate CAR-T cells in vivo, additional targeting strategies that can deliver genetic material to the target T cells are considered. Introduction of pDNA and mRNA delivery using T cell targeting antibody to PNPs. It was confirmed that the anticancer effect of in vivo T-cell manipulation was similar to that of the existing method of infusing ex vivo engineered CAR-Ts [31]. In contrast, CD5 targeting LNP encapsulated with FAP-specific CAR-encoding mRNA prevented the progression of fibrosis and restored cardiac function by removing FAP [32]. This research is significant in that CAR-T engineering in vivo is not limited to cancer immunotherapy but has potential as a therapeutic agent for various diseases.
NP-based CAR-NK therapy uses the unique properties of T cells and other NK cells. NK cells have an MHC class I independent recognition method, allowing them to maintain aggression against tumor cells with reduced MHC expression. NK cells have a significantly lower transfection efficiency using viral vectors than T cells, and NPs can be an alternative. The CART system delivers CAR mRNA more successfully to isolated resting human NK cells than the commercially available transfection reagent Lipofectamine [33]. PEI-coated magnetic NPs have also been utilized in CAR-NK therapy in a multifunctional manner [34]. These MF-NPs have improved stability and efficiency compared with viral vectors or EPs, and the biological behavior of CAR-NK cells in vivo can be observed by MR. Furthermore, CAR-modified NK cells can act as carriers of drugs by themselves, showing synergistic anticancer effects with chemotherapy.
CAR-M therapy involves the delivery of specific CAR genes to macrophages with the ability to bind to the tumor cell surface through specific antigen identification and subsequently activate macrophage activity against tumor cells. CAR-M shares several characteristics and limitations with CAR-T but has two unique advantages over CAR-T treatment: immune cell trafficking and infiltration into the immunosuppressive TME. The MPEI/pDNA nanocomplex was engineered for in vivo CAR-M generation, and its effective anti-solid tumor capability showed that CAR expression in vivo can be applied to other immune cells as well as T cells [35]. In addition, effective CAR expression in primary macrophages through LNP-based mRNA delivery has been reported previously [36]. This study highlights the potential of adoptive cell therapy for CAR-M B lymphoma in vitro through LNP formulation and mRNA modification.
During the past five years, NP-based CAR therapy strategies have shown remarkable growth and potential. Despite high interest, NP-based CAR strategies still fall short compared with viral vectors in terms of experience and sufficient skills to understand and interact with the cellular machinery. Continued research on CAR applications and a better understanding of nanotechnology's interaction with cells will contribute to the development of effective CAR-based anticancer drugs. New nanoparticles designed based on basic research results are expected to maximize the therapeutic effect of not only cancer but also various incurable diseases through effective CAR expression. Recently, gene delivery and vaccine technologies using LNPs encapsulated in mRNA or siRNA have been clinically approved, and LNP-based SARS-CoV-2 vaccines have been a huge success. With this, NP-based genetic engineering technology is expected to develop more rapidly and grow as a promising cancer treatment. Taken together, we believe that the NP-based CAR Therapy introduced in this review will be an effective cancer treatment option in the future.
References
Mullard A. FDA approves first CAR T therapy. Nat Rev Drug Discovery. 2017;16:669.
Reagan PM, Friedberg JW. Axicabtagene ciloleucel and brexucabtagene autoleucel in relapsed and refractory diffuse large B-cell and mantle cell lymphomas. Future Oncol. 2021;17:1269–83.
Mullard A. FDA approves fourth CAR-T cell therapy. Nat Rev Drug Discov. 2021;20:166.
Sharma P, Kanapuru B, George B, Lin X, Xu Z, Bryan WW, et al. FDA Approval summary: Idecabtagene vicleucel for relapsed or refractory multiple myeloma. Clin Cancer Res. 2022;28:1759–64.
Moretti C. CAR-T Cell Therapy May Have ‘Big Future’in Relapsed/Refractory Multiple Myeloma. 2022.
Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–26.
Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol. 2021;12:636568.
Watanabe K, Kuramitsu S, Posey AD Jr, June CH. Expanding the therapeutic window for CAR T Cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front Immunol. 2018;9:2486.
Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunol. 2019;8:e1049.
Charrot S, Hallam S. CAR-T cells: future perspectives. Hemasphere. 2019;3:e188.
