Abstract
BACKGROUND:
The fabrication of microchannels in hydrogel can facilitate the perfusion of nutrients and oxygen, which leads to guidance cues for vasculogenesis. Microchannel patterning in biomimetic hydrogels is a challenging issue for tissue regeneration because of the inherent low formability of hydrogels in a complex configuration. We fabricated microchannels using wire network molding and immobilized the angiogenic factors in the hydrogel and evaluated the vasculogenesis in vitro and in vivo.
METHODS:
Microchannels were fabricated in a hyaluronic acid-based biomimetic hydrogel by using “wire network molding” technology. Substance P was immobilized in acrylated hyaluronic acid for angiogenic cues using Michael type addition reaction. In vitro and in vivo angiogenic activities of hydrogel with microchannels were evaluated.
RESULTS:
In vitro cell culture experiment shows that cell viability in two experimental biomimetic hydrogels (with microchannels and microchannels + SP) was higher than that of a biomimetic hydrogel without microchannels (bulk group). Evaluation on differentiation of human mesenchymal stem cells (hMSCs) in biomimetic hydrogels with fabricated microchannels shows that the differentiation of hMSC into endothelial cells was significantly increased compared with that of the bulk group. In vivo angiogenesis analysis shows that thin blood vessels of approximately 25–30 μm in diameter were observed in the microchannel group and microchannel + SP group, whereas not seen in the bulk group.
CONCLUSION:
The strategy of fabricating microchannels in a biomimetic hydrogel and simultaneously providing a chemical cue for angiogenesis is a promising formula for large-scale tissue regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vascularization in regenerating tissues is essential to make fully functional tissues with proper oxygen and nutrient supply [1]. For adequate growth and survival, cells in regenerating tissues should be located within 200 µm around blood vessels [2]. Two strategies have been developed in tissue engineering for vascularization in damaged tissues. One is to facilitate angiogenesis by the controlled release of angiogenic factors, and the other is to fabricate hollow vascular structures by 3D micro fabrication technologies.
The controlled release of chemical cues such as growth factors and angiogenic peptides from the scaffolds to the surrounding tissues induces angiogenesis for regeneration [3]. Regarding studies that used a growth factor, Tabata et al. [4] incorporated vascular endothelial growth factor (VEGF) into the collagen hydrogel and controlled the release rate to induce angiogenesis around the implanted hydrogel. Mooney et al. [5] suggested that the dual delivery of VEGF and platelet-derived growth factor (PDGF) could form dense and stable vessels compared with the delivery of VEGF or PDGF alone. The combined delivery of fibroblast growth factor-2 and VEGF via star-shaped poly (ethylene glycol) heparin hydrogels appeared to have proangiogenic effects in vitro and in vivo [6]. In addition, when BMP-2 and VEGF were combined with hydrogel, it was confirmed that bone regeneration and angiogenesis were significantly increased in bone defect model as compared with hydrogel alone [7]. Several groups reported that incorporating three angiogenic growth factors into a hydrogel formed a mature vascular network in vitro and in vivo by controlling their sequential release [8,9,10,11]. Meanwhile, angiogenic peptides, such as secretoneurin, N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP), and substance P (SP) are also used in immobilized hydrogels to induce angiogenesis. According to several studies, secretoneurin acted as an angiogenic cytokine and improved angiogenesis in vivo [12]. Ac-SDKP in myocardial infarction models has been reported as effective in angiogenesis by its controlled release in hyaluronic acid (HA)-based hydrogels [13]. For the acrylated HA hydrogel, SP was chosen and immobilized for biochemical cues. SP is composed of the tachykinin family of neuropeptides, which are small molecules secreted from the peripheral terminals of sensory nerve fibers. SP, which is also known as a neuropeptide, is involved in neurogenesis and angiogenesis. Kohara et al. [14] confirmed that SP incorporated into hydrogel induces angiogenesis by the enhanced recruitment of angiogenic cells. Kim et al. [15] suggested that SP could promote angiogenesis through its recruitment of autologous mesenchymal stem cells (MSCs). However, the facilitation of blood vessel network formation by using only angiogenic chemical cues is limited by the size and volume of the hydrogel.
