Abstract
The optimal cell culture method of autologous oral mucosal epithelial cell sheet is not well established for a safe transplantation on to the patients’ ocular surface. Animal serum and 3T3 mouse feeder cells are currently being used to stimulate the growth of the epithelial cells. However, the use of animal compounds can have potential side effects for the patient after transplantation of the engineered cell sheet. In the present study, we focused on engineering a rabbit oral mucosal epithelial cell sheet without 3T3 mouse feeder cells using a mix of Dulbecco’s Modified Eagle Medium/Bronchial Epithelial Cell Growth Medium culture media (DMEM/BEGM). Autologous oral mucosal epithelial cell sheets, engineered with DMEM/BEGM feeder cell free culture media, were compared to those cultured in presence of serum and feeder cells. Using a DMEM/BEGM mix culture media, feeder cell free culture condition, autologous oral mucosal epithelial cells reached confluence and formed a multilayered sheet. The phenotype of engineered cell sheets cultured with DMEM/BEGM were characterized and compared to those cultured with serum and feeder. Hematoxylin and eosin staining showed the formation of a similar stratified multilayer cell sheets, in both culture conditions. The expression of deltaN-p63, ABCG2, PCNA, E-cadherin, Beta-catenin, CK3, CK4, CK13, Muc5AC, was similar in both culture conditions. We demonstrated that rabbit autologous oral mucosal epithelial cell sheet can be engineered, in feeder cell free conditions. The use of the DMEM/BEGM culture media to engineer culture autologous oral mucosa epithelial cell sheet will help to identify key factors involved in the growth and differentiation of oral mucosal epithelial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Limbal stem cells, located in the periphery of the cornea, migrate inwards regenerating and renewing the corneal epithelium [1]. In limbal stem cell deficiency (LSCD) however, the corneal epithelium is not renewed properly and the cornea is invaded by blood vessels and conjunctival cells/tissue inducing corneal cloudiness and opacity [2,3,4]. LSCD can be caused by external factors that destroy limbal epithelial stem cells (LESC), such as chemical or thermal injuries, Stevens-Johnson syndrome, ocular cicatricial pemphigoid, multiple surgeries or cryotherapies, prolonged contact lens wear or extensive microbial infection [5].
Long term restoration of visual function requires constant renewal of the corneal epithelium through the replacement by the stem cell population, which led to the development of two different therapies. The first therapy has been achieved by limbal autograft procedure [6]. However, limbal autografts may cause damage to the healthy eye from which the autologous tissue is taken for transplantation. Allografts from cadaver tissue require long-term immunosuppression, which can cause other serious medical complications. In order to avoid the problems associated with limbal autografts and allografts, cell culture laboratories have developed in vitro cultured LESC to engineer “cornea-like epithelium” to be grafted on the LSCD eye. Various materials have been used for culturing and transplanting LESC, such as amniotic membrane, fibrin, or Mebiol gel-A thermo-reversible gelation polymer [7, 8]. Different types of cells also were used to engineer ocular surface tissue for transplantation and to reverse the LSCD phenotype such as [9]: conjunctival epithelial cells [10], embryonic stem cells [11], hair follicle stem cells [12], limbal cells [13] and oral mucosal epithelial cells (OMEC) [14]. The biological mechanism of efficacy experienced by recipients of the cultured LESC and OMEC are unclear, but the clinical results are very promising [15, 16].
Human cell culture of progenitors cells have been used in many cases for autologous grafting, especially in the case of a patient with bilateral LSCD [9]. Rheinwald and Green developed a culture medium called epithelial cell culture medium (ECCM) using 3T3 fibroblast to stimulate growth [17]. Animal serum and 3T3 mouse feeder cells are widely used to stimulate growth of the epithelial cells, however, xeno-contamination is a risk to patients, blocking the translational potential of this technology [18]. In addition to 3T3 mouse feeder cells, OMEC are cultured in presence of fetal bovine serum (FBS) as a key compound for their survival and proliferative effects. The Food and Drug Administration (FDA) has concerns about the use of animal products in engineering tissues for human grafting (http://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/fda/fda_gtindcmc.pdf), even though the use of animal products is currently tolerated, as long as they have been tested for adventitious agents. The use of xenogeneic cells and animal serum is very useful for laboratory studies, and showed much success in the past 30 years [12, 15, 19]. Because of the FDA requirement, more and more laboratories and companies are working on developing a serum and feeder free culture using animal-free compounds for culturing stem cells.
