Abstract
Background:
Some scholars have found that dermal papilla spheroid–derived exosomes could promote the development of hair follicles. However, whether adipose-derived stem cell exosomes (ADSC-Exos) have a similar effect on hair growth has not been determined yet. Thus, the purpose of this article was to detect whether ADSC-Exos could promote hair regeneration.
Methods:
Adipose-derived stem cells (ADSCs) were isolated from 6-week-old C57BL/6 mice. Then, ADSC-Exos were isolated from the ADSCs. Western blotting was used to detect specific exosome markers. The particle size and distribution of the exosomes were analyzed by NanoSight dynamic light scattering. A total of 12 nude mice were randomly divided into two groups (n = 6 each): the ADSC-Exos group and the control group. For the control group, a mixture of freshly isolated dermal cells (DCs) and epidermal cells (ECs) was grafted. For the ADSC-Exos group, a mixture of DCs, ECs, and 50 μg/ml of ADSC-Exos was grafted. Gross evaluation of the hair regeneration was carried out 2–3 weeks after the transplantation, and the graft site was harvested for histology at the third week.
Results:
The existence of exosomes derived from ADSCs was evidenced by CD63, ALX1, and CD9 expression. Two or three weeks after the grafting, the number of regenerated hairs in the ADSC-Exos group was higher than that in the control group (p < 0.001). Histologically, the terminal hairs were remarkable in the ADSC-Exos group, whereas the hair follicles observed in the control group were comparatively immature. The ADSC-Exos group had a higher number of regenerated follicles than the control group (p < 0.001). In addition, we found that the skin tissues in the ADSC-Exos group had higher PDGF and vascular endothelial growth factor expressions and lower transforming growth factor beta 1 levels than those in the control group.
Conclusion:
Our results indicated that ADSC-Exos could promote in vivo hair follicle regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Adipose-derived stem cells (ADSCs) separated from adipose tissue are an important stem cell type and have properties of multilineage differentiation and self-renewal [1]. They exert their biological functions via the paracrine actions of various growth factors and cytokines to help cells develop and proliferate. Due to this, ADSCs have been extensively researched and applied in tissue engineering and regenerative medicine. Hair loss is a prevalent medical problem in both men and women and is one of the most common complaints presented to dermatologists and plastic surgeons [2]. Accumulating evidence suggests that ADSC-based regenerative medicine helps hair regrowth in patients with hair loss [2, 3]. The growth factors secreted by ADSCs, such as hepatocyte growth factor, vascular endothelial growth factor (VEGF), insulin‐like growth factor, and platelet‐derived growth factor (PDGF), have been reported to increase the size of hair follicles during hair development [4]. Thus, ADSCs are considered one of the most common treatments for hair loss [5].
Exosomes are a small subset of extracellular vesicles that range from 50 to 200 nm. They have properties that regulate the biological behaviors of cells, including their proliferation and migration [6]. Moreover, ADSC-derived exosomes (ADSC-Exos) are important components released by ADSC paracrine that possess multiple biological activities. For example, previous studies have demonstrated that ADSC-Exos exert crucial functions in cutaneous wound healing [7], tissue repair [8], and inflammatory response regulation [6]. In recent years, dermal papilla–derived exosomes have been reported to promote hair growth. Kwack et al. [9] demonstrated that exosomes derived from human dermal papilla cells could promote hair growth in cultured human hair follicles. Hu et al. [10] also found that dermal papilla spheroid–derived exosomes could upregulate β-catenin, promoting the development of hair follicles. However, whether ADSC-Exos have a similar effect on hair growth has not been determined yet.
Considering ADSCs’ effect on hair growth, we hypothesized that ADSC-Exos could promote hair regeneration. To verify our hypothesis, we isolated and characterized exosomes from ADSCs. Then, we demonstrated ADSC-Exos as a new method to support hair induction and improve the outcome of hair loss treatment.
2 Materials and methods
2.1 Animals and ethics statement
All animal procedures were approved by the Animal Care and Utilization Committee of Shanghai Medical College, Fudan University (IACUC no. 20190400S). A total of 6 time-dated, pregnant C57BL/6 mice, 6 male adult C57BL/6 mice, and 12 male Nu/nu athymic nude mice (6 weeks old) were purchased from SLAC Laboratory Animal Co. Ltd. (Shanghai, China). The mice were housed in individual cages with free access to laboratory food and water at a temperature range of 20–23 °C under a 12:12-h light–dark cycle.