Recently, various attempts have been made to fabricate microchannels in hydrogels that can be applied to large-scale tissue regeneration. Manufactured microchannels play an important role in delivering oxygen and nutrients to the cells in the hydrogel to improve cell viability and function. To fabricate microchannels in a large-scale hydrogel, various techniques such as fiber bonding [16], electrospinning [17], PDMS molding [18] and 3D printing [19,20,21] have been introduced in hydrogels by using 3D fabrication technologies [22]. Recently, 3D printing techniques have been attracting attention because of the merits of solid freeform fabrication and guaranteed interconnectivity. Agarose fibers printed in a branching architecture were removed from a gelatin methacryloyl hydrogel to obtain branched fluidic networks [23]. The 3D-printed carbohydrate glass lattices are embedded in a cell-loaded extracellular matrix (ECM) and create microchannels that can be perfused by dissolution after perfusion [24]. Furthermore, a blood vessel network was created in the hydrogel by using sacrificial material [25]. However, in the case of environmentally sensitive materials, the manufacturing process of a 3D hydrogel scaffold via a 3D printing technique has a different gelation time owing to the pathway of the printing heads [19]. Therefore, the use of a hydrogel that is sensitive to the gelation rate is limited in 3D fabrication with interconnective pores, and this is one of main disadvantages of 3D printing techniques. To overcome this problem, a fabrication method called ‘Wire network molding (WNM) was introduced in our previous study [26]. Given that the method is based on a molding technology that guarantees interconnectivity without having a different gelation time, it is an effective method for fabricating 3D scaffolds or microchannels by using a material that is sensitive to the gelation rate.
Consequently, in this study, we combined two strategies of a chemical signal and physical cue. For the chemical signal, the angiogenic peptide SP was immobilized on acrylated HA and allowed to be released continuously. For the physical cue, microchannels in the HA-based hydrogel were fabricated by the WNM technique. To assess the angiogenesis and vascularization of biomimetic hydrogels, the bulk hydrogel (no microchannels or SP), hydrogel with microchannels, and hydrogel with microchannels + SP were compared via in vitro and in vivo evaluations.
2 Materials and methods
2.1 Materials
Molds were designed using CATIA V5, and metallic molds (STS 304) were manufactured by a wire-cutting process. A bottom mold was printed by Objet 30 prime (Stratasys, USA), and the used material (MED 610) is known to be a biocompatible material. Needles (STS 304) with a diameter of 500 µm were prepared. PDMS and its curing agent were obtained from Dow Corning (Sylgard 184; Midland, MI, USA). Sodium hyaluronate (MW: 200 kDa) was purchased from Lifecore Biomedical. 1-Ethyl-3-(3 dimethylaminopropyl) carbodiimide, adipic acid dihydrazide, and triethanolamine (TEA) were acquired from Sigma-Aldrich (St. Louis, USA). 1-Hydroxybenzotriazole hydrate was purchased from Fluka Chemical (Buchs, Switzerland). N-Acryloxysuccinimide was purchased from Polyscience (Warrington, PA). SP (RPKPQQFFGLMC) and the matrix metalloprotease (MMP)-sensitive peptide (GCRDGPQGIWGQDRCG) were synthesized by Anygen (GwangJu, Korea). Trizol, penicillin/streptomycin, Gibco Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and phosphate-buffered saline (PBS; pH 7.4) were purchased from Invitrogen-Life Technologies. FBS, penicillin/streptomycin, trypsin, low-glucose DMEM, and PBS (pH 7.4) were purchased from GIBCO BRL (Grand Island, NY). An endothelial growth medium (EGM-2) Bullet kit was purchased from Lonza. A LIVE/DEAD Viability/Cytotoxicity Assay Kit was purchased from Molecular Probes (Eugene, OR, USA). The PrimeScript first-strand cDNA synthesis kit and PrimeScript RT reagent kit were purchased from Takara Bio Inc. Von Willebrand factor (vWF) (A0082), and α-smooth muscle actin (α-SMA) (M0851) were purchased from Abcam (Cambridge, MA, USA).
