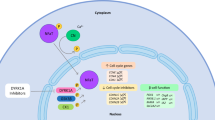
Abstract
Background:
Endogenous pancreatic β-cell regeneration is a promising therapeutic approach for enhancing β-cell function and neogenesis in diabetes. Various findings have reported that regeneration might occur via stimulating β-cell proliferation, neogenesis, or conversion from other pancreatic cells to β-like cells. Although the current scenario illustrates numerous therapeutic strategies and approaches that concern endogenous β-cell regeneration, all of them have not been successful to a greater extent because of cost effectiveness, availability of suitable donors and rejection in case of transplantation, or lack of scientific evidence for many phytochemicals derived from plants that have been employed in traditional medicine. Therefore, the present study aims to investigate the effect of gymnemic acid (GA) on β-cell regeneration in streptozotocin-induced type 1 diabetic rats and high glucose exposed RIN5-F cells.
Methods:
The study involves histopathological and immunohistochemical analysis to examine the islet’s architecture. Quantitative polymerase chain reaction (qPCR) and/or immunoblot were employed to quantify the β-cell regeneration markers and cell cycle proliferative markers.
Results:
The immunoexpression of E-cadherin, β-catenin, and phosphoinositide 3-kinases/protein kinase B were significantly increased in GA-treated diabetic rats. On the other hand, treatment with GA upregulated the pancreatic regenerative transcription factor viz. pancreatic duodenal homeobox 1, Neurogenin 3, MafA, NeuroD1, and β-cells proliferative markers such as CDK4, and Cyclin D1, with a simultaneous downregulation of the forkhead box O, glycogen synthase kinase-3, and p21cip1 in diabetic treated rats. Adding to this, we noticed increased nuclear localization of Pdx1 in GA treated high glucose exposed RIN5-F cells.
Conclusion:
Our results suggested that GA acts as a potential therapeutic candidate for endogenous β-cell regeneration in treating type 1 diabetes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder caused by immune-mediated destruction of pancreatic β-cells, resulting in lifelong dependency on exogenous insulin therapy [1]. Despite its association with numerous side effects (injection site complications, insulin resistance and allergies), insulin therapy cannot prevent or delay the progression of diabetes in patients with uncontrolled hyperglycemia [2, 3]. Another therapeutic approach called islet transplantation might overcome the dependency of exogenous insulin, but it has more limitations including, inadequate islet donors, transplantation rejection, a lifelong requirement of immune suppression, and poor patient survival after transplantation [4]. These facts accentuate the need for research on endogenous β-cell regeneration through proliferation, neogenesis, reprogramming of non β-cells into differentiated β-like cells, transdifferentiation from progenitor cells, and differentiation of induced pluripotent stem cells (iPSCs) to new β-cells [5, 6].
Several researchers have shown that small molecules mediate stem cell differentiation into insulin producing cells by activating pancreatic endocrine markers such as pancreatic duodenal homeobox 1 (Pdx1), sex determining region Y-box 9 (SOX9), forkhead box A2 (FOXA2), Nkx6.1, insulin, and C peptide [7, 8]. From the scientific investigations, it is clear that usage of biological/chemical inducers and phytochemicals promote β-cell neogenesis through the activation of Pdx1, neurogenin 3, and musculoaponeurotic fibrosarcoma oncogene family protein A (MafA), which were the most widely explored factors for β-cell regeneration [9,10,11,12]. Despite being cost-effective and easy to intake, they have minimal risk complications, making them a better choice than other synthetic agents/chemicals.
Pdx1, is a crucial transcription factor that regulates gene expression of Neurogenin 3, MafA, and neurogenic differentiation D1 (NeuroD1), is required for the β-cell development and regeneration [13], where MafA, NeuroD1 acts as a marker of β-cell maturation [14, 15]. In addition, MafA binds to the insulin gene's promoter region, promoting insulin expression in response to glucose [16]. Further, E-cadherin, a calcium-dependent transmembrane protein, is a member of the cadherin superfamily that is predominantly expressed in the epithelial cells of numerous tissues, including the endocrine pancreas [17] is involved in homotypic cell–cell interactions [18]. One of the intracellular mechanisms of ECAD in regulating the β-cell proliferation is known to occur through β-catenin, phosphoinositide 3-kinases (PI3Ks)/protein kinase B(AKT) and maintains the cellular architecture [19, 20]. The cytoplasmic domain of E-cadherin is bound to intracellular proteins known as catenin’s that assist in linking the complex to the actin cytoskeleton [21, 22], to other proteins involved in signal transduction and nuclear transcription factors [23].
Gymnemic acid (GA) is triterpenoid saponin isolated from the Gymnema sylvestre leaf [24], and its extract was reported to have anti-hyperglycemic activity in T2DM patients [25]. However, the exact mechanism of action through its primary bioactive compound is not clearly elucidated. Therefore, the present study aimed to investigate GA's role in promoting the β-cell regeneration, making them a possible approach for endogenous β-cells regeneration in treating type 1 diabetes.
2 Materials and methods
2.1 Animals and chemicals
Male albino Wistar rats were procured from Central Animal House facility, Dr. AL Mudaliar Post Graduate Institute of Basic Medical Sciences, taramani campus, University of Madras, Chennai, India. The experiments were conducted with the guidelines approved by the Institutional Animal Ethical Committee (IAEC No: 01/10/2020). The animals were housed by maintaining three rats per cage in the large spacious sterile cage under maintaining controlled temperature (25 ± 2 °C) with 12/12 h light/dark cycle, and the animals were given commercial rat feed and water ad libitum. A single dose of intraperitoneal injection (i.p) of streptozotocin (STZ) (60 mg/kg b.w) dissolved in buffer (0.1 M citrate buffer, pH 4.5) was used to induce type 1 diabetes in rats [26].