The goal of this study was to use commercially available culture media and compounds to engineer CAOMECS, in feeder cell free conditions. We proposed to mix Dulbecco’s Modified Eagle Medium culture (DMEM) with Bronchial Epithelial Cell Growth Medium culture (BEGM), and expected that this DMEM/BEGM mix would help the growth of the oral mucosal epithelial cells. To confirm the efficacy of DMEM/BEGM culture media, morphology and phenotype of CAOMECS engineered with commercially available culture media were compared to CAOMECS grown in the traditional epithelial cell culture media (ECCM).
2 Materials and methods
2.1 Animal studies
New Zealand white rabbits weighing between 2.5 and 3 kg were used. They were maintained according to the Guidelines of Animal Care, as described by the National Academy of Sciences and published by the Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. This study was approved by the Institutional Animal Care and Use (IACUC) of the Los Angeles Biomedical Research Institute (IACUC No. 20381).
2.2 Isolation of oral mucosal epithelium cells (OMEC)
To perform the interior cheek biopsy, rabbits were lightly sedated and a 6 mm in diameter biopsy was done. The biopsy was taken to a cell culture room to isolate OMEC. OMEC were isolated previously described in [20]. Briefly, after incubating the biopsy with Dispase I for 1 h at 37 °C (Roche Diagnostics GmbH, Mannheim, Germany), the epithelium was peeled off from the lamina propria and subjected to trypsin digestion in order to separate the epithelial cells. Isolated cells were then incubated with Trypan blue (Invitrogen Corp., Grand Island, NY), and counted using Hemocytometer (Incyto, Covington, GA).
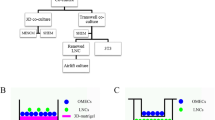
2.3 Engineering cell sheets with oral mucosal epithelium cells on transwell
The Epithelial Cell Culture media (ECCM) is a culture media developed by Rheinwald and H. Green, in 1975. Since that time, no major modification of the culture media composition was made [17]. We decided to use a feeder cell free condition, based on a mix of Dulbecco’s Modified Eagle Medium and Bronchial Epithelial Cell Growth Medium (DMEM/BEGM). BEGM culture media is supplemented with bovine pituitary extract (BPE). BPE is from New-Zealand and it is controlled for infection diseases for humans. We call this mix of DMEM/BEGM: Feeder Cell Free Media (FCFM).
The isolated epithelial cells were seeded on a 6 well plate Transwell (Corning, Tewksbury, MA), at 90,000 cells/cm2, in co-culture with or without mitomycin C (MMC)-treated NIH/3T3 feeder cells [14]. As follows, cells were cultured in presence of 10 ng/ml of EGF with Epithelial Cell Culture Medium (ECCM) and 0.5 ng/ml of epidermal growth factor (EGF) for Dulbecco’s Modified Eagle Medium/Bronchial Epithelial Cell Growth Medium (DMEM/BEGM). All conditions were done in triplicate.
-
Condition 1 OMECs, were cultured with (MMC)-treated NIH/3T3 feeder cells, in ECCM culture media previously described in [14].
-
Condition 2 OMEC, were cultured with (MMC)-treated NIH/3T3 feeder cells, in similar culture media used in previous publications [14, 21]. This culture media is called ECCM. Phosphate Buffered Saline/Ca2+ (PBS/Ca2+) at 1 mM supplementation was also added, to improve the stratification of the cell sheet.
-
Condition 3 OMECs were cultured with BEGM culture media (Lonza, Anaheim, CA) to reach confluence in Corning® Transwell® insert. Once they reached confluence, cell sheets were cultured in a 1:1 mix of DMEM/BEGM culture media in the well, and PBS/Ca2+ at 1 mM in the Corning® Transwell® insert. This culture media is called Feeder Cell Free Media (FCFM).
2.4 H&E and immunocytochemistry staining
After about 2 weeks of growth, engineered multilayer cell sheets were fixed with 10% Neutral Buffered Formalin, for future immunocytochemistry analysis. A portion of the cell sheets were suspended in lysis buffer for protein analysis.