2.2 Preparation of a biomimetic HA hydrogel
The acrylation of HA followed that of a previous study [27]. Synthesized HA-ac (200 kDa, 3 wt. %) was dissolved in a 0.3 M TEA-buffered solution. For gel preparation, an MMP-sensitive peptide was added to an acrylated HA solution as a cross-linker with the same molar ratio of acryl and thiol groups. The HA hydrogel was formed via a Michael-type addition reaction (Fig. 1A). SP was also immobilized on the acrylated HA with the molar ratio of 20% of the acryl groups of the HA (Fig. 1B).
Schematic of the biomimetic hydrogel fabrication procedure with microchannels using a WNM technique. A Molecular structure of the HA hydrogel, B inserting cell-encapsulated HA hydrogel in a PDMS mold assembled with wires, C cross-linking of the assembled mold in an incubator, D fabricated HA hydrogel with cells and microchannels after demold, E assessment of in vitro cell culture, F molecular structure of acrylated HA with an angiogenic peptide, G inserting HA hydrogel in the assembled WNM mold, H cross-linking of the assembled WNM mold in an incubator, I a fabricated HA hydrogel sheet after demold, J in vivo evaluation of dicing HA hydrogels with microchannels using mice
2.3 Fabrication of a scaffold using the WNM technique
To fabricate hydrogel for the in vitro assessment, a cylindrical hydrogel with microchannels was prepared by a simple PDMS mold with wires, as shown in Fig. 1C–F. First, to prepare the upper PDMS mold with holes and the bottom PDMS mold, the PDMS material was mixed with a cross-linker and was placed in Petri dishes. Plate PDMS molds were prepared by baking in a vacuum oven. Second, the prepared PDMS mold was punched with 5 mm diameter and 3 mm thickness holes to fabricate an upper mold with holes. The prepared upper and bottom molds were then assembled by staking, as depicted in Fig. 1C. Then, two stainless-steel wires with 500 µm diameter were placed perpendicular to each other on the surface of the bottom mold. To fabricate a cylindrical biomimetic hydrogel with microchannels, the HA hydrogel was placed in the prepared PDMS mold. The mold with HA hydrogel and assembled wires was cross-linked for 30 min at 37 °C in an incubator (Fig. 1D). The wires and PDMS mold were then removed (Fig. 1E). Thereafter, for the in vitro experiment, the HA hydrogel with microchannels was manufactured, as depicted in Fig. 1F.
For the in vivo experiment, the WNM method was applied to the fabricated hydrogel with microchannels, as depicted in Fig. S1. First, four metallic mold parts (Fig. S1A) were assembled to create a 3D mold (Fig. S1B). The assembled metallic mold and the bottom mold (MED 610) were then assembled (Fig S1C). To minimize hydrogel leakage, the mold column was wrapped with Parafilm (Fig. S1D). Cylindrical wires were inserted through the wrapped Parafilm at mold slots that were positioned perpendicular to the wire network configuration (Fig. S1E). The prepared HA hydrogel mixture was injected using a syringe into the wire-networked mold (Figs. 1G and S1F) and baked for 30 min at 37 °C in an incubator (Figs. 1H and S1G). Wires and molds were then removed and a HA hydrogel with microchannels was fabricated (Figs. 1I and S1H). For the in vivo experiment, the HA hydrogel with microchannels was then divided into four equal parts (Fig. S1I) and implanted in the back of a mouse (Fig. S1J). The bulked HA hydrogel with the same dimensions was fabricated with a simple PDMS mold as a control.