2.2 Isolation and culture of ADSCs
Adipose tissue was harvested from the adult C57BL/6 mice. The tissue was digested by 0.2% collagenase I (Sigma-Aldrich, Milwaukee, WI, USA) for 60 min at 37 °C with intermittent shaking and terminated using Dulbecco's Modified Eagle Medium (DMEM; Sigma-Aldrich) containing 15% fetal bovine serum (FBS). A cell-debris pellet was obtained by centrifugation at 1000 g for 10 min. Then, the cell pellet was resuspended in DMEM supplemented with 15% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin in a 37 °C incubator with 5% CO2. Upon reaching 80% confluence, the ADSCs were detached with Accutase (Sigma-Aldrich) for 5 min and then replated. Finally, the ADSCs were cultured under normoxic conditions in 95% air (20% O2) and 5% CO2.
2.3 Exosome isolation
After 48 h in serum-free culture, the medium of the ADSCs was harvested and centrifuged at 500 g for 10 min to remove the cells. Then, the supernatant was centrifuged at 12,000 g for 20 min to remove apoptotic bodies and cell debris, followed by filtration through a 0.22-mm filter to remove any molecules larger than 200 nm [11]. Using an exosome isolation reagent (Qiagen, Dusseldorf, Germany), exosomes were extracted from the ADSC supernatant without the cell debris in accordance with the manufacturer’s guidelines.
2.4 Exosome characterization
Transmission electron microscopy (TEM; JEOL Ltd., JEM2010-HT; Tokyo, Japan) was performed to visualize and examine the morphology of the isolated exosomes. The size, distribution, and number of particles in the ADSC-Exos were evaluated by NanoSight dynamic light scattering using a nanoparticle tracking analyzer (v3.1, Malvern Instruments, Ltd., Worcestershire, UK). A western blot analysis of the proteins in the exosomes (i.e., CD9, CD63, and ALX1) was conducted as described previously [5], and the following primary antibodies were used: CD9 (1:1000; Abcam, Cambridge, UK), CD63 (1:1000; Abcam), and ALX1 (1:1000; Abcam).
2.5 Graft chamber assay
A graft chamber assay was performed to evaluate the hair-inductive capacity of the ADSC-Exos, as described previously [12]. Briefly, newborn C57BL/6 mice were sacrificed within the first 24 h after birth, and their trunk skin was harvested. The epidermal and dermal layers were separated by floating on 1.5 U/mL dispase II (Roche Applied Science, Indianapolis, IN, USA) in PBS at 37 °C. The epidermal layer was peeled off and incubated in a 0.25% trypsin solution (Invitrogen, Carlsbad, CA, USA) with gentle shaking. Then, the lysates were filtered through 40-μm filters, and the epidermal cells (ECs) were collected. The dermis was digested in 0.35% collagenase I (Invitrogen) at 37 °C for 45 min. The dermal cell (DC) pellets were then resuspended in DMEM.
A total of 12 nude mice were randomly divided into two groups (n = 6 each): the ADSC-Exos group and the control group. After a wound with a diameter of 1.0 cm was made on the backs of the nude mice, a sterile silicon chamber was inserted. For the control group, a mixture of freshly isolated DCs (1.5 × 106 cells) and ECs (1 × 106 cells) was grafted. For the ADSC-Exos group, a mixture of DCs, ECs, and 50 μg/ml of ADSC-Exos was grafted. After 1 week, the chamber was removed. Gross evaluation of the hair regeneration was carried out 2–3 weeks after the transplantation, and the graft site was harvested for histology at the third week. Gross and dermoscopic evaluations were carried out (Dermlite FOTO, San Juan Capistrano, CA, USA). Under a microscope, we manually counted the number of regenerated hairs.
2.6 Histology
To investigate the density of the hair follicles, the tissues at the grafted sites were analyzed histologically. The skin tissues were fixed in Fekete's acid–alcohol formalin overnight and then transferred to 70% ethanol until trimming. The tissues were embedded in paraffin and sectioned at 4 μm both horizontally and vertically. Then, they were stained with hematoxylin and eosin (HE).