Cell sheets were fixed in 10% neutral buffered formalin, embedded in paraffin, and tissue sections were stained with H&E or used for immunofluorescent staining using deltaN-p63 (Biocare Medical, Concord, CA), ABCG2 (Abcam, Cambridge, MA), CK3 (Immunquest, England), CK4, CK13 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), Muc5AC (LifeSpan BioSciences, Inc., Seattle, WA), E-cadherin, Beta-catenin (BD Biosciences, San Jose, CA) and PCNA (DAKO, Carpinteria, CA). Alexa Fluor® 488 donkey anti-mouse conjugated second antibody (Invitrogen, Eugene, OR) was used. Propidium iodine (Invitrogen, Eugene, OR) was used to stain nuclear DNA. A Nikon 400 fluorescent microscope was used to analyze the slides (Nikon Inc., Melville, NY).
2.5 Western blot
Five micrograms of protein from harvested and lysed CAOMECS were analyzed. Cell homogenates were separated by SDS-PAGE electrophoresis using 12% polyacrylamide gels. Proteins were transferred to a PVDF membrane (Bio-Rad, Hercules, CA) for 1 h in 25 mM Tris-HCl (pH = 8.3), glycine 192 mM and 20% methanol. Antibodies against deltaN-p63 (Biocare Medical, Concord, CA), CK4, PCNA (Abcam Cambridge, MA), E-cadherin (Sigma-Aldrich, St Louis MO), Beta-catenin (Sigma-Aldrich, St Louis, MO), Beta-Actin (Sigma-Aldrich, St. Louis, MO) were used as primary antibodies. Goat anti-mouse and sheep anti-goat antibody (Bio-Rad, Hercules, CA) were used as secondary antibodies. Immunodetection was done using ECL plus (Amersham Bioscience Corp., Piscataway, NJ) or an alkaline phosphatase kit (Bio-Rad, Hercules, CA). Bands were scanned using a GS-800 imaging densitometer (Bio-Rad, Hercules, CA).
3 Results
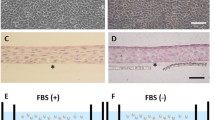
Our first step was to compare the growth of OMECS in different cell culture conditions. OMEC cultured with ECCM reached confluence in the transwell in 6 days, while OMEC cultured with Feeder Cell Free Media (FCFM) reached confluence in 10 days. This indicates that FCFM stimulates OMEC proliferation at a slower pace. ECCM and FCFM had a similar effect on the OMEC differentiation phase to engineer a multistratified cell sheet after it reached confluence. H&E staining was performed on the rabbit oral epithelial mucosa, rabbit eye, cell sheet engineered with 3T3 fibroblast, and cell sheets engineered with FCFM (Fig. 1). Cell sheets engineered with FCFM were very similar to the cell sheets engineered using ECCM, showing both the formation of multilayered cell sheets (Fig. 1D–F). The morphology of the cell sheets was similar in structure to the rabbit epithelial cornea (Fig. 1B).
H&E staining of: A oral mucosa epithelium; B central corneal epithelium; C limbal region of the rabbit eye. D Cell sheet engineered in ECCM culture media; E cell sheet engineered in ECCM culture media, with PBS/Ca2+; F cell sheet engineered with SFM1 culture media. Scale bar = 254 µm. Multilayer CAOMECS could be engineered in all cell culture conditions, and their morphology is similar to the central cornea
Another important aspect of the successful CAOMECS production is to ensure that the multilayered cell sheet can renew itself, to mimic the renewal of the corneal epithelium by the limbal stem cells. We studied the expression of potential progenitor stem cells markers: deltaN-p63, and ABCG2. DeltaN-p63 is normally expressed on the basal cells of the microvilli of the oral mucosa (Fig. 2A) and on the limbal area of the rabbit eye (Fig. 2C), but its expression is absent on the corneal epithelium (Fig. 2B). All cell sheets express deltaN-p63 on the basal side indicating the renewal properties of the cells and of the cell sheet (Fig. 2D–F). ABCG2 was expressed in the basal cells of the oral mucosa (Fig. 2G) in the nucleus and on the membrane, where it formed speckles. Similar result was found on the cell sheet, where ABCG2 was expressed in the nucleus (Fig. 2J–L). ABCG2 was also well expressed at the membrane of limbal stem cells (Fig. 2I), but not on the epithelium of the central cornea (Fig. 2H). As noticed on the oral mucosa, ABCG2 was expressed on the basal side of the cell sheets and in the nucleus (Fig. 2J–L). FCFM culture media did not affect the expression of deltaN-p63 and ABCG2 in the cell sheets, when compared to cell sheets grown using ECCM. PCNA was well expressed in the basal cells of the oral mucosa, indicating the high proliferative rate of these cells, which are involved in the renewal of the epithelium mucosa (Fig. 2M) [22]. PCNA was also expressed on the limbal region, as was deltaN-p63, for renewal of the corneal epithelium (Fig. 2O). All engineered cell sheets expressed PCNA which could be involved in cell proliferation and DNA repair (Fig. 2P–R).
