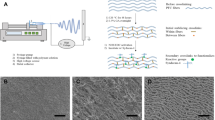
Abstract
Expanded polytetrafluoroethylene (ePTFE) polymers do not support endothelialization because of nonconductive characteristics towards cellular attachment. Inner surface modification of the grafts can improve endothelialization and increase the long-term patency rate of the ePTFE vascular grafts. Here we reported a method of inner-surface modification of ePTFE vascular graft with extracellular matrix (ECM) and CD34 monoclonal antibodies (CD34 mAb) to stimulate the adhesion and proliferation of circulating endothelial progenitor cells on ePTFE graft to enhance graft endothelialization. The inner surface of ECM-coated ePTFE grafts were linked with CD34 mAb in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) solution and the physicochemical properties, surface morphology, biocompatibility, and hemocompatibility of the grafts were studied. The hydrophilicity of CD34 mAb-coated graft inner surface was significantly improved. Fourier transform infrared spectroscopy analysis confirmed ECM and CD34 mAb cross-linking in the ePTFE vascular grafts with our method. Scanning electron microscopy analysis showed protein layer covering uniformly on the inner surface of the modified grafts. The cell-counting kit-8 (CCK-8) assay confirmed that the modified graft has no obvious cytotoxicity. The modified graft showed a low hemolytic rate (0.9%) in the direct contact hemolysis test, suggesting the modification improved hemocompatibility of biopolymers. The modification also decreased adhesion of platelets, while significantly increased the adhesion of endothelial cells on the grafts. We conclude that our method enables ePTFE polymers modification with ECM and CD34 mAb, facilitates endothelialization, and inhibits platelet adhesion on the grafts, thus may increase the long-term patency rate of the prosthetic bypass grafts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Synthetic vascular grafts are commonly used as surgical bypass to accomplish arterial revascularization. Expanded polytetrafluoroethylene (ePTFE) is one of the most commonly used polymers for vascular grafts. Although large-diameter ePTFE grafts have gained great success in clinical applications, small-diameter (<6 mm) synthetic vascular grafts are unsatisfactory, due to their poor long-term patency rate, which is usually attributed to the comparatively lower blood-flow velocity in small arteries [1,2,3]. Low blood flow in the synthetic vessel graft can cause luminal thrombosis and intimal hyperplasia [4, 5]. One approach to address this issue is to facilitate the endothelialization of the graft inner surface [6]. Endothelial cells maintain a homeostatic defense mechanism that regulates inflammation and anticoagulation [7, 8]. Thus, various researches have been done to endothelialise vascular grafts in vitro using autologous endothelial cells before implantation in in vivo tissue engineering [9, 10]. However, the ePTFE polymers do not support endothelialization because of the nonconductive characteristics towards cellular attachment [11]. Therefore, there is a need to modify the inner surface properties of ePTFE vascular grafts to improve endothelialization of the grafts.
The discovery of circulating endothelial progenitor cells (EPCs) opened up new perspectives for in vitro endothelialization of synthetic vascular grafts before implantation [12]. EPCs are a subset of CD34 + circulating mononuclear cells that have the potential to proliferate and differentiate into mature endothelial cells [9, 12, 13]. Synthetic vascular grafts biofunctionalized with capture molecules for circulating EPCs can be used as attracting agents to mimic selective homing factors. Circulating EPCs in the blood stream remain positive for CD34 and VEGFR-2, but obviously lose CD133 [14]. Thus, antibodies against CD34 that immobilized on the surfaces of vascular grafts may have a high affinity and selectivity to EPCs. This modification may effectively promote rapid in situ endothelialization [10, 15]. Nevertheless, in situ endothelialization of ePTFE vascular grafts can be hampered by the highly hydrophobic property of the ePTFE polymers [16, 17]. Extracellular matrix (ECM) can support endothelial growth and plays a critical role in the adhesion, migration and proliferation of endothelial cells [18, 19]. Therefore, ECM modification of ePTFE can mimic native vessel architecture and achieve enhanced endothelialization of vascular grafts [20,21,22].