Streptozotocin (STZ) was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Gymnemic acid was procured from Amalth lifecare, India. The primary antibodies for Pdx1(1:1000), NeruroD1 (1:500), Neurogenin 3 (1:1000), MafA, CyclinD1(1:1000), CDK4 (1:1000), p21cip1 (1:1000), E-cadherin (1:1000), β-catenin (1:1000), PI3K/AKT (1:1000), pAKT (1:1000), forkhead box A2 (FOXA2) (1:500), glycogen synthase kinase-3 (GSK3β) (1:1000), β-actin (1:1000), Lamin B1(1:1000), and secondary anti-rabbit and anti-mouse antibodies were purchased from Abcam, Cambridge, UK. The enhanced chemiluminescence (ECL) kit was purchased from Bio-Rad (Hercules, CA, USA). AccuBind ELISA kit was purchased from Monobind Inc. (Lake Forest, CA, USA). Primers were procured from Bioserve Biotechnologies (India) Pvt Ltd. (Secunderabad, India). Bovine serum albumin (BSA) and other fine chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA). Lipofectamine, Pdx1 (Validated siRNA), Roswell Park Memorial Institute (RPM medium), Glucose, Antibiotics and Fetal Bovine Serum, rabbit Alexa Fluor-conjugated secondary antibody, were purchased from Invitrogen (Thermo Fisher Scientific Inc., Waltham, MA, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Himedia Pvt Ltd. (Mumbai, India).. RIN5-F cells were procured from National Centre for Cell Science (NCCS), Pune. All chemicals and consumables used were of analytical grade and were purchased from Medox Biotech (Chennai, India). Sisco Research Laboratories Pvt. Ltd (SRL, India), Genei (Bangalore), and CDH (Central Drug House Pvt. Ltd) Mumbai, India.
2.2 In vivo studies
Young rats (2 months old, weighing around 130–150 g) were used for this study. All animals were divided into three groups with six animals in each as; Group 1—young healthy control rats; Group 2—rats were administered with streptozotocin (STZ) 60 mg/kg b.w (i.p) and fasting blood glucose was measured after 48 h, animals which showed above 300 mg/dl served as diabetic control; Group 3—Diabetic rats supplemented with GA (150 mg/kg b.w; oral administration) from 3rd day of the experiment for 30 days. GA was dissolved in distilled water (dH2O) and supplemented by oral gavage. The effective GA dose was fixed based on the earlier studies conducted in our laboratory [27] and there is no changes observed in the drug control rats (data not shown) compared to that of control group.
2.3 Biochemical analysis
The pancreas was excised and washed with ice-cold saline for histopathological and biochemical analyses. Fasting blood glucose level was measured by the One Touch SelectSimple BGMS [28] at the different time periods (0th, 15th and 30th day), and Plasma insulin levels were determined by the ELISA kit (Accu bind) method, and values were expressed in µU/ml.
2.4 Histopathological examination
The pancreatic tissue was fixed with 10% formalin for 48 h and then tissues were embedded in molten paraffin wax. Then, sections of the pancreas (5 μm thick) were obtained using a microtome, The section were deparaffinized using xylene for 10 min and followed by rehydration through descending grades of 100% ethanol for 3 min, 95% and 80% ethanol for 1 min at each concentration respectively. After rehydration the sections were rinsed with distilled water followed by staining with haematoxylin and eosin (H&E) dye. Then, the slides were finally examined under a light microscope (Accu Scope, Commack, NY, USA) for histopathological observation.
2.5 Immunohistochemistry analysis
The immuno-expression analysis of insulin in pancreatic tissues was done by Immunostaining [29]. The sections were cut at 5 µm thickness using weswox semi-automated microtome, mounted on slides, and dried at 37 °C. Dried sections were rehydrated by xylene to 100%, 70%, 50% ethanol, and dis.H2O. Then, heat activated antigen retrieval step was done using antigen retrieval buffer (Tris–EDTA buffer, pH 9 (10 mM Tris Base, 1 mM EDTA and 0.05% Tween 20) through pressure cooker and allowed to boil for 5 min. After, slides were cooled to room temperature for 30 min. Then the sections were rinsed with 0.1 M TBS three times (Tris buffered saline pH 7.4) followed by incubation in 1% H2O2 in TBS for 5 min and permeabilized with 0.4% Triton X-100 for 30 min. The sections were blocked by 3% BSA (Bovine Serum Albumin, containing 0.2% Triton X-100 in TBS) for 30 min. After, sections were incubated with the primary antibody in TBS, pH 7.4 for 24 h at 4 °C. Then, sections were washed in TBS thrice, and then incubated with secondary IgG-conjugated horseradish peroxidase antibody (1:1000) in TBS, pH 7.4, for 1 h at room temperature. Visualization analysis was performed by incubation in 3,3-diaminobenzidine (DAB) for 5 min. To test the specificity of the immunostaining, control sections were processed in an identical manner devoid of both primary and secondary antibody. All sections were then washed for 10 min in TBS, mounted on slides, dried, dehydrated in increasing grades of ethanol, cleared in xylene, and mounted with DPX and cover slipped. These sections were scanned and examined under a light microscope (Accu scope).