A–F Show the expression of DeltaN-p63 on oral mucosa epithelium, central corneal epithelium, limbus, and engineered cell sheet. G–L Show the expression of ABCG2 on oral mucosa epithelium, central corneal epithelium, limbus, and engineered cell sheet. M–R Show the expression of PCNA on oral mucosa epithelium, central corneal epithelium, limbus, and engineered cell sheet. Nuclear DNA was stained with propidium iodine. (Scales bars are from 50–100 µm). Stem cells markers and proliferative markers were preserved in the FCFM culture media compared to ECCM. Nota Bene: In Fig. 2G, the tissue in the rectangle is a higher modification of the framed oral mucosa epithelium
Another important criterion of the successful CAOMECS production is the formation of a stratified multilayer, similar to the stratified epithelium covering the corneal stroma (Fig. 1). We observed the presence of CK3 not only on the oral mucosa epithelium, but also on the corneal epithelium and the limbus (Fig. 3A–C). However, none of culture media conditions maintained the expression of CK3 during the entire culture and cell sheet engineering process (Fig. 3D–F). CK4, another type II keratin filament, was well expressed in the stratified layer of the oral mucosa and on the limbus region of the eye (Fig. 3G/I). It is normally not expressed on the central cornea (Fig. 3H). ECCM culture media maintained CK4 expression in the apical layers of the cell sheet (Fig. 3J), while the expression was much weaker when PBS/Ca2+ was added to ECCM (Fig. 3K). The expression of CK4 was decreased using FCFM culture medium compared to ECCM, but slightly higher compared to ECCM/PBS/Ca2+. These two observations were confirmed by the Western blot. PBS/Ca2+ supplementation might have helped in the cell sheets’ final differentiation step, to a phenotype similar to that of a wild type cornea (Fig. 3B/H/N). We observed a similar expression pattern of CK13 with CK4 for all the tissue (Fig. 3M–R).
Expression of CK3 (A–F), CK4 (G–L) and CK13 (M–R) on oral mucosa epithelium, central corneal epithelium, limbus, and engineered cell sheet. Nuclear DNA was stained with propidium iodine. (Scales bars are 100 µm). The expression of differentiation markers are preserved in the in SFM1, compared to ECCM culture media conditions
E-cadherin is an important transmembrane protein involved in the formation of adherens junctions. E-cadherin was well expressed on the oral mucosa (Fig. 4A), on the limbus region (Fig. 4C) but less on the central cornea (Fig. 4B). All cell sheets expressed E-cadherin, showing strong cell-cell connection (Fig. 4D–F). However, we noticed that PBS/Ca2+ supplementation disturbed the localization of E-cadherin all over the cell sheet (Fig. 4D–F). A similar expression pattern was observed with Beta-catenin. Beta-catenin and E-cadherin were expressed in the same area on the oral mucosa, central cornea, and limbus (Fig. 4G–I). Muc5AC, a specific marker of goblets cells present only on the conjunctiva [23], was detected only on the conjunctiva (Fig. 5C) but not in the oral mucosa and cell sheets (Fig. 5A–F).
Expression of E-cadherin (A–F), Beta-catenin (G–L) on oral mucosa, corneal epithelium, limbus, and engineered cell sheet. Nuclear DNA was stained with propidium iodine. (Scales bars are 100 µm). The expression of cell–cell connection is preserved in the in SFM1, compared to ECCM culture media conditions
Expression of Muc5AC on A oral mucosa; B corneal epithelium; C limbal region of the rabbit eye. D Cell sheet engineered in ECCM culture media; E cell sheet engineered in ECCM culture media, with PBS/Ca2+; F cell sheet engineered with SFM1 culture media. Nuclear DNA was stained with propidium iodine. (Scale bar is 100 µm)
We confirmed the results obtained by immunostaining with western blots (Fig. 6). The expression of Beta-catenin, PCNA and E-cadherin were stable in the three culture conditions (Fig. 6). The expression of all deltaN-p63 isoforms was unchanged in the ECCM and condition 2 culture conditions. However, the expression of deltaN-p63 g inexplicably decreased in the ECCM/PBS/Ca2+ condition, when the expression of deltaN-p63 alpha was increased. The expression of PCNA, E-cadherin, and Beta-catenin were stable in all cell culture conditions. CK4 expression decreased in the ECCM/PBS/Ca2+ condition, confirming immunocytostaining observations (Fig. 3J–L).