2.4 Swelling of hydrogel with microchannels
The swelling of hydrogel with microchannels was incubated in PBS overnight. The swelling rate was measured by comparing the difference in weight before and after incubation. The percentage of absorbed water (Wa) was calculated by the following formula: Swelling rate (%) = (Ww − Wi)/Wi × 100% (Ww: wet weight of hydrogel; Wi: initial weight of hydrogel).
2.5 Cell preparation and culture
Fibroblast cells (L929) were purchased from Thermo Fisher and maintained in DMEM supplements with 10% FBS and 1% penicillin/streptomycin. Human MSCs (hMSCs) were obtained from Lonza and maintained in MSCGM medium. All cells were cultured at 37 °C in humidified 5% CO2 incubator. The medium was refreshed every 2–3 days.
2.6 Evaluation of cell viability
L929 cells were trypsinized and washed in PBS. Biomimetic hydrogels (200 kDa HA-ac 3 wt. %) with or without microchannels and SP peptide were mixed with L929 suspensions (1 × 106 cells/100 μl). The biomimetic hydrogel mixture containing the cells was injected into the WNM mold. It was then gelled in an incubator for 30 min. The cells in the hydrogels were cultured in DMEM media with 10% FBS and 1% penicillin/streptomycin in a humidified incubator (5% CO2, 37 °C) for 7 days. After 7 days, viability was measured by the LIVE/DEAD Viability/Cytotoxicity Assay Kit. Live and dead cells were examined under fluorescence microscopy. The viability of L929 in the biomimetic hydrogel with microchannels was determined by counting the live cells that appeared green from the total cell number.
2.7 Quantitative real-time polymerase chain reaction
The biomimetic hydrogel samples with hMSCs that were cultured for 14 days were soaked in 1 ml of a TRI reagent. Purified RNA was reverse-transcribed to cDNA with the RimeScript RT reagent kit. To determine vWF factor expression (forward: CCAGCTTCTGAAGAGCACCT; reverse: GTACAGCACCATTCCCTCCT), PECAM-1 (CD31, forward: GAG AGG ACA TTG TGC AA; reverse: TCT GTT GAA GGC TGT GCA GT), and a-SMA (forward: CCT ATC CCC GGG ACT; reverse: ACC CAG TGC TGT CCT CTT CT) genes as angiogenic differentiation markers, the final cDNAs were subjected to real-time polymerase chain reaction. As an internal control, the expression of the β-actin gene (forward: ATTGGCAATGAGCGGTTC; reverse: GGATGCAGGACTCCAT) was determined. Gene expression was calculated as the fold change by comparing the cycle threshold values for the β-actin gene and mRNAs of interest.
2.8 Implantation of a biomimetic hydrogel with microchannels
The animal experiment procedures were approved by the institutional animal care and use committee of Korea University College of Medicine (Protocol Number: KUIACUC-2015-165). All institutional and national guidelines for the care and use of laboratory animals were followed. Five-week-old BALB/c-nu Slc mice were obtained from OrientBio (Sungnam, Kungkido, Korea). Subcutaneous pockets were made to the right and left of two incisions on their backs. The experimental biomimetic hydrogels (bulk, microchannels, and both microchannels and SP) were implanted into the dorsal skin of nude mice. Six animals were used for in vivo experiment. The implanted scaffolds were harvested a month after implantation. The implanted scaffolds were retrieved and fixed in 10% formalin.
2.9 Three-dimensional immunostaining of the biomimetic hydrogel with microchannels
The expression of α-SMA and vWF was evaluated to assess angiogenesis. The hydrogel fixed on formalin was frozen by liquid nitrogen. The frozen hydrogel was then sectioned to a thickness of 1.5–2 mm. The sectioned hydrogel with channels for 1 h at 37 °C with the primary antibodies vWF (1:500) and α-SMA (1:500). Immunofluorescence detection was performed by Alexa Fluor 488 and 568 secondary antibodies.