In this study, we developed a method for coating ePTFE vascular grafts with ECM scaffolds and CD34 monoclonal antibodies (CD34 mAb) to improve endothelialization and increase the long-term patency rate of ePTFE vascular grafts. Physisorption was used to coat ECM proteins in the inner surfaces of ePTFE grafts, and then CD34 mAb was crosslinked to the coated-ECM. The modified ePTFE grafts showed good biocompatibility and hemocompatibility, and facilitated endothelial cell adhesion as evaluated by static and dynamic cell adhesion assays. Here we describe our method and discuss the advantage of the ECM/CD34 mAb-modified ePTFE graft in in vivo vascular engineering.
2 Materials and methods
2.1 Materials
The ePTFE grafts were purchased from W. L. Gore & Associates (USA). Growth factor-reduced Matrigel (BD Biosciences; USA) was five-fold diluted with dulbecco’s modified eagle medium (DMEM; Gibco, USA). EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride), NHS (N-hydroxysuccinimide), and MES [2-(N-morpholino) ethanesulfonic acid] were obtained from Thermo Fisher Scientific (USA). Anti-human CD34 monoclonal antibody (IgG2a, epitope class III) was purchased from Biolegend (USA). Human umbilical vein endothelial cells (HUVECs) were purchased from Cascade Biologics-Thermo Fisher Scientific (USA). Fetal bovine serum (FBS) was purchased from Gibco (USA). Chemicals and histopaque 1077 used for the experiments were purchased from Sigma-Aldrich (USA) unless noted otherwise. Water was deionized using a Milli-Q reagent water system (Millipore, USA). Endothelial growth medium (EGM-2) bullet Kit were purchased from Lonza (Walkersville, MD).
2.2 Surface preparation and modification
Synthetic ePTFE grafts were presoaked in methanol and washed with sterile water. Then they were exposed to acidified glycerol (7.48 M H2SO4 in 20% glycerol) in boiling water bath for 4 h with intermittent shaking. They were thoroughly washed with sterile deionized water before exposing to 10% citric acid solution (Wt/V in sterile water) at 37 °C for 3 h. After these pretreatments, ePTFE grafts were ready for the fabrication of ECM scaffolds, as depicted in Supplementary Figure S1. Briefly, ePTFE grafts were immersed in five-fold diluted Matrigel solution at 37 °C for 1 h. Then the coated grafts were gently rinsed with phosphate-buffered saline (PBS) once, followed by nitrogen stream drying. Finally, we repeat the soaking and drying steps again, permitting solidification of Matrigel on the luminal surface of the grafts.
CD34 mAb were covalently crosslinked with Matrigel proteins by activating carboxyl group through the functionalities of EDC/NHS solution. Matrigel-coated ePTFE grafts were incubated in 0.1 M MES buffer solution (pH 6.0) containing 2 mM EDC and 5 mM NHS for 15 min at room temperature and centrifuged at 1500 rpm for 5 min to eliminate air bubbles. Next, the CD34 mAb solution (50 μg/ml) was added and the solution was gently stirred for 2 h at room temperature. At the end of this process, the Matrigel/CD34 mAb-coated grafts were washed three times with sterile deionized water.
2.3 Surface characterization
2.3.1 Contact angle
Water contact angle measurement helps to understand the hydrophilicity of the ePTFE surface. The ePTFE grafts were flattened and square pieces of 5 × 5 mm were obtained. The water contact angle was measured using the sessile drop method at room temperature in air. A drop volume of 4 μl deionized water was dropped on the sample and the angle between the edge of the drop and the surface was measured with OCA20 contact angle goniometer (DataPhysics, Germany). A total of five different samples were measured in the analyses: bare ePTFE, EDC/NHS, solution-acidified ePTFE (acidified ePTFE), ECM-coated ePTFE (ECM-ePTFE), and ECM/CD34 mAb-coated ePTFE (ECM/CD34 mAb-ePTFE). Data are represented as mean contact angle of the samples.