Dana H, Chalbatani GM, Jalali SA, Mirzaei HR, Grupp SA, Suarez ER, et al. CAR-T cells: early successes in blood cancer and challenges in solid tumors. Acta Pharm Sin B. 2021;11:1129–47.
Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev. 2019;33:98–110.
Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, et al. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40:367.
Lukjanov V, Koutná I, Šimara P. CAR T-cell production using nonviral approaches. J Immunol Res. 2021;2021:6644685.
Balakrishnan PB, Sweeney EE. Nanoparticles for enhanced adoptive T cell therapies and future perspectives for CNS tumors. Front Immunol. 2021;12:600659.
Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–59.
Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2012;64:24–36.
Zhang J, Li C, Zhang X, Huo S, Jin S, An FF, et al. In vivo tumor-targeted dual-modal fluorescence/CT imaging using a nanoprobe co-loaded with an aggregation-induced emission dye and gold nanoparticles. Biomaterials. 2015;42:103–11.
Saeed M, Gao J, Shi Y, Lammers T, Yu H. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics. 2019;9:7981–8000.
Shin S, Lee J, Han J, Li F, Ling D, Park W. Tumor Microenvironment modulating functional nanoparticles for effective cancer treatments. Tissue Eng Regen Med. 2022;19:205–19.
Park YM, Lee SJ, Kim YS, Lee MH, Cha GS, Jung ID, et al. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 2013;13:177–83.
Sanità G, Carrese B, Lamberti A. Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization. Front Mol Biosci. 2020;7:587012.
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193.
Park W, Heo YJ, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24.
Gammon JM, Dold NM, Jewell CM. Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget. 2016;7:15421–43.
Nawaz W, Xu S, Li Y, Huang B, Wu X, Wu Z. Nanotechnology and immunoengineering: how nanotechnology can boost CAR-T therapy. Acta Biomater. 2020;109:21–36.
Balakrishnan PB, Sweeney EE. Nanoparticles for Enhanced Adoptive T Cell Therapies and Future Perspectives for CNS Tumors. Front Immunol. 2021;12:600659.
Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41:119.
Yu Q, Zhang M, Chen Y, Chen X, Shi S, Sun K, et al. Self-assembled nanoparticles prepared from low-molecular-weight PEI and low-generation PAMAM for EGFRvIII-chimeric antigen receptor gene loading and T-cell transient modification. Int J Nanomedicine. 2020;15:483–95.
Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–89.
Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–20.
Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–6.
Wilk AJ, Weidenbacher NL, Vergara R, Haabeth OAW, Levy R, Waymouth RM, et al. Charge-altering releasable transporters enable phenotypic manipulation of natural killer cells for cancer immunotherapy. Blood Adv. 2020;4:4244–55.
Kim KS, Han JH, Park JH, Kim HK, Choi SH, Kim GR, et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials. 2019;221:119418.
Kang M, et al. Nanocomplex-mediated in vivo programming to chimeric antigen receptor-M1 macrophages for cancer therapy. Adv Mater. 2021;33:e22103258.
Ye Z, Chen J, Zhao X, Li Y, Harmon J, Huang C, et al. In vitro engineering chimeric antigen receptor macrophages and T cells by lipid nanoparticle-mediated mRNA delivery. ACS Biomater Sci Eng. 2022;8:722–33.
Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor designmaking better chimeric antigen receptors. Cancer Discov. 2013;3:388–98.
Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017;108:1109–18.
Guedan S, Calderon H, Posey AD Jr, Maus MV. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–56.
Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22.
Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120:26–37.
Yu S, Yi M, Qin S, Wu K. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer. 2019;18:125.
Stock S, Schmitt M, Sellner L. Optimizing manufacturing protocols of chimeric antigen receptor T cells for improved anticancer immunotherapy. Int J Mol Sci. 2019;20:6223.
Labbé RP, Vessillier S, Rafiq QA. Lentiviral vectors for T cell engineering: clinical applications, bioprocessing and future perspectives. Viruses. 2021;13:1528.
Rodríguez-Otero P, Prósper F, Alfonso A, Paiva B, San Miguel JF. CAR T-cells in multiple myeloma are ready for prime time. J Clin Med. 2020;9:3577.
Irving M, Lanitis E, Migliorini D, Ivics Z, Guedan S. Choosing the right tool for genetic engineering: clinical lessons from chimeric antigen receptor-T cells. Hum Gene Ther. 2021;32:1044–58.