4 Discussion
For the past 10 years, companies and laboratories have been trending towards developing serum free culture media for translational purposes. Companies are trying different methodologies to culture cells and engineer cell sheets in animal-free product conditions. One of the first steps to overcome the use of animal serum was the use of autologous patient serum. This showed promising results and lead to a viable alternative for growing keratinocytes in vitro [24]. Okano’s group in Japan succeeded in engineering cell sheets in the absence of feeder cells, using human or dog OMEC and autologous serum [21, 25]. Cell sheets were well stratified and their phenotypes were similar to cell sheets engineered with serum and 3T3 feeder cells [26, 27]. Autologous serum is an alternative approach to not use animal serum for the culture of human cells; however, it requires large volume of blood volume to obtain a sufficient amount of serum for cell culture. This process also requires more safety testing to avoid contamination during cell culture increasing the price of production. Sugiyama et al. [28] used adipocyte tissue-derived mesenchymal stem cells to replace feeder cells with success. The use of adipocyte tissue-derived mesenchymal stem cells is an interesting approach because of their high availability and low immunoreactivity, but it involves additional steps in the control of cell sheet production such as their isolation and their characterization from each patient. Kolli et al. used the patient’s serum and amniotic membrane as feeder cells. Cells cultured on amniotic membrane grew faster than isolated OMEC co-cultured with 3T3J2. However, it is important to note that biopsies were placed entirely on the amniotic membrane, rather than isolated OMEC [20]. Amniotic membrane is a good support for cell growth but their availability is limited. The amniotic membrane’s ability to act as a cell sheet support vector can also affect reproducibility due to lot variability [29]. Our laboratory tried to engineer CAOMECS, using completed ECCM culture media without 3T3 feeder cells but we could not engineer multilayer cell sheets (data not shown). This result indicated that fetal bovine serum (FBS) alone is not enough to stimulate the growth of cells, but shows the importance of factors secreted by 3T3 feeder cells in stimulating the proliferation and the trans-differentiation of the OMEC. These exocrine signal factors are not yet identified, but it could be very interesting to identify them to develop new serum free culture media for epithelial cells. In our experiments, we used Feeder Cell Free Media (FCFM) in absence of 3T3 feeder cells, and we succeeded in engineering CAOMECS. Their phenotype was similar to that of CAOMECS engineered with ECCM in the presence of 3T3 feeder cells. The expression of progenitor stem cell markers or proliferative markers was confirmed. DeltaN-p63 is a transcription factor involved in the renewal of the ectoderm and appendage development [30, 31]. Its expression was detected in the basal layer of the CAOMECS and the oral mucosa epithelium, playing a critical role in the renewal of the CAOMECS upper layer. However, Western blot analysis showed that we detected the expression of the three deltaN-p63 isoforms. Di Lorio et al. described the expression of the three isoforms in the cornea and limbus [32, 33]. DeltaN-p63g is mainly expressed on the top cell layers of the limbus and cornea. The expression of deltaN-p63g is increased in the limbus and cornea when the cornea is wounded, and when cells need to be replaced. In our conditions, deltaN-p63g expression was decreased, indicating a decrease in proliferation however, this reduction in proliferation was not noticed during the growth of the cells. On the contrary, deltaN-p63g was less expressed in the condition ECCM/PBS, where the cells grew slower. It is not clear what the exact role of deltaN-p63g is, but its function and expression seem to be controlled by the microenvironment of the cells. ABCG2, another progenitor stem cell marker was detected on CAOMECS. ABCG2 belongs to the superfamily of ATP-Binding Cassette (ABC) transporters and is involved in cell drug resistance. PCNA, a proliferative marker, was also detected on the basal layers of the oral mucosa epithelium and the CAOMECS. The combination of the expression of Delta-Np63 and PCNA indicated the capacity of CAOMECS and oral mucosa epithelium to be renewed. In addition, PCNA was also detected on the central cornea, which might indicate a potential role of PCNA in DNA repair, under the exposure of corneal epithelial cells to ultra violet light [34].