2.6 RNA extraction and quantitative RT-PCR
The pancreatic tissue RNA was isolated by using the Trizol (RNAiso Plus) method. The total RNA to cDNA conversion was carried out with the Bio-Rad–iScript cDNA synthesis reagent kit. Real-time quantitative PCR was carried out with KAPA SYBR Green Master Mix in ABI Quantstudio 6 Flex system. The following primers were used, Pdx1: forward, 5′-TTCCCGAATGGAACCGAGAC-3′ and reverse 5′-TCCACTTCATGCGACGGTTT-3′; NeuroD1: forward, 5′-AAAAGCCCAGACCTCGTCTC-3′ and reverse 5′-AAGGGCTGGTGCAATCAGTT-3′; Neurogenin 3: forward, 5′-CCCGGATGACGCCAAACTTA-3′ and reverse 5′-TCGAGTGCCTCCACTACCTT -3′; MafA: forward, 5′-TTTGGTGCAGGGACGATCTG-3′ and reverse 5′-CCACACTTCTGTACCACGCT-3′; Insulin: forward, 5′-CCAAGTCCCGTCGTGAAGT-3′ and reverse 5′-GGTGCAGCACTGATCCACAA-3′; β-actin: forward, 5′-ATCATTGCTCCTCCTGAGCG-3′ and reverse 5′-GAAAGGGTGTAAAACGCAGCTC-3′. The thermal cycling condition: 50 °C for 2 min and 95 °C for 10 min once, then followed by 95 °C for 15 s and 60 °C for 1 min for 40 cycles. β-actin served as an endogenous control. All the reactions were performed in triplicates, mean Ct was used for analysis the 2 − ΔΔCt [30] method was applied to evaluate the relative mRNA expression level.
2.7 Western blotting analysis
Pancreatic tissue samples were homogenized in Tris–HCl buffer (0.01 M, pH 7.4), and protein concentrations were determined [31]. For immunoblotting, pancreatic tissue lysate was mixed with sample loading buffer where each sample contained 50 μg concentration. The protein samples were separated by SDS–Polyacrylamide Gel Electrophoresis [32]. Followed by transferring to the polyvinyl difluoride membrane (PVDF). Membranes were blocked in Tris-buffered saline with Tween-20 (TBST) containing 5% (w/v) BSA (Bovine serum albumin) for 1 h at room temperature. Then, the transferred membrane was incubated with primary antibodies Pdx1, Neurogenin 3, MafA, NeruroD1, cyclin D1, CDK4, p21cip1, E-cadherin, β-catenin, PI3K, AKT, pAKT, FOXO1, GSK3β, β-actin, and Lamin B1 for overnight at 4 °C. After that, incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Immuno expression bands were detected by enhanced chemiluminescence reagent mixture (an equal volume of luminol with H2O2), and images were captured on a Bio-Rad Chemidoc XRS imaging system.
2.8 In vitro studies
Monolayer cultures of rat insulinoma cell lines (RIN5-F) (NCCS, Pune, India) were cultured in RPMI medium supplemented with heat-inactivated fetal bovine serum (10% FBS), 1% sodium pyruvate, 1% Non-Essential Amino Acids (NEAA), and 1% L-glutamine and Antibiotics (mixture of 1% penicillin/streptomycin/nystatin). Cells were incubated in T25 tissue culture flasks at 37 °C in a humidified atmosphere (5% CO2 & 95% air environment) with and without high glucose (25 mM) in the medium. Gymnemic acid was dissolved in the RPMI medium. Preliminary studies were done with different concentrations of gymnemic acid to fix the exposure time as 24 h and optimum (data not shown) dosage of 1 µM using cell viability by MTT assay. Based on the dose fixation studies (Data shown), the optimum concentration of GA was about 1 μM for 24 h in RIN5-F cells, while 25 mM high glucose treatment in RIN5-F cells was found to be 48 h and these concentrations were chosen for further studies. (1) Control—RIN5-F cells were cultured with RPMI-1640 medium; (2) High glucose (HG)—RIN5-F cells were cultured in RPMI-1640 medium with high glucose (25 mM) concentration for 48 h; (3) HG + GA—RIN5-F cells were culture in RPMI-1640 medium with high glucose (25 mM) concentration for 48 h and treated with GA (1 µM) for last 24 h.
2.9 Transient transfection with Pdx1-specific siRNA in RIN5-F cells
For gene silencing, RNA primers are complementary to rat Pdx1 (Validated siRNA). Cells were effectively transfected with the annealed RNA primer pair using Lipofectamine RNAiMAX reagent (Invitrogen Life Technologies) following the guidelines provided by the manufacturer. The cells were transfected with scrambled siRNA (30 pmol) as controls. Further, the transfection efficiency was confirmed by qPCR of Pdx1 at different time intervals (0 h, 6 h, 12 h, 24 h, and 48 h). Wherein, approximately 70% knockdown of Pdx1 is observed at 24 h. For immunofluorescence studies (Pdx1 localization), cells were divided into five groups as follows: (1) Control—RIN5-F cells were culture with RPMI-1640 medium; (2) Scrambled—RIN5-F cells were treated with scrambled siRNA (30 pmol); (3) High glucose (HG)—RIN5-F cells were culture in RPMI-1640 medium with high glucose (25 mM) concentration for 48 h; (4) siRNA—RIN5-F cells were treated with Pdx1-specific siRNA(30 pmol) alone for last 24 h; (5) siRNA + GA—RIN5-F cells were treated with Pdx1-specific siRNA and 1 µM GA for last 24 h; (6) HG + siRNA + GA—RIN5-F cells were treated with HG (25 mM) for 48 h, transfected with Pdx1-specific siRNA and for the last 24 h co-treatment with 1 µM GA. At the end of the experiment period, cells were washed with PBS and analysed for the localization of Pdx1 by immunofluorescence assay. Pdx1 localization was used to elucidate the mechanism of GA in the activation of Pdx1.