2.3.2 Fourier transform infrared spectroscopy (FTIR)
FTIR is one of the main tools for the quantitative study of the composition in material surface modifications. Here, we analyzed the ePTFE samples of each steps with a Nicolet 6700 FTIR spectrometer (Thermo Scientific, USA) equipped with a horizontal attenuated total reflectance attachment (Ge prism crystal plate, PIKE Technologies, USA). Spectra were collected by 128 scans with a 4 cm−1 resolution ranging from 400 to 4000 cm−1. Curve fitting was carried out by Origin 8.0.
2.3.3 Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was conducted to visually observe whether ECM is uniformly coated on the ePTFE surface. Samples were fixed in 4% paraformaldehyde and 2.5% glutaraldehyde, dehydrated in an ethanol series, dried in a CO2 critical point dryer (Hitachi, Japan), and coated with gold plate by a sputterer (JFC-1600; 15 mA, 60 s) prior to imaging. SEM images were obtained with a JSM-6380LA scanning electron microscope (JEOL, Japan) using an accelerating voltage of 20 kV. Each sample was analyzed at high magnification (1000×) at predetermined location.
2.3.4 Cytotoxicity test
Human umbilical vein endothelial cells (HUVECs) were adopted to evaluate the cytotoxicity of the surface modification procedure. HUVECs were maintained in DMEM plus 10% FBS with 100 U/ml penicillin, 100 g/ml streptomycin. The cytotoxicity tests were carried out by indirect contact. The leaching solutions of each ePTFE samples were prepared using serum-free DMEM media as the extraction medium with the surface area of extraction medium ratio 1.0 ml/cm2 in a humidified atmosphere with 5% CO2 at 37 °C for 72 h. The supernatant fluid was withdrawn and centrifuged to prepare the extraction medium, then refrigerated at 4 °C, and the supernatant was harvested for the cytotoxicity tests.
The Cell Counting Kit-8 (CCK-8) assay kit was used in the cytotoxicity tests according to the manufacturer’s instructions (Dojindo, Japan). Briefly, HUVECs were seeded at 5 × 103 cells/well into 96-well plates and cultured in a 5% CO2 incubator at 37 °C for 12 h to allow attachment. The medium in each well was then replaced with different leaching solutions and cultured for 24 h. The cells were subsequently incubated with 10 μl of CCK-8 reagent for 1 h at 37 °C and the supernatant was then transferred to a new 96-well plate. The O.D.450 value was measured with a microplate absorbance reader (Tecan Sunrise, Switzerland). The control groups involved the use of DMEM medium as negative controls and 0.64% phenol DMEM medium as positive controls. Six replicates (n = 6) were performed for each sample.
2.3.5 Hemolysis test
Hemolysis test was performed by modification of the direct contact method [23]. Briefly, healthy human blood from a volunteer was collected into plastic tubes with sodium citrate (3.8 wt.%) in the ratio of 9:1. The ePTFE sample (about 1 g) was dipped in a standard tube containing 9 ml of PBS at 37 °C for 30 min. Then 1 ml blood was added to the standard tube and the mixture was incubated at 37 °C for 1 h. Similarly, 1 ml of blood diluted with 9 ml of normal saline solution and 9 ml of sterilized deionized water was used as negative (0% hemolysis) and positive (100% hemolysis) control, respectively. Then the tubes were centrifuged at 3000 rpm for 5 min and the supernatants were taken for the estimation of free hemoglobin. Absorbance (O.D.) was determined using a spectrophotometer at 545 nm.
The hemolysis was calculated as follows:
2.3.6 Platelet adhesion test
Whole blood was drawn from healthy adult volunteers by venipuncture into ACD anticoagulant (BD Biosciences, USA). The blood was centrifuged at 250g for 15 min to obtain platelet-rich plasma (PRP) supernatant. The platelet concentration of PRP was measured by an automatic CBC analyzer and then adjusted to 5 × 107 cells/ml in the platelet suspension buffer (PSB) as described previously [24]. Samples of ePTFE grafts were cut into disks using a cork borer to match the surface area of 96-well plates and gently pinned down to remain in place. Then these disks were incubated with the diluted PRP for 1 h at 37 °C under static conditions. The suspension was aspirated and each well was rinsed carefully three times with PBS.