2.10 Statistical analyses
All data are reported as mean ± standard deviation. Statistical analyses were performed using a single factor ANOVA with SPSS version 22.0 software (IBM Corp., Armonk, NY). Differences between experimental groups were analyzed by two-sample t tests, and p < 0.05 was considered statistically significant.
3 Results
3.1 Fabrication and evaluation of microchannels in a biomimetic hydrogel
To make a biomimetic hydrogel, HA, which is one of the major components of ECM, was acrylated, and MMP-sensitive peptides degradable by cell-secreted MMP were used as a cross-linker [28]. Angiogenic peptide SP was immobilized on the acrylated HA to induce angiogenic activity in the hydrogel. The WNM technique was used to fabricate microchannels in the biomimetic hydrogel. To maintain the microchannels in the biomimetic hydrogel even after swelling, an HA concentration optimization test was performed. The HA concentration was variously tested from 3 to 10 wt. %. The molecular weight, which affects the viscoelastic properties of the gel, was changed from 50 to 200 kDa (Fig S2). After the test, we found that hydrogels with 3 wt. % and 200 kDa HA was ideal to maintain the microchannels even after the swelling of the gel (Fig. 2A). To assess angiogenesis and vascularization, in vitro and in vivo analyses were performed using the optimal hydrogel composition.
For the in vitro assessment, biomimetic hydrogels with two channels layers were prepared using 500 µm wires. The fabricated hydrogel was 5 mm in diameter and 3 mm in height. The diameters of the formed microchannels in biomimetic hydrogel were approximately 500 and 600 µm before and after swelling, respectively (Fig. S3). The swelling ratio was measured to evaluate the swelling property of the hydrogel with channels. As depicted in Fig. 2B, the swelling ratio values of the bulk, channel, and channel + SP group were 51.0 ± 6.0%, 58.0 ± 3.7%, and 58.0 ± 1.8%, respectively. There was no substantial difference in the swelling ratio between the channel and channel + SP groups. However, their ratios were a little higher than that of the bulk group. It can be assumed that the differences in swelling property between the experimental groups was due to the formed channels and not by the swelling properties of HA or a side effect of SP.
Cell viability in hydrogels is an important issue. In general, cell viability in the central medial region of the hydrogel is lower than that in the boundary region because of the low permeability of oxygen and nutrients. Our previous study showed that the viability of hMSCs cultured in high-molecular-weight hydrogel (200 kDa) was lower than 80% [27]. We compared the viability of L929 cells encapsulated in each biomimetic hydrogel group. As shown in Fig. 3A, in the bulk group, dead cells (shown as red) were observed inside the gel rather than outside the gel. In the channel group, living cells (shown as green) were observed around the microchannels and fewer dead cells were observed compared with the bulk group even inside the gel. In the channel + SP group, living and dead cells showed similar results to that of the channel group. As shown in Fig. 3B, the bulk group showed a cell viability lower than approximately 80%, which is similar to the results of the previous study. However, the channel group showed a cell viability of > 90% at 7 days of culture. This result means that cell viability was enhanced in the channel groups because the media contains oxygen and nutrients that can be transferred through the channels and delivered to the cells by diffusion.
3.2 Differentiation of cells in the biomimetic hydrogel with microchannels
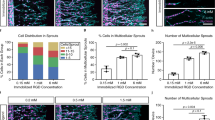
The differentiation and morphology of hMSCs in the biomimetic hydrogel with microchannels are shown in Fig. 4A. Cells were sparsely spread in the bulk group, whereas cells were spread along the microchannels and formed similar vascular structures in the channel group and channel + SP group. In particular, in the channel + SP group, the channel shape was changed because of dense cell migration and cell interaction. The analysis of angiogenic specific genes, such as vWF, CD31, and α-SMA, shows that the expression of vWF in the cells of the channel group, which is an endothelial cell (EC)-specific marker, was three times higher than that in the bulk group (Fig. 4B). In particular, in the channel + SP group, vWF expression was 14 times higher than that in the bulk group. CD31 was also highest in the channel + SP group compared with the other two experimental groups. By contrast, the expression of α-SMA, which is a smooth muscle cell marker of vessel maturation, was highest in the bulk group compared with the other two groups. These results show that the combination of SP (a chemical cue to promote angiogenesis) and the microchannel structure (a physical cue to enhance oxygen and nutrients delivery) facilitates hMSCs differentiation into ECs.