2.10 Immunofluorescence
Cells were seeded onto chambered slides upon coverslip. After 24 h, the RIN5-F cells were treated with GA for 24 h, and then the RIN5-F cells were washed thrice with PBS for 5 min and fixed with 400 µl of 4% formaldehyde in PBS for 20 min at room temperature. The cells were permeabilized with 0.25% Triton-X-100 for 10 min and rinsed thrice with PBS. Then the cells were blocked with 1% BSA in PBS-T (0.1% Tween-20) for 30 min and were treated with primary antibody (1:1000) at 4 °C at the humid chamber overnight. After rinsing with PBS thrice, the cells were incubated with an Alexa Fluor-conjugated secondary antibody (1:1000) for 1 h at dark and counterstained with Hoechst for 10 min. Finally, rinsed with PBS and the slides were mounted. Immunofluorescence images were captured by fluorescence microscope using a 40 × objective.
2.11 Statistical analysis
The statistical data are presented as Mean ± Standard Error of Mean (SEM). Differences between each group were analysed by one-way analysis of variance (ANOVA) using the Graphpad Prism version 8.0. Tukey's post-hoc-test was performed for inter-group comparisons; Group 2 rats are compared with group 1 rats; Group 3 rats are compared with group 2 rats. Statistical significance represented with asterisks (*represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, ns no significant).
3 Results
3.1 Effects of GA on FBG, plasma insulin and insulin mRNA levels
The increased sugar level is the critical hallmark for diabetes. Our results on fasting blood glucose (FBG) depicted a substantially increased (p < 0.001) level in diabetic rats compared with that of control rats at different intervals of the experimental period. On the other hand, diabetic rats treated with GA exhibited a significant decrease (p < 0.001) in FBG levels on day 15th and day 30th when compared with that of the untreated group (Fig. 1A). In addition to increased FBG, diabetic rats also presented with reduced plasma insulin levels and decreased insulin mRNA expression in the pancreatic tissues. However, diabetic rats administered with GA exhibited significantly (p < 0.001) increased plasma insulin (Fig. 1B) and higher pancreatic insulin mRNA levels (Fig. 1C) compared to diabetic rats. Therefore, our data suggest that the gymnemic acid can control blood glucose and improve plasma insulin levels in GA treated rats.
A Assessment of fasting blood glucose, B Plasma insulin levels and C Insulin mRNA expression levels in control and experimental groups. Data are shown as mean ± SEM, n = 6 for each group. Group 2 compared with Group 1; Group 3 compared with Group 2. Statistical significance represented with asterisks (*represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, ns no significant)
3.2 Impact of GA on histopathological and immunohistochemical analysis of insulin in rat pancreas
The histopathological examination of pancreatic tissues from control rats showed a distinctive cellular structure of the pancreas. In comparison, diabetic rats indicated severe vacuolation and devastation of the islets. However, diabetic rats supplemented with GA depicted a lessened cellular degeneration of pancreatic islets than diabetic rats (Fig. 2A). Immunohistochemical analysis of insulin in the pancreas of various experimental groups showed positive insulin expression in control rats, while the number of positive insulin expression was found to be decreased in the diabetic rats. A remarkable restoration was observed in the GA treated diabetic rats compared to streptozotocin treated animals (Fig. 2B).
A Pancreatic tissues histology of diabetic rats and treatment with GA (H & E × 40). Group 1 (G1)—Tissue section of control rats showed normal cellular architectures with the islet (dotted line) of Langerhans and healthy blood vessels. Group 2 (G2)—STZ induced diabetic rats showed severe vacuolation, degranulation, and massive destruction of the islets were observed (arrowheads). Group 3 (G3)—Diabetic rats supplemented with gymnemic acid showed significant improvement in cellular architecture with reduced degeneration of pancreatic islets. B Immunohistochemistry evaluation of insulin in pancreatic tissues of experimental rats. G1—Control rats showed the typical cellular architecture and positive insulin islets (dotted line). G2—Diabetic rats show a drastic decline of insulin content (arrowheads). G3—Diabetic rats treated with gymnemic shows increase of insulin content (arrowheads) and the moderate number of positive islets
3.3 Beneficial role of GA on pancreatic β-cell regenerative markers in pancreas of experimental rats
To study the effect of GA on β-cell regeneration, the mRNA and protein expression studies were performed in pancreatic tissues. Our results revealed that the expressions (Pdx1, Neurogenin 3, MafA and NeuroD1) were significantly reduced at the levels of both transcription (p < 0.01) and translation (p < 0.001) in response to streptozotocin administration. Impressively, we observed a significant increase in expression of β-cell regeneration markers in GA treated diabetic rats (Fig. 3A, B), emphasizing the role of GA in boosting the β-cell regeneration by modulating regenerative markers.