There have only been a few OMEC studies using animal serum free and feeder cell free define culture conditions to engineer a multistratified cell sheet. Ilmarinen et al. used human oral mucosal epithelial cells without 3T3 feeder cells and in serum free conditions [26]. Using similar conditions, we did not succeed in engineering cell sheets, possibly because we used rabbit cells, which usually have a lower colony forming efficiency (CFE) than human cells [35]. Another important factor that could explain the difference of results is the percentage of deltaN-p63 positive cells after isolation of oral mucosal epithelial cells. Rama et al. [36] showed that the percentage of limbal stem cells expressing deltaN-p63 could predict the success or the failure of cell sheet engineering. In all reported papers, multilayer cell sheet were successfully engineered, in absence of feeder cell and/or serum. Stratification of the cell sheet is an important criterion for a different reason. First, the structure of the cell sheet must be similar to the targeted epithelium to be replaced. Second, it is important that the proliferative progenitor stem cells of the basal layer are capable to differentiate into flat squamous epithelial cells. The expression of cytokeratin was confirmed by immunostaining and western blot. CK3, a type II keratin filament, is mainly expressed on differentiated layers of the corneal epithelium. Its expression was not maintained in our cell culture conditions, but it was also observed in another publications [37]. Nonetheless, after grafting on an LSCD rabbit model, we did observe the return of CK3 expression [38]. CK13, a type I keratin filament, is associated with CK4. CK4 and CK13 were both expressed in the limbus area, and also on the apical layers of the cell sheets. Cytokeratins are important proteins, forming a network going through the entire cells, from the membrane to the nucleus [39]. They are part of the intermediate filament and play a role in barrier function on the epithelium [39]. When CAOMECS engineered with ECCM were grafted back onto rabbit cornea with LSCD, the expression of CK13 was lower on the central cornea compared to the CAOMECS [38]. This indicates that the contact with lacrimal fluid, air contact or eye lid, or the combination of these can reduce the expression of CK13 in grafted cell sheets. CK13 was also expressed in presence of FCFM culture media. Third, the most important criterion is to show that the cell-cell connection is well established, demonstrating that the migratory potential of the cells is very low. In all the cell culture conditions, we showed the presence of adherens junction. Engineered CAOMECS expressed E-cadherin and Beta-catenin, which are a very important compound of the adherens junction [40,41,42]. These junctions play a major role to stabilize the cell-cell interaction, avoiding a random migration of cells [43, 44].
Airlifting is also used for the epithelium cell culture, to improve the trans-differentiation of the epithelial cells [45]. We observed that the airlifting method caused dryness and keratinization of our cell sheets (data not shown), while Illmarinen et al. did not observe this phenomenon [26]. We decided to cover the confluent cell sheet with PBS supplemented with 1 mM Ca2+ to avoid its keratinization of the apical side of the cell sheet and to stimulate the differentiation with Ca2+ supplementation [46]. However, Ca2+ did not seem to play a major role in the differentiation processes of the cell sheet when the cell sheet’s morphology was compared (Fig. 1D/E). Instead, it seemed to disturb the cellular distribution of E-cadherin and Beta-catenin. These two proteins are crucial in maintaining the integrity of the cell sheet, preventing uncontrolled cell migration (Fig. 4D–F/J–L) [47]. ABCG2 is an important protein, usually localized at the surface of the membrane, and is involved in drug-resistance. ABCG2 protein was detected at the membrane of limbal progenitor stem cells (Fig. 2I). However, in the oral epithelium mucosa and in all engineered cells sheet, ABCG2 was localized at the nucleus of basal cells. It was recently shown that ABCG2 localized in the nucleus and acts as a transcription factor, binding the E-Box of E-cadherin promoter, and increased the expression of E-cadherin [48]. Due to cell mobility being a major concern for patient safety, E-cadherin expression might reduce this risk preventing cell migration to an ectopic location in the body [48].
Also, the composition of the culture media has a major role in the proliferation and differentiation of the OMEC into CAOMECS. We decided to modify the composition of the culture media to observe its influence on the growth of OMEC by mixing DMEM and BEGM culture media. DMEM is a classic culture media used to amplify many types of cells in vitro. However, BEGM is a more specific culture medium used to amplify epithelial cells. Using the protocol described in Jeon et al. [49], we successfully cultured rabbit OMEC (instead of human conjunctival cells) without 3T3 feeder cells, but supplemented with BPE. We demonstrated that the production of CAOMECS can be done in absence of 3T3 feeder cells, by using FCFM culture media. In the presence of 3T3 feeder cells and animal serum, OMEC grew at a higher rate than OMEC grown in FCFM, but the time needed to engineer CAOMECS was similar. Furthermore, the morphology and the phenotype of the two types of cell sheets were very similar, indicating that feeder cells free culture conditions could replace epithelial cell culture conditions with animal serum and feeder cells. Kolli et al. [20] showed that with a well-controlled environment and steps for cell culture OMEC can grow much faster, up to 70% faster than OMEC cultured in regular laboratories.