3.3 Evaluation of vascularization in hydrogel with microchannels in vivo
To evaluate the vascularization in vivo, the biomimetic hydrogel with four micro-channels for in vivo test was fabricated by WNM technique using 500 μm wires. The fabricated hydrogel has 10 mm × 10 mm × 5 mm dimensions as shown in Fig. 5. Hydrogels of each groups were implanted in the back of a mouse for one month. As the result, in the bulk group, tissues and blood vessels were not observed throughout hydrogels without microchannels. However, tissues and blood vessels were observed throughout the implanted hydrogels in the channel group and channel + SP group (Fig. S4). Particularly, solid and long blood vessels were observed in the channel + SP group. Microscopic observations showed that there was no blood vessel in growth in the hydrogel of the bulk group (Fig. 6A). Meanwhile, in groups with channels, lines with a tubular structure were confirmed to have generated along the channels. Images were stitched together to observe whole images (Fig. 6B), which confirmed that the ingrowth lines were connected from the beginning to the end of the microchannels. However, there was no significant difference in line coverage in the hydrogels between the channel group and the channel + SP group. To confirm whether these line were cells, cellular actin proteins were stained, as depicted in Fig. 6C. When the channel group was stained by CD31 and α-SMA, the outside region of the tubular structure was dominantly stained by α-SMA (Fig. 7). Furthermore, in the central medial region, EC was stained with CD31. Consequentially, it was confirmed that these were new blood vessels that were generated in the biomimetic hydrogel. Furthermore, it is quite clear that the fabricated microchannels serve as a guidance cue to facilitate vessel formation in the hydrogel.
Immunofluorescence staining of ECs and vascular smooth muscle cells (VSMCs) in channel group four weeks after implantation. In immunofluorescence, ECs are stained with VWF (green), and VSMCs are stained with a-SMA (red). White dotted line: Micro channel line, black square: Part of the blood vessel line to be confirmed by fluorescent staining. (Color figure online)
4 Discussion
Although angiogenesis is an important issue in tissue regeneration, challenges remain, such as hypo- and hypervascularization and vascular patterning during the regeneration of damaged tissues. Channel fabrication for guiding angiogenesis using 3D fabrication technology is in line with classical pore-forming technologies, but it is attracting attention as a novel technology that attempts to overcome the limitations of existing methods of unity, regularity, and interconnection of pores. Attempts to regenerate damaged tissue by increasing angiogenesis via immobilized or encapsulated growth factors have evolved in a variety of ways from drug delivery for controlled release. In this study, we used the WNM technique to construct microchannels in biomimetic hydrogel immobilized with angiogenic peptides and analyzed cell viability, angiogenesis, and vascularization in vitro and in vivo.
We found that cell viability in large-scale biomimetic hydrogels was increased in the experimental group that had microchannels in the hydrogel compared with those without channels. It is known that oxygen and nutrients are transferred to cells in the hydrogel through the fabricated channels [29,30,31]. In our results, groups with microchannels showed a higher cell survival rate than the bulk group (without microchannels). Our results also suggest that hydrogel immobilized with SP showed a substantial increase in the survival rate of cells because SP is known to be involved in increasing cell viability and proliferation [32].
The fabrication of channels and the immobilization of SP in the gel also showed differential cell migration in 3D. When hMSCs were cultured in a hydrogel with microchannels, hMSCs migrated along the preformed channels, whereas hMSCs showed partial spreading in the bulk group. In particular, our results show that hMSCs in the channel + SP group migrated toward the central region and along the channels. It can be postulated that when cell activity around microchannels is improved, more hydrogel is degraded by the secreting MMPs and more SP is released, thus facilitating angiogenesis and migration [33, 34].