Pancreatic mRNA and protein expression levels of regeneration markers viz. Pdx1, Neurognin 3, NeuroD1, and MafA in GA treated diabetic rats. A Relative mRNA expression levels and B protein expression levels. Data are shown as mean ± SEM of three independent observations in each group. Group 2 compared with Group 1; Group 3 compared with Group 2. Statistical significance represented with asterisks (*represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, ns no significant)
3.4 Effects of GA on cell cycle markers and E-cadherin, β-catenin, PI3K, AKT, pAKT, FOXO1, GSK3β in rat pancreas
We performed immunoblot analysis for E-cadherin, β-catenin, PI3K, AKT and pAKT, and cell cycle progression markers such as Cyclin D1 and CDK4 in the pancreas of various experimental rats. Downregulated protein expressions were observed for E-cadherin, β-catenin, PI3K, AKT, pAKT and cell cycle markers (Cyclin D1 and CDK4) and upregulated protein expression were detected in cell cycle inhibitor proteins (FOXO1, GSK3β, and p21cip1) in diabetic rats. Nevertheless, upon GA treatment to diabetic rats showed significantly higher expressions of E-cadherin, β-catenin, PI3K, AKT, pAKT, Cyclin D1 and CDK4, and reduced expression levels of FOXO1, GSK3β and p21cip1 (Fig. 4A–C). These data revealed that GA promotes the proliferation of β-cells and maintains the islet architecture via persuading the expression of E-cadherin, β-catenin, and PI3K/AKT.
GA mediated activation of PI3K/AKT and cell cycle proliferative markers in various experimental groups. A Immunoblot analysis of E-cadherin, β-catenin, PI3K, AKT, pAKT and B FOXO1, GSK3β. C Cell cycle markers Cyclin D1, CDK4, and p21cip1. Data are shown as mean ± SEM of three independent observations in each group. Group 2 compared with Group 1; Group 3 compared with Group 2. Statistical significance represented with asterisks (*represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, ns no significant)
3.5 Role of GA in modulating insulin mRNA expression and Pdx1 in RIN5-F cells exposed to high glucose
Pdx1 is generally involved in the regulation of insulin transcription, β-cell neogenesis, proliferation, differentiation and β-cell functioning. Therefore, to explore the insulin mRNA expression and cellular localization of Pdx1 were carried out in high glucose exposed RIN5-F cells. The lowered expressions of Pdx1 and insulin were witnessed in high glucose exposed cells than that of the control cells. While GA treatment to high glucose exposed cells showed slightly higher nuclear localization of Pdx1 and increased insulin mRNA expression than that of high glucose exposed cells (Fig. 5A, B). This results evidence that GA is capable to assist in β-cell regeneration and its function by eliciting expression of Pdx1 and insulin in RIN5-F cells.
3.6 Influence of GA on RNAi-mediated gene silencing of Pdx1 in RIN5-F cells
To confirm the role of GA in stimulating β-cell regeneration by Pdx1, we performed siRNA mediated transient knockdown of Pdx1 in RIN5-F cells treated with/without high glucose and GA treatment, and the nuclear localization of Pdx1 was confirmed by immunofluorescence assay. The transfection efficiency of siRNA (transient method) was confirmed by qPCR analysis (Fig. 6A), where cells transfected with Pdx1 specific siRNA at a concentration of 30 pmol for 24 h, showed a marked decline in the levels of Pdx1 mRNA and this time period was used for further studies. Cells transfected with scrambled siRNA were used as a negative control (Fig. 6B). Our immunofluorescent results demonstrated that Pdx1 siRNA alone treated cells and high glucose treated cells depicted a decrement in the expression of Pdx1 when compared to that control cells. On the other hand, GA treatment to both siRNA and/or high glucose treated cells recorded a slightly increase in the levels of nuclear Pdx1 (Fig. 6B). Therefore, our results suggested that GA enhances β-cell regeneration by increasing the expression of Pdx1.
A The siRNA transfection efficiency analysis by qPCR. Cells were transfected with scrambled (Scr) siRNA and siRNA targeting Pdx1 for various experimental time (6 h, 12 h, 24 h, 48 h and 72 h). Statistical significance represented with asterisks (*represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, ns no significant). B Influence of GA on RNAi mediated gene silencing of Pdx1 in RIN-5F cells. Cells were transfected with scrambled (Scr) siRNA and siRNA targeting Pdx1 for 24 h in the concentration of 30 pmol. Subcellular localization of Pdx1 in RNAi mediated transient knockdown in RIN-5F cells along with treated with/without high glucose and GA treatment (Magnification: 40×)
4 Discussion
Diabetes is a severe public health problem, with more than 552 million people afflicted by 2030, as a report given by the International Diabetes Federation [33]. The major life-threatening diabetic complications such as micro and macro-vascular diseases, including retinopathy, neuropathy, and nephropathy are associated with an uncontrolled hyperglycemic condition in diabetic patients [34]. The challenges in insulin therapy, initial therapeutic treatment for T1DM have made the researchers work on several small molecules that mediate self-renewal, lineage differentiation, reprogramming, and regeneration of β-cells [35]. Various studies emphasized the anti-diabetic properties of phytochemicals obtained from plants, promoting pancreatic β-cell functions [36, 37]. However, scientific evidence in the biological mechanism behind their β-cell reprogramming and regeneration has not been established. As a result of which these small molecules cannot be taken up widely accepted as a drug. The current study investigated the β-cell regeneration in streptozotocin-induced diabetic rats after exogenous oral supplementation of GA. Induction of diabetes was confirmed by hyperglycemia and supplementation with GA consistently brought down FBG levels on 15th and 30th days in GA treated rats. These observations demonstrated the anti-diabetic potential of GA, which is consistent with our previous report of the glucose-lowering effect of GA in high-fat diet induced diabetic rats [27]. The simultaneous increase in the plasma insulin along with the increased insulin mRNA expression in the rat pancreas might suggest the potential of GA to control hyperglycemia by increasing the insulin secretion in the GA-treated rats and obtained a similar observation of insulin expression in invitro studies, where high glucose exposed RIN5-F cells treated with GA showed increased insulin expression.