The Food and Drug Administration (FDA) has recently raised questions about cell culture conditions, such as phenotype, cell potency and genomic mutations, and xenogeneic contamination during cell culture. The FDA established regulations for the use of stem cells for human cell therapy, mainly to protect patients from contamination and diseases. Drabiak-Syed et al. [50] published a complete review of FDA concerns about ex vivo use conditions of stem cells for human therapy. Indeed, laboratories are still culturing the cells in vitro as they are culturing cells lines, by using animal serum and/or xenogeneic cells (such as 3T3 mouse NIH fibroflast). We think that the culture of cells in serum and xeno free culture condition is a very important step in translational research. Each cell type will require a specific culture condition that will take time to develop for human applications. The development of animal serum free and feeder cell free well-controlled culture conditions are primordial for human application, but this will require many tests on animal studies or artificial human organs. We understand it will not be easy to use the discussed culture condition for translational purposes; however, our results show that we can avoid the use mitomycin C (MMC)-treated NIH/3T3 feeder cells, and that BPE factors are enough to stimulate the growth of the cells, raising the question: What are these BPE factors? It will be very interesting to determine these factors by comparing the clean-tested BPE lots and human autologous serum, to develop a well chemically-defined culture media for human application.
References
Tseng SC. Concept and application of limbal stem cells. Eye (Lond). 1989;3:141–57.
Bertalanffy FD, Lau C. Mitotic rate and renewal time of the corneal epithelium in the rat. Arch Ophthalmol. 1962;68:546–50.
Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–85.
Haddad A. Renewal of the rabbit corneal epithelium as investigated by autoradiography after intravitreal injection of 3H-thymidine. Cornea. 2000;19:378–83.
Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1:110–5.
Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22.
Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–75.
Sitalakshmi G, Sudha B, Madhavan HN, Vinay S, Krishnakumar S, Mori Y, et al. Ex vivo cultivation of corneal limbal epithelial cells in a thermoreversible polymer (Mebiol Gel) and their transplantation in rabbits: an animal model. Tissue Eng Part A. 2009;15:407–15.
He H, Yiu SC. Stem cell-based therapy for treating limbal stem cells deficiency: a review of different strategies. Saudi J Ophthalmol. 2014;28:188–94.
Silber PC, Ricardo JR, Cristovam PC, Hazarbassanov RM, Dreyfuss JL, Gomes JA. Conjunctival epithelial cells cultivated ex vivo from patients with total limbal stem cell deficiency. Eur J Ophthalmol. 2015;25:60–4.
Meyer-Blazejewska EA, Call MK, Yamanaka O, Liu H, Schlötzer-Schrehardt U, Kruse FE, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells. 2011;29:57–66.
Lam PK, Chan ES, Yen RS, Lau HC, King WW. A new system for the cultivation of keratinocytes on acellular human dermis with the use of fibrin glue and 3T3 feeder cells. J Burn Care Rehabil. 2000;21:1–4.
Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol. 2000;84:273–8.
Hayashida Y, Nishida K, Yamato M, Watanabe K, Maeda N, Watanabe H, et al. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46:1632–9.
Burillon C, Huot L, Justin V, Nataf S, Chapuis F, Decullier E, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53:1325–31.
Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3.
Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–43.
Llames S, García-Pérez E, Meana Á, Larcher F, del Río M. Feeder layer cell actions and applications. Tissue Eng Part B Rev. 2015;21:345–53.
Miyashita H, Shimmura S, Higa K, Yoshida S, Kawakita T, Shimazaki J, et al. A novel NIH/3T3 duplex feeder system to engineer corneal epithelial sheets with enhanced cytokeratin 15-positive progenitor populations. Tissue Eng Part A. 2008;14:1275–82.
Kolli S, Ahmad S, Mudhar HS, Meeny A, Lako M, Figueiredo FC. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells. 2014;32:2135–46.
Takagi R, Murakami D, Kondo M, Ohki T, Sasaki R, Mizutani M, et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253–9.
Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–40.
Ma DH, Yeh LK, Chen HC, Chang AM, Ho YJ, Chang SH, et al. Epithelial phenotype in total sclerocornea. Mol Vis. 2014;20:468–79.