For vascularization in the hydrogel, many researchers fabricated the channels and seeded ECs to facilitate the formation of a monolayer inside the channels [35, 36]. Miller et al. [24] showed that HUVEC in the prevascularized matrix affects the metabolic activity of hepatocytes, and Tseng et al. [25] showed the formation of AVANTI by the interaction with NCs. However, there was little evidence that biomolecules in the prevascularized channels had an effect on cell differentiation. Our results show that hydrogels immobilized with SP with microchannels facilitated the differentiation of stem cells into an endothelial cell lineage because the angiogenic factors of EGM-2 media [37] and oxygen are transmitted through the microchannels, and the activation and differentiation of cells are increased, thereby promoting hydrogel degradation by cells and increasing the release of SP [14].
In large tissue defects, rapid ingrowth and penetration of the host vessels is important for regeneration. Rnjak-kovacina et al. [38] fabricated hollow channels in a porous silk hydrogel and confirmed tissue penetration into the hydrogel. We fabricated a network of interconnected pore structures, which is a more complex structure, and applied it in vivo. We showed that all the vessels penetrated along the channels in the matrix. However, in our experiment, unlike the in vitro results, no difference was observed in the vessel formation between the channel + SP group and the channel group. Zhang [39] cultured HUVEC in a hollow channel structure, prevascularized it, and implanted it into the back of mice. As a result, many blood vessel infiltrations were observed in the HUVEC + channel structure, in which HUVECs that secreted nutrients and oxygen through channels secreted more VEGF, and large and stable blood vessels infiltrated the channel. In our in vitro system, the hydrogel is degraded, and the immobilized SP is released by the action of MMPs secreted from the encapsulated cells [28, 40], thus inducing the effects of SP. However, given that there were no cells in the hydrogel in the in vivo experiment, the initial attenuation of the degradation and release of SP affected the degradation and formation of angiogenesis in the gel. The encapsulation of cells secreting MMPs could trigger the effects of SPs in the channel + SP group, as we demonstrated in our in vitro experiments.
We have shown that oxygen and nutrients are delivered through channels, thus resulting in increased cell viability and differentiation. It was also confirmed that blood vessels enter the hydrogel through the channels. This is an important strategy to overcome the limitations of hydrogels, which are difficult to use for large-scale tissue regeneration owing to limited vascularization. Although cells cultured in vitro differentiated into an endothelial cell lineage, the effects of SP immobilized in the hydrogel is still controversial. Nevertheless, the fabrication of microchannels in hydrogel by the WNM technique is a great approach to pattern the vascular structure for the regeneration of large tissue defects.
References
Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–41.
Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–3.
Hall H. Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr Pharm Des. 2007;13:3597–607.
Tabata Y, Miyao M, Ozeki M, Ikada Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J Biomater Sci Polym Ed. 2000;11:915–30.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Zieris A, Chwalek K, Prokoph S, Levental KR, Welzel PB, Freudenberg U, et al. Dual independent delivery of pro-angiogenic growth factors from starpeg-heparin hydrogels. J Control Release. 2011;156:28–36.
Kim SK, Cho TH, Han JJ, Kim IK, Park Y, Hwang SJ. Comparative study of BMP-2 alone and combined with VEGF carried by hydrogel for maxillary alveolar bone regeneration. Tissue Eng Regen Med. 2016;13:171–81.
Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B–PDGFRbeta signaling. J Cell Sci. 2005;118:3759–68.
Chwalek K, Tsurkan MV, Freudenberg U, Werner C. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci Rep. 2014;4:4414.
Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb). 2014;6:555–63.