Reprogramming non β-cells into insulin producing β-like cells is a possible regenerative approach for T1D therapy [38]. The regeneration processes could be induced by replicating pre-existing β-cells, neogenesis from endogenous progenitors, or trans-differentiation from differentiated non β-cells, revealing a surprising degree of cell plasticity in the mature pancreas [39, 40]. Furthermore, reports indicated neogenesis of β-cell in adult rodents after inducing pancreatic injury by streptozotocin [41], partial pancreatectomy [42], pancreatic duct ligation [43], cellophane wrapping of the pancreas head [44], and in sucrose-induced insulin resistance [45]. Although the mechanism for the β-cell regeneration through neogenesis has not been clarified, transdifferentiation into β-cells from duct cells, acinar cells, centro acinar cells, and other endocrine cells such as α cells and δ cells has been reported [46].
Several transcription factors (TFs) such as Pdx1, Neurogenin 3, MafA, NeuroD1 were reported to play a role in the development of endocrine cells in the pancreas [47]. In addition, Pdx1 is implicated in β-cell neogenesis, being expressed in the duct and duct-associated cells in models of pancreas regeneration [48,49,50]. Holland et al., reported that Pdx1 expression was associated with increased cell proliferation primarily in the exocrine pancreas and upregulation of genes involved in pancreas regeneration [51]. Furthermore, the enforced simultaneous expression of three key developmental transcription factors, Pdx1, Neurogenin 3, and MafA, induced acinar to β-cell conversion and rescued hyperglycemia in streptozotocin-induced diabetic animals [52]. The present study showed that the reduced relative mRNA and protein expression levels of pancreatic regenerative markers such as Pdx1, Neurogenin 3, MafA and NeuroD1 in diabetic rats with upregulation of these markers on supplementation with gymnemic acid in diabetic rats. In supporting this statement, Zhou et al., reported re-expressing these critical regulators of β-cell development Pdx1, Neurogenin 3, NeuroD1 and MafA can be reprogramming pancreatic exocrine cells into cells that closely resemble β-cells in adult mice [53]. Thereby it’s clear that reduced expression of Pdx1 is contributing to β-cell loss and dysfunction in diabetes. Thus, promoting the expression of Pdx1 can be an effective strategy to conserve β-cell mass and function [54]. During glucotoxicity and lipotoxicity, the expression of Pdx1 in pancreatic islets is significantly reduced [55]. Similarly, in our in vitro study on high glucose treated RIN5-F cells, there was a decreased expression of Pdx1, while reverted upon GA supplementation. To further confirm the GA mediated upregulation of Pdx1, we conducted a siRNA-mediated gene silencing of Pdx1 in RIN5-F cells, where GA treatment in Pdx1 silenced cell dictates slightly increased Pdx1 level, suggesting the ability of GA to restore Pdx1 expression. So, both our in vitro and in vivo experiments demonstrated that GA enhanced β-cell regeneration via Pdx1 activation.
The correlation between E-cadherin and insulin levels in adult rodent β-cells suggests the importance of tight cell-to-cell junctions for the function of β-cells [56]. Numerous studies reported that the normal insulin secretory responses are consequences of islet aggregations, mediated by a principal adhesion molecule found in islets of Langerhans called E-cadherin (ECAD), which maintains islet architecture [57, 58] and insulin secretion [59, 60]. So, we analyzed ECAD levels in the various experimental groups, where we obtained a significant upregulation of ECAD in GA supplemented rats, indicating its capability of restoring islet architecture via ECAD. The same has been confirmed by histopathological and immunohistochemical examination of islet architecture in treated and untreated rats with the notable restoration of islet architecture in GA-treated rats. Thus, E-cadherin has been shown to play an essential role in developing and maintaining epithelial morphology, cell differentiation, migration proliferation, and apoptosis [61]. The results from the western blot analysis observed an increased expression of β-catenin in GA-treated diabetic rats compared to streptozotocin treated rats. The above observations collectively represent the role of GA in maintaining the architecture and β-cell proliferation by upregulating the ECAD and β-catenin. In addition, E-cadherin-mediated cell–cell adhesion is augmented by short-term β-catenin activation in association with PI3K/AKT signaling and enhances human embryonic stem cells (hESC) self-renewal and cell proliferation [62]. Fatrai et al., reported the β-cell proliferation via Akt activation in a CDK4-dependent manner by regulating Cyclin D1, D2 and p21 [63]. Furthermore, Kushner et al., reported the preservation of β-cell mass most predominantly from the proliferation of pre-existing β-cell depends on activation of the Cyclin D1/CDK4 complex [64]. Interestingly, we observed that the levels of cell cycle markers such as Cyclin D1, CDK4 and AKT, pAKT were upregulated in the GA-treated group while cell cycle inhibitor p21cip1 is downregulated. This is a further promising observation that suggested the role of GA in promoting β-cell proliferation via PI3K/AKT activation. Besides this activation, it is an essential regulator of the pancreatic β-cells mass and function [65]. Furthermore, studies by Norman and Cecilia suggest that the proteins such as FOXO1 and GSK3-β are critical cell cycle regulators that negatively regulate proliferative and cell survival signals in response to the activation of PI3K/AKT signaling pathway [66]. Our study also showed decreased levels of GSK3β and FOXO1 in GA treated rats compared to the diabetic rats. Collectively, our observations reported that GA administration could have been responsible for β-cell proliferation and survival.