Smola H, Reinke M, Shephard P, Krieg T, Hess S. Autologous patient serum for the culture of keratinocyte transplants reduces risk of transmittable disease. Lancet. 1999;353:641–2.
Murakami D, Yamato M, Nishida K, Ohki T, Takagi R, Yang J, et al. The effect of micropores in the surface of temperature-responsive culture inserts on the fabrication of transplantable canine oral mucosal epithelial cell sheets. Biomaterials. 2006;27:5518–23.
Ilmarinen T, Laine J, Juuti-Uusitalo K, Numminen J, Seppänen-Suuronen R, Uusitalo H, et al. Towards a defined, serum- and feeder-free culture of stratified human oral mucosal epithelium for ocular surface reconstruction. Acta Ophthalmol. 2013;91:744–50.
Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, Tsuzuki M, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–16.
Sugiyama H, Maeda K, Yamato M, Hayashi R, Soma T, Hayashida Y, et al. Human adipose tissue-derived mesenchymal stem cells as a novel feeder layer for epithelial cells. J Tissue Eng Regen Med. 2008;2:445–9.
Wang JS, Xie HT, Zhang MC. Characterization of ex vivo expanded oral mucosal epithelium cells on acellular porcine corneal stroma for ocular surface reconstruction. J Ophthalmol. 2017;2017:6761714.
Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8.
Ince TA, Cviko AP, Quade BJ, Yang A, McKeon FD, Mutter GL, et al. p63 Coordinates anogenital modeling and epithelial cell differentiation in the developing female urogenital tract. Am J Pathol. 2002;161:1111–7.
Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A. 2005;102:9523–8.
Lindsay J, McDade SS, Pickard A, McCloskey KD, McCance DJ. Role of DeltaNp63gamma in epithelial to mesenchymal transition. J Biol Chem. 2011;286:3915–24.
Iyama T, Wilson DM 3rd. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst). 2013;12:620–36.
Kondo M, Yamato M, Takagi R, Murakami D, Namiki H, Okano T. Significantly different proliferative potential of oral mucosal epithelial cells between six animal species. J Biomed Mater Res A. 2014;102:1829–37.
Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55.
Joseph A, Powell-Richards AO, Shanmuganathan VA, Dua HS. Epithelial cell characteristics of cultured human limbal explants. Br J Ophthalmol. 2004;88:393–8.
Bardag-Gorce F, Oliva J, Wood A, Hoft R, Pan D, Thropay J, et al. Carrier-free cultured autologous oral mucosa epithelial cell sheet (CAOMECS) for corneal epithelium reconstruction: a histological study. Ocul Surf. 2015;13:150–63.
Salas PJ, Forteza R, Mashukova A. Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity. Tissue Barriers. 2016;4:e1178368.
Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–66.
Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:4734–41.
Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–17.
Pećina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17.
Gallagher SJ, Rambow F, Kumasaka M, Champeval D, Bellacosa A, Delmas V, et al. Beta-catenin inhibits melanocyte migration but induces melanoma metastasis. Oncogene. 2013;32:2230–8.
Osei-Bempong C, Henein C, Ahmad S. Culture conditions for primary human limbal epithelial cells. Regen Med. 2009;4:461–70.
Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–46.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22.
Liang SC, Yang CY, Tseng JY, Wang HL, Tung CY, Liu HW, et al. ABCG2 localizes to the nucleus and modulates CDH1 expression in lung cancer cells. Neoplasia. 2015;17:265–78.
Jeon S, Choi SH, Wolosin JM, Chung SH, Joo CK. Regeneration of the corneal epithelium with conjunctival epithelial equivalents generated in serum- and feeder-cell-free media. Mol Vis. 2013;19:2542–50.
Drabiak-Syed K. Challenging the FDA’s authority to regulate autologous adult stem cells for therapeutic use: celltex therapeutics’ partnership with RNL Bio, substantial medical risks, and the implications of United States v. Regenerative Sciences. Health Matrix Clevel. 2013;23:493–535.
Acknowledgement
Supported by Emmaus Medical, Inc (Torrance, California, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
This study was approved by the Institutional Animal Care and Use of the Los Angeles Biomedical Research Institute (IACUC No. 20381).
Rights and permissions
About this article
Cite this article
Oliva, J., Ochiai, K., Florentino, A. et al. Feeder Cells Free Rabbit Oral Mucosa Epithelial Cell Sheet Engineering. Tissue Eng Regen Med 15, 321–332 (2018). https://doi.org/10.1007/s13770-017-0108-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0108-4