Fesnak A, O’Doherty U. Clinical development and manufacture of chimeric antigen receptor t cells and the role of leukapheresis. Eur Oncol Haematol. 2017;13:28–34.
Allen ES, Stroncek DF, Ren J, Eder AF, West KA, Fry TJ, et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion. 2017;57:1133–41.
Zhang H, Zhao P, Huang H. Engineering better chimeric antigen receptor T cells. Exp Hematol Oncol. 2020;9:34.
June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–12.
Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015.
Jin S, Leach JC, Ye K. Nanoparticle-mediated gene delivery. In: Foote RS, Lee JW, editors. Micro and nano technologies in bioanalysis: methods and protocols. Totowa: Humana Press; 2009. p. 547–57.
Riley MK, Vermerris W. Recent advances in nanomaterials for gene delivery-a review. Nanomaterials (Basel). 2017;7:94.
Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107:459–66.
Kumar ARK, Shou Y, Chan B, L K, Tay A. Materials for improving immune cell transfection. Adv Mater. 2021;33:e2007421.
Rai R, Alwani S, Badea I. Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers (Basel). 2019;11:745.
Kafshdooz T, Kafshdooz L, Akbarzadeh A, Hanifehpour Y, Joo SW. Applications of nanoparticle systems in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol. 2016;44:581–7.
Cardle II, Cheng EL, Jensen MC, Pun SH. Biomaterials in chimeric antigen receptor T-cell process development. Acc Chem Res. 2020;53:1724–38.
Garber K. Alnylam launches era of RNAi drugs. Nat Biotechnol. 2018;36:777–8.
Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14:1084–7.
Riley RS, Kashyap MV, Billingsley MM, White B, Alameh MG, Bose SK, et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci Adv. 2021;7:eaba1028.
Han X, Zhang H, Butowska K, Swingle KL, Alameh MG, Weissman D, et al. An ionizable lipid toolbox for RNA delivery. Nat Commun. 2021;12:7233.
Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–34.
Zou S, Scarfo K, Nantz MH, Hecker JG. Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells. Int J Pharm. 2010;389:232–43.
Oh S, Kessler JA. Design, assembly, production, and transfection of synthetic modified mRNA. Methods. 2018;133:29–43.
Xu X, Huang S, Xiao X, Sun Q, Liang X, Chen S, et al. Challenges and clinical strategies of CAR T-cell therapy for acute lymphoblastic leukemia: overview and developments. Front Immunol. 2021;11:569117.
Gomes-Silva D, Ramos CA. Cancer immunotherapy using CAR-T cells: from the research bench to the assembly line. Biotechnol J. 2018;13:10.1002/biot.201700097.
Dinauer N, Balthasar S, Weber C, Kreuter J, Langer K, von Briesen H. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26:5898–906.
Parayath NN, Stephan SB, Koehne AL, Nelson PS, Stephan MT. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11:6080.
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–3.
Rosenberg J, Huang J. CD8(+) T cells and NK cells: parallel and complementary soldiers of immunotherapy. Curr Opin Chem Eng. 2018;19:9–20.
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975.
Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124.
Graham C, Jozwik A, Pepper A, Benjamin R. Allogeneic CAR-T cells: more than ease of access? Cells. 2018;7:155.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99.
Sanber K, Savani B, Jain T. Graft-versus-host disease risk after chimeric antigen receptor T-cell therapy: the diametric opposition of T cells. Br J Haematol. 2021;195:660–8.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor t cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–53.
Elahi R, Heidary AH, Hadiloo K, Esmaeilzadeh A. Chimeric antigen receptor-engineered natural killer (CAR NK) cells in cancer treatment; recent advances and future prospects. Stem Cell Rev Rep. 2021;17:2081–106.
Zhang L, Meng Y, Feng X, Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10:12.
Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147.
Marofi F, Rahman HS, Thangavelu L, Dorofeev A, Bayas-Morejón F, Shirafkan N, et al. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther. 2021;12:200.
Lowry LE, Zehring WA. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front Immunol. 2017;8:1061.
Thielens A, Vivier E, Romagné F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr Opin Immunol. 2012;24:239–45.
Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73.
Dębska-Zielkowska J, Moszkowska G, Zieliński M, Zielińska H, Dukat-Mazurek A, Trzonkowski P, et al. KIR receptors as key regulators of NK cells activity in health and disease. Cells. 2021;10:1777.