In conclusion, the present study points out the plausible role of GA in maintaining normoglycemia in diabetic rats by boosting the insulin secretion via β-cells regeneration markers (Pdx1, Neurognin 3, MafA and NeuroD1) and consecutively maintaining the proliferation through upregulation of PI3K/AKT, ECAD and β-catenin, which also maintains islet’s architecture by cell adhesion. Moreover, further studies are warranted to check whether GA can modulate the other diabetic complication in diabetic rats.
References
Zhu Y, Liu Q, Zhou Z, Ikeda Y. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res Ther. 2017;8:240.
Jiang WJ, Peng YC, Yang KM. Cellular signaling pathways regulating β-cell proliferation as a promising therapeutic target in the treatment of diabetes. Exp Ther Med. 2018;16:3275–85.
Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4:661–7.
Farney AC, Sutherland DE, Opara EC. Evolution of islet transplantation for the last 30 years. Pancreas. 2016;45:8–20.
Zhong F, Jiang Y. Endogenous pancreatic β cell regeneration: a potential strategy for the recovery of β cell deficiency in diabetes. Front Endocrinol. 2019;20:101.
Kim HS, Lee MK. β-Cell regeneration through the transdifferentiation of pancreatic cells: Pancreatic progenitor cells in the pancreas. J Diabetes Investig. 2016;7:286–96.
Thakur G, Lee HJ, Jeon RH, Lee SL, Rho GJ. Small molecule-induced pancreatic β-like cell development: mechanistic approaches and available strategies. Int J Mol Sci. 2020;21:2388.
Wang W, Walker JR, Wang X, Tremblay MS, Lee JW, Wu X, et al. Identification of small-molecule inducers of pancreatic β-cell expansion. Proc Natl Acad Sci U S A. 2009;106:1427–32.
Yuan Y, Hartland K, Boskovic Z, Wang Y, Walpita D, Lysy PA, et al. A small-molecule inducer of PDX1 expression identified by high-throughput screening. Chem Biol. 2013;20:1513–22.
Ma X, Zhu S. Chemical strategies for pancreatic β cell differentiation, reprogramming, and regeneration. Acta Biochim Biophys Sin (Shanghai). 2017;49:289–301.
Wickramasinghe AS, Kalansuriya P, Attanayake AP. Herbal medicines targeting the improved β-cell functions and β-cell regeneration for the management of diabetes mellitus. Evid Based Complement Alternat Med. 2021;15:2021.
Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med. 2015;21:383–8.
Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–11.
Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, et al. MafA and MafB regulate genes critical to β-cells in a unique temporal manner. Diabetes. 2010;59:2530–9.
Bohuslavova R, Smolik O, Malfatti J, Berkova Z, Novakova Z, Saudek F, et al. NEUROD1 is required for the early α and β endocrine differentiation in the pancreas. Int J Mol Sci. 2021;22:6713.
Gagliardino JJ, Del Zotto HH, Massa ML, Flores LE, Borelli MI. Pancreatic duodenal homeobox-1 and islet neogenesis-associated protein: a possible combined marker of activateable pancreatic cell precursors. J Endocrinol. 2003;249–59.
Rouiller DG, Cirulli V, Halban PA. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol. 1991;148:233–42.
Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–41.
Carvell M, Marsh P, Persaud S, Jones P. E-cadherin interactions regulate β-cell proliferation in islet-like structures. Cell Physiol Biochem. 2007;20:617–26.
Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent β-catenin transcriptional activity. J Cell Biol. 2001;154:1185–96.
Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–96.
Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23.
Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42.
Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: a memoir. J Clin Biochem Nutr. 2007;41:77–81.
Baskaran K, Ahamath BK, Shanmugasundaram KR, Shanmugasundaram ER. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol. 1990;30:295–300.
Cheng D, Liang B, Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int. 2013;2013:162724..
Chakrapani LN, Periandavan K. Protective role of gymnemic acid in curbing high fat diet and high fructose induced pancreatic oxidative stress mediated type-2 diabetes in wistar rats. Int J Pharm Sci Res. 2018;9:2130–9.
Philis-Tsimikas A, Chang A, Miller L. Precision, accuracy, and user acceptance of the OneTouch SelectSimple blood glucose monitoring system. J Diabetes Sci Technol. 2011;5:1602–9.
Campbell-Thompson ML, Heiple T, Montgomery E, Zhang L, Schneider L. Staining protocols for human pancreatic islets. J Vis Exp. 2012. https://doi.org/10.3791/4068
Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71–85.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21.
Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–35.
Li K, Zhu S, Russ HA, Xu S, Xu T, Zhang Y, et al. Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell. 2014;14:228–36.
Srivastava A, Dadheech N, Vakani M, Gupta S. Swertisin ameliorates diabetes by triggering pancreatic progenitors for islet neogenesis in Streptozotocin treated BALB/c mice. Biomed Pharmacother. 2018;100:221–5.