2.7 Western blot analysis for cytokines
Skin tissues homogenized in cold RIPA buffer were centrifuged at 16,000 ×g for 10 min at 4 °C to obtain supernatants. To determine the protein expression, an equal the mount proteins were separated by 10–12% SDS-PAGE and then transferred to polyvinylidene fluoride membranes using a semi-dry transfer system (Bio-Rad, Hercules, CA, USA). The membranes were incubated with the appropriate primary antibodies overnight and then with horseradish peroxidase-conjugated secondary antibodies. Finally, protein bands were visualized using enhanced chemiluminescence western blotting reagents (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The primary antibodies used were as follows: PDGF (1:1000; Abcam), transforming growth factor beta 1 (TGF-β1, 1:1000; Abcam), VEGF (1:1000, Abcam). GAPDH was used as an internal loading control.
2.8 Statistical analyses
The statistical analyses were performed using the SPSS 25.0 software (SPSS, Inc., Chicago, IL, USA). All data were expressed as the mean ± standard deviation. Comparisons between the two groups were performed using t tests. The statistical significance was set at p < 0.05.
3 Results
3.1 Characterization of ADSC-Exos
The morphology of the ADSC-Exos was examined by TEM. We found that the exosomes from the ADSCs exhibited a sphere-shaped morphology approximately 20–130 nm in diameter (Fig. 1A). The results of the NanoSight dynamic light scattering analysis demonstrated that the size of most ADSC-Exos was in the range of 30–300 nm (Fig. 1B). The western blotting detection further confirmed the expression of exosome markers CD63, ALX1, and CD9 in the ADSC-Exos (Fig. 1C), which suggested that these nanoparticles were in fact exosomes.
3.2 Gross evaluation of the hair regeneration in the two groups
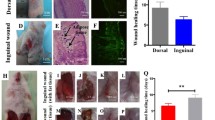
Two weeks after the grafting, a small number of hairs regenerated in the control group (DC–EC mixture), and the number of regenerated hairs in the ADSC-Exos group was higher than that in the control group (40.11 ± 4.22 vs. 31.00 ± 3.77, respectively; p < 0.001). Three weeks after the grafting, the number of regenerated hairs in both groups had increased. A large, uniform amount of hair regeneration was observed in the ADSC-Exos group (p < 0.001 vs. control group, Fig. 2).
3.3 The results of the histological analysis in the two groups
In the ADSC-Exos group, normal-looking hairs were observed in both the vertical and transverse sections (Fig. 3A). The terminal hairs were remarkable in the ADSC-Exos group, whereas the hair follicles observed in the control group were comparatively immature (Fig. 3A). Furthermore, the number of regenerated follicles per vertical section was significantly higher in the ADSC-Exos group than in the control group (17.70 ± 2.67 vs. 11.22 ± 2.37, respectively; p < 0.001, Fig. 3B). Therefore, our results showed that the ADSC-Exos promoted in vivo hair follicle regeneration.
Histological analysis. A Representative images of histological analysis in both vertical and transverse sections (×200); arrows indicated hair follicles. B The comparison of hair follicles numbers between two groups. The number of regenerated follicles per vertical section was significantly higher in the ADSC-Exos group than the control group (p < 0.001)
3.4 The results of the cytokine detection in the two groups
As shown in Fig. 4, the expressions of PDGF and VEGF were much higher in the ADSC-Exos group than in the control group (p < 0.05). However, compared with the control group, the expression of TGF-β1 in the ADSC-Exos group was much lower (p < 0.001).
4 Discussion
In the present study, we successfully isolated ADSC-Exos from ADSCs and demonstrated that ADSC-Exos could promote in vivo hair follicle regeneration.
Hair is a feature of mammals that plays a great role in their beauty, social acceptance, and self-esteem. Hair loss is therefore a threat to beauty and a psychological challenge [5]. Although medical technology has made great progress in promoting hair growth over the past decades, a satisfactory treatment for hair loss is still in blank [13]. Adipose-derived stem cells are regarded as multipotent stem cells, which can be harvested in large quantities [14]. Various cytokines secreted by ADSCs have been reported to play a key role in stimulating hair follicles and inducing hair growth [13]. Thus, ADSC-based regenerative medicine has been suggested to treat patients with hair loss [2, 3].