Kim SK, Lee J, Song M, Kim M, Hwang SJ, Jang H, et al. Combination of three angiogenic growth factors has synergistic effects on sprouting of endothelial cell/mesenchymal stem cell-based spheroids in a 3D matrix. J Biomed Mater Res B Appl Biomater. 2016;104:1535–43.
Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, et al. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation. 2004;109:777–83.
Song M, Jang H, Lee J, Kim JH, Kim SH, Sun K, et al. Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor SDF-1 and angiogenic peptide Ac-SDKP. Biomaterials. 2014;35:2436–45.
Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010;31:8617–25.
Kim JH, Jung Y, Kim BS, Kim SH. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013;34:1657–68.
Charles PT, Goldman ER, Rangasammy JG, Schauer CL, Chen MS, Taitt CR. Fabrication and characterization of 3d hydrogel microarrays to measure antigenicity and antibody functionality for biosensor applications. Biosens Bioelectron. 2004;20:753–64.
Kim G, Park K. Alginate-nanofibers fabricated by an electrohydrodynamic process. Polym Eng Sci. 2009;49:2242–8.
Liu C, Kray J, Chan C. Schwann cells enhance penetration of regenerated axons into three-dimensional microchannels. Tissue Eng Regen Med. 2018;15:351–61.
Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905–25.
Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep. 2016;6:24474.
Fedorovich NE, de Wijn JR, Verbout AJ, Alblas J, Dhert WJ. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng Part A. 2008;14:127–33.
Muehleder S, Ovsianikov A, Zipperle J, Redl H, Holnthoner W. Connections matter: channeled hydrogels to improve vascularization. Front Bioeng Biotechnol. 2014;2:52.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–11.
Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–74.
Tseng TC, Hsieh FY, Theato P, Wei Y, Hsu SH. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials. 2017;133:20–8.
Lee SH, Jo AR, Choi GP, Woo CH, Lee SJ, Kim B, et al. Fabrication of 3d alginate scaffold with interconnected pores using wire-network molding technique. Tissue Eng Regen Med. 2013;10:53–9.
Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830–7.
Kim J, Park Y, Tae G, Lee KB, Hwang SJ, Kim IS, et al. Synthesis and characterization of matrix metalloprotease sensitive-low molecular weight hyaluronic acid based hydrogels. J Mater Sci Mater Med. 2008;19:3311–8.
Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–89.
Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–70.
Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–24.
Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–78.
Hribar KC, Meggs K, Liu J, Zhu W, Qu X, Chen S. Three-dimensional direct cell patterning in collagen hydrogels with near-infrared femtosecond laser. Sci Rep. 2015;5:17203.
Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28:677–84.
Kinstlinger IS, Miller JS. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip. 2016;16:2025–43.
Richards D, Jia J, Yost M, Markwald R, Mei Y. 3D bioprinting for vascularized tissue fabrication. Ann Biomed Eng. 2017;45:132–47.
Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, et al. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7:e46842.
Rnjak-Kovacina J, Wray LS, Golinski JM, Kaplan DL. Arrayed hollow channels in silk-based scaffolds provide functional outcomes for engineering critically-sized tissue constructs. Adv Funct Mater. 2014;24:2188–96.
Zhang W, Wray LS, Rnjak-Kovacina J, Xu L, Zou D, Wang S, et al. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials. 2015;56:68–77.
Kim J, Kim IS, Cho TH, Kim HC, Yoon SJ, Choi J, et al. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A. 2010;95:673–81.
Acknowledgements
This study was supported by a grant from the Ministry of Health and Welfare in the Republic of Korea (HI14C2143).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal experiment procedures were approved by the institutional animal care and use committee of Korea University College of Medicine (KUIACUC-2015-165).
Additional information
Jaeyeon Lee and Se-Hwan Lee contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J., Lee, SH., Lee, BK. et al. Fabrication of Microchannels and Evaluation of Guided Vascularization in Biomimetic Hydrogels. Tissue Eng Regen Med 15, 403–413 (2018). https://doi.org/10.1007/s13770-018-0130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-0130-1