Salehi B, Ata A, Anil Kumar VN, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9:551.
Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med. 2013;2:328–36.
Desgraz R, Bonal C, Herrera PL. β-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab. 2011;22:34–43.
Márquez-Aguirre AL, Canales-Aguirre AA, Padilla-Camberos E, Esquivel-Solis H, Díaz-Martínez NE. Development of the endocrine pancreas and novel strategies for β-cell mass restoration and diabetes therapy. Braz J Med Biol Res. 2015;10:765–76.
Fernandes A, King LC, Guz Y, Stein R, Wright CV, Teitelman G. Differentiation of new insulin-producing cells is induced by injury in adult pancreatic islets. Endocrinology. 1997;138:1750–62.
Lee HC, Bonner-Weir S, Weir GC, Leahy JL. Compensatory adaption to partial pancreatectomy in the rat. Endocrinology. 1989;124:1571–5.
Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207.
Vinik A, Pittenger G, Rafaeloff R, Rosenberg L. Factors controlling pancreatic islet neogenesis. Yale J Biol Med. 1992;65:471–91.
Del Zotto H, Massa L, Rafaeloff R, Pittenger GL, Vinik A, Gold G, et al. Possible relationship between changes in islet neogenesis and islet neogenesis-associated protein-positive cell mass induced by sucrose administration to normal hamsters. J Endocrinol. 2000;165:725–33.
Miyazaki S, Tashiro F, Miyazaki JI. Transgenic expression of a single transcription factor Pdx1 induces transdifferentiation of pancreatic acinar cells to endocrine cells in adult mice. PLoS One. 2016;11:e0161190.
Lima MJ, Muir KR, Docherty HM, McGowan NW, Forbes S, Heremans Y, et al. Generation of functional beta-like cells from human exocrine pancreas. PLoS One. 2016;11:e0156204.
Waguri M, Yamamoto K, Miyagawa JI, Tochino Y, Yamamori K, Kajimoto Y, et al. Demonstration of two different processes of β-cell regeneration in a new diabetic mouse model induced by selective perfusion of alloxan. Diabetes. 1997;46:1281–90.
Kritzik MR, Jones E, Chen Z, Krakowski M, Krahl T, Good A, et al, Sarvetnick N. PDX-1 and Msx-2 expression in the regenerating and developing pancreas. J Endocrinol. 1999;163:523–30.
Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–13.
Holland AM, Góñez LJ, Naselli G, MacDonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of β-cells in the adult pancreas. Diabetes. 2005;54:2586–95.
Orlando G, Gianello P, Salvatori M, Stratta RJ, Soker S, Ricordi C, et al. Cell replacement strategies aimed at reconstitution of the β-cell compartment in type 1 diabetes. Diabetes. 2014;63:1433–44.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–32.
Yao X, Li K, Liang C, Zhou Z, Wang J, Wang S, et al. Tectorigenin enhances PDX1 expression and protects pancreatic β-cells by activating ERK and reducing ER stress. J Biol Chem. 2020;295:12975–92.
Shimo N, Matsuoka TA, Miyatsuka T, Takebe S, Tochino Y, Takahara M, et al. Short-term selective alleviation of glucotoxicity and lipotoxicity ameliorates the suppressed expression of key β-cell factors under diabetic conditions. Biochem Biophys Res Commun. 2015;467:948–54.
Roscioni SS, Migliorini A, Gegg M, Lickert H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat Rev Endocrinol. 2016;12:695–709.
Rogers GJ, Hodgkin MN, Squires PE. E-cadherin and cell adhesion: a role in architecture and function in the pancreatic islet. Cell Physiol Biochem. 2007;20:987–94.
Dahl U, Sjødin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895–902.
Bosco D, Orci L, Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res. 1989;184:72–80.
Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes. 2000;49:233–43.
Bosco D, Rouiller DG, Halban PA. Differential expression of E-cadherin at the surface of rat β-cells as a marker of functional heterogeneity. J Endocrinol. 2007;194:21–9.
Huang TS, Li L, Moalim-Nour L, Jia D, Bai J, Yao Z, et al. A regulatory network involving β-C atenin, e-C adherin, PI3k/A kt, and S lug balances self-renewal and differentiation of human pluripotent stem cells in response to wnt signaling. Stem Cells. 2015;33:1419–33.
Fatrai S, Elghazi L, Balcazar N, Cras-Méneur C, Krits I, Kiyokawa H, et al. Akt induces β-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–25.
Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, et al. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol. 2005;25:3752–62.
Elghazi L, Bernal-Mizrachi E. Akt and PTEN: β-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20:243–51.
Balcazar Morales N, Aguilar de Plata C. Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb Med (Cali). 2012;43:235–43.
Acknowledgements
The work is supported by UGC-UPE Phase-II, UNOM & ICMR-SRF (ID: 2019-6897), New Delhi. We thank DHR-MRU, University of Madras for providing the qPCR facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The experiments were conducted with the guidelines approved by the Institutional Animal Ethical Committee (IAEC No: 01/10/2020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kannan, P., Raghunathan, M., Mohan, T. et al. Gymnemic Acid Ameliorates Pancreatic β-Cell Dysfunction by Modulating Pdx1 Expression: A Possible Strategy for β-Cell Regeneration. Tissue Eng Regen Med 19, 603–616 (2022). https://doi.org/10.1007/s13770-022-00435-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00435-7