Additionally, ADSC-conditioned medium (CM), which is secreted by stem cells and rich in paracrine factors, has been widely explored as a hair regenerative medicine for hair loss [13, 15]. Despite the good results of stem-cell-derived CM in the treatment of various diseases, the short half-life of the cytokines and growth factors of CM is a disadvantage in regenerative therapy [16]. In the field of dermatology, exosomes have been shown to play an effective role in healing wounds, preventing scarring, and inducing hair growth. One of the specific advantages of the therapeutic application of exosomes instead of CM is protection from degradation [17]. It has been demonstrated that exosomes may offer safer and more efficient treatment approaches for patients compared to CM and stem cell therapy [18]. Exosomes are released from cells and can carry messenger RNA, microRNA, and proteins to target cells. In previous studies, exosomes derived from human hair outer root sheath cells [5], DP cells [19], and myeloid-derived suppressor cells [20] were reported to regulate hair follicle growth and development. Exosomes derived from ADSCs exert crucial functions in cutaneous wound healing [7], tissue repair [8], and inflammatory response regulation [6]. However, whether ADSC-Exos could also promote hair growth has not been determined.
As ALX1, CD63, and CD9 are important expression biomarkers of exosomes [21], in the present study, the existence of the exosomes derived from the ADSCs was evidenced by CD63, ALX1, and CD9 expression. Moreover, TEM revealed that the ADSC-Exos exhibited a sphere-shaped morphology approximately 20–130 nm in diameter. Then, the mixture of ECs, DCs, and ADSC-Exos was grafted onto the nude mice; the mixture of ECs and DCs was used as the control. In 2011, Lee et al. reported that a mixture of ECs and DCs self-organized and eventually formed well-oriented hairs when the slurry was grafted onto nude mice [22]. In this study, a small number of hairs regenerated in the mice grafted with the mixture of ECs and DCs (control group) 2 weeks after the grafting, consistent with the data reported by other researchers [5, 23]. Moreover, as expected, for the mice grafted with the mixture of ECs, DCs, and ADSC-Exos (ADSC-Exos group), the number of regenerated hairs appeared to be higher. Histologically, the terminal hairs were remarkable in the ADSC-Exos group, whereas the hair follicles observed in the control group were comparatively immature. More importantly, the ADSC-Exos group had more regenerated follicles than the control group. These results indicated that ADSC-Exos could accelerate the onset of anagen and promote hair follicle growth. Previous studies have demonstrated that the exosomes derived from DP cells promote hair growth and hair regeneration by regulating the activity of follicular DCs and ECs [9]. Thus, ADSC-Exos may also regulate DCs and ECs to exert a hair growth-promoting effect.
Furthermore, in this study, we found that the skin tissues in the ADSC-Exos group had higher PDGF and VEGF expressions and lower TGF-β1 levels than those in the control group. As VEGF and PDGF are known growth modulators of the follicular papillae in the anagen stage [24, 25], the induction of these two growth factors by an ADSC-Exos treatment could promote the anagen stage or activate hair growth. The downregulation of TGF-β1 may partially contribute to the prevention of hair loss because it is involved in the catagen stage of hair development or the apoptosis of hair follicle cells [24]. Thus, our results indicated that ADSC-Exos may promote hair growth by modulating growth regulators.
One limitation of this study was that we only performed a morphological observation. The mechanism of why ADSC-Exos can promote hair growth was not detected in this study. Further studies should focus on this field.
In conclusion, we successfully isolated ADSC-Exos from ADSCs and demonstrated that they could promote in vivo hair follicle regeneration. Thus, ADSC-Exos could be applied as a new method to support hair induction and improve the outcome of hair loss treatment.
References
Xiong M, Zhang Q, Hu W, Zhao C, Lv WC, Yi Y, et al. Exosomes from adipose-derived stem cells: the emerging roles and applications in tissue regeneration of plastic and cosmetic surgery. Front Cell Dev Biol. 2020;8:574223.