Capuano C, Pighi C, Battella S, De Federicis D, Galandrini R, Palmieri G. Harnessing CD16-mediated NK cell functions to enhance therapeutic efficacy of tumor-targeting mAbs. Cancers (Basel). 2021;13:2500.
Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells. Methods Mol Biol. 2016;1441:333–46.
Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25:1769–81.
Robbins GM, Wang M, Pomeroy EJ, Moriarity BS. Nonviral genome engineering of natural killer cells. Stem Cell Res Ther. 2021;12:350.
Lee NK, Kim SN, Park CG. Immune cell targeting nanoparticles: a review. Biomater Res. 2021;25:44.
Hou KK, Pan H, Schlesinger PH, Wickline SA. A role for peptides in overcoming endosomal entrapment in siRNA delivery - A focus on melittin. Biotechnol Adv. 2015;33:931–40.
McKinlay CJ, Vargas JR, Blake TR, Hardy JW, Kanada M, Contag CH, et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc Natl Acad Sci U S A. 2017;114:E448–56.
Benner NL, Near KE, Bachmann MH, Contag CH, Waymouth RM, Wender PA. Functional DNA delivery enabled by lipid-modified charge-altering releasable transporters (CARTs). Biomacromolecules. 2018;19:2812–24.
Testa S, Haabeth OAW, Blake TR, Del Castillo TJ, Czerwinski DK, Rajapaksa R, et al. Fingolimod-conjugated charge-altering releasable transporters efficiently and specifically deliver mRNA to lymphocytes in vivo and in vitro. Biomacromolecules. 2022;23:2976–88.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55.
Zhou X, Liu X, Huang L. Macrophage-mediated tumor cell phagocytosis: opportunity for nanomedicine intervention. Adv Func Mater. 2021;31:2006220.
Galván-Peña S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420.
Sylvestre M, Crane CA, Pun SH. Progress on modulating tumor-associated macrophages with biomaterials. Adv Mater. 2020;32:1902007.
Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–65.
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–53.
Villanueva MT. Macrophages get a CAR. Nat Rev Immunol. 2020;20:273.
Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011.
Sarwar HS, Ashraf S, Akhtar S, Sohail MF, Hussain SZ, Rafay M, et al. Mannosylated thiolated polyethylenimine nanoparticles for the enhanced efficacy of antimonial drug against Leishmaniasis. Nanomedicine (Lond). 2018;13:25–41.
Makita S, Imaizumi K, Kurosawa S, Tobinai K. Chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma: opportunities and challenges. Drugs Context. 2019;8:212567.
Gregory TK, Schade H, Berdeja JG. Latest developments in cellular therapy for multiple myeloma. Oncol Hematol Rev. 2020;16:111–8.
Xin T, Cheng L, Zhou C, Zhao Y, Hu Z, Wu X. In-Vivo induced CAR-T cell for the potential breakthrough to overcome the barriers of current CAR-T cell therapy. Front Oncol. 2022;12:809754.
Wang S, Yang Y, Ma P, Zha Y, Zhang J, Lei A, et al. CAR-macrophage: an extensive immune enhancer to fight cancer. EBioMedicine. 2022;76:103873.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
Acknowledgements
This study was supported by the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (MSIT), Republic of Korea (2021R1A2C4001776 to W.P., 2020H1D3A1A04105814 to W.P., 2019R1C1C1006300 to C.G.P., and 2022R1A4A2000776 to C.G.P.), a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (Ministry of Science and ICT, Ministry of Health & Welfare) (KFRM 21A0501L1 to W.P. and C.G.P.), and by Sungkyunkwan University and BK21 FOUR (Graduate School Innovation) funded by the Ministry of Education (MOE) and NRF of Korea.
Author information
Authors and Affiliations
Contributions
SS, PL, JH, S-NK, JL, D-HP, and TP collected data and evidence. SS and PL drew the figures. D-HP, TP, JM, CGP, and WP formulated the study concept and design. SS and PL wrote the manuscript. JM, CGP, and WP revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
No animal experiments were carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, S., Lee, P., Han, J. et al. Nanoparticle-Based Chimeric Antigen Receptor Therapy for Cancer Immunotherapy. Tissue Eng Regen Med 20, 371–387 (2023). https://doi.org/10.1007/s13770-022-00515-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00515-8