Xiao S, Deng Y, Mo X, Liu Z, Wang D, Deng C, et al. Promotion of hair growth by conditioned medium from extracellular matrix/stromal vascular fraction gel in C57BL/6 mice. Stem Cells Int. 2020;2020:9054514.
Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl Med. 2020;9:839–49.
Ramdasi S, Tiwari SK. Human mesenchymal stem cell-derived conditioned media for hair regeneration applications. J Stem Cells. 2016;11:201–11.
Nilforoushzadeh MA, Aghdami N, Taghiabadi E. Human hair outer root sheath cells and platelet-lysisexosomes promote hair inductivity of dermal papilla cell. Tissue Eng Regen Med. 2020;17:525–36.
Wang WM, Wu C, Jin HZ. Exosomes in chronic inflammatory skin diseases and skin tumors. Exp Dermatol. 2019;28:213–8.
He L, Zhu C, Jia J, Hao XY, Yu XY, Liu XY, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci Rep. 2020;40:BSR20192549.
Xing X, Han S, Cheng G, Ni Y, Li Z, Li Z. Proteomic analysis of exosomes from adipose-derived mesenchymal stem cells: a novel therapeutic strategy for tissue injury. Biomed Res Int. 2020;2020:6094562.
Kwack MH, Seo CH, Gangadaran P, Ahn BC, Kim MK, Kim JC, et al. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp Dermatol. 2019;28:854–7.
Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, et al. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685.
Bai Y, Han YD, Yan XL, Ren J, Zeng Q, Li XD, et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun. 2018;500:310–7.
Paik SH, Choi SJ, Jang S, Jo SJ, Kim KH, Kwon O. Skin equivalent assay: an optimized method for testing for hair growth reconstitution capacity of epidermal and dermal cells. Exp Dermatol. 2019;28:367–73.
Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12:531–4.
Lin YC, Grahovac T, Oh SJ, Ieraci M, Rubin JP, Marra KG. Evaluation of a multi-layer adipose-derived stem cell sheet in a full-thickness wound healing model. Acta Biomater. 2013;9:5243–50.
Yuan AR, Bian Q, Gao JQ. Current advances in stem cell-based therapies for hair regeneration. Eur J Pharmacol. 2020;881:173197.
Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849.
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852.
Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:2402916.
le Riche A, Aberdam E, Marchand L, Frank E, Jahoda C, Petit I, et al. Extracellular vesicles from activated dermal fibroblasts stimulate hair follicle growth through dermal papilla-secreted norrin. Stem Cells. 2019;37:1166–75.
Zöller M, Zhao K, Kutlu N, Bauer N, Provaznik J, Hackert T, et al. Immunoregulatory effects of myeloid-derived suppressor cell exosomes in mouse model of autoimmune alopecia areata. Front Immunol. 2018;9:1279.
Melzer C, Ohe JV, Hass R. Anti-tumor effects of exosomes derived from drug-incubated permanently growing human MSC. Int J Mol Sci. 2020;21:7311.
Lee LF, Jiang TX, Garner W, Chuong CM. A simplified procedure to reconstitute hair-producing skin. Tissue Eng Part C Methods. 2011;17:391–400.
Kanayama K, Takada H, Saito N, Kato H, Kinoshita K, Shirado T, et al. Hair regeneration potential of human dermal sheath cells cultured under physiological oxygen. Tissue Eng Part A. 2020;26:1147–57.
Boisvert WA, Yu M, Choi Y, Jeong GH, Zhang YL, Cho S, et al. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement Altern Med. 2017;17:109.
Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43:105–15.
Acknowledgements
Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (Project No 19441909900); Strategic Priority Research Program of the Chinese Academy of Sciences Grant No. XDA* (XDA16040400); Shanghai Engineering Technology Research Center of Hair Medicine (19DZ2250500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare(s) that there is no conflict of interest.
Ethical statement
All animal procedures were approved by the Animal Care and Utilization Committee of Shanghai Medical College, Fudan University (IACUC no. 20190400S).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, J., Yang, Q., Wu, S. et al. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng Regen Med 18, 685–691 (2021). https://doi.org/10.1007/s13770-021-00347-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00347-y