Platelet adhesion levels were quantified using a lactate dehydrogenase (LDH) assay [24]. Briefly, adherent platelets were lysed by incubation with 0.1% Triton X-100/PSB buffer for 15 min at 37 °C. A colorimetric substrate for LDH (Roche, Germany) was added and incubated for 20 min at 37 °C. The reaction was stopped with the addition of the stop solution. The optical density was measured at 490 nm with reference wavelength of 650 nm. A calibration curve was generated from a series of serial dilutions of a known platelet concentration and used to determine the number of adhered platelets. The values were expressed as the average number of adhered platelets per cm2 of surface.
The morphology of the adhered platelets was visualized using scanning electron microscopy (SEM). Briefly, the samples were fixed using 2.5% glutaraldehyde in PBS for 1 h, subjected to critical-point drying, mounted on stubs, and coated by sputtering with a thin layer of gold and examined by SEM with a JSM-6380LA scanning electron microscope (Japan).
2.3.7 Static and dynamic cell adhesion assays
To evaluate the retention of cells on the surfaces of modified ePTFE samples, static adhesion assays were performed with HUVECs and EPCs. EPCs were isolated form mononuclear cells of healthy volunteers by Histopaque 1077 and cultured in EGM-2 bulletKit as pervious report [25,26,27]. The 5.9 mm diameter disks of bare ePTFE, acidified ePTFE, ECM-ePTFE, and ECM-CD34Ab ePTFE were prepared and put in a 96-well tissue culture plate, with the treated face up. Empty plate wells were set as blank controls. The wells were blocked with 10 mg/ml BSA for 1 h at room temperature, to prevent any nonspecific cell adhesion, and washed twice with PBS. The HUVECs in culture were harvested by trypsinization, and the seeding density was adjusted to 2 × 104 cells/well. Similarly, the seeding density for EPCs was 1 × 104 cells/well. After 30 min of incubation at 37 °C, the wells were washed with warm PBS to remove the non-adherent cells, while adherent cells were trypsinized and counted using a hemocytometer. In all experiments triplicate measurements were taken.
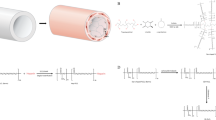
Before the dynamic adhesion assay, HUVECs were collected from culture flasks and marked with a CellTracker CM-DiI (Invitrogen, USA). The cell suspension was adjusted to a density of 1 × 106 cells/ml. The in vitro perfusion system (Fig. 7C, D) consisted of a peristaltic pump (Millipore, USA) upstream of the graft, and an outflow reservoir downstream of the graft. The ePTFE graft was connected by sterilized glass tubing and silicone tubing to the system. The medium can flow through the ePTFE graft and recirculated. The entire apparatus except for the peristaltic pump was installed in an incubator at 37 °C in a humidified environment with 5% CO2. The pump setting was 120 ml/min, which yielded a shear stress of 8 dynes/cm2 according to the Hagen-Poiseuille equation. Shear stress was applied for 24 h. Then the retrieved graft was fixed with 4% w/v paraformaldehyde before examined with an upright fluorescent microscope (BX53 Olympus, Japan).
2.4 Statistical analysis
Data are presented as mean ± standard deviation (SD). All means were compared by one-way ANOVA with Fisher’s least significant difference test as post hoc. The significance level of all tests was set at p < 0.05. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad software, USA).
3 Results
3.1 Surface characterization and comparison of biomaterials
Water contact angle measurement is the most common method for determining the wettability of a material. Figure 1 presents the changes of the water contact angles in each surface modification step. We observed that the luminal surface of untreated ePTFE graft was highly hydrophobic and its original water contact angle was 125.5° ± 2.9° (Fig. 1B). After exposing to acidified glycerol and citrate, the contact angle of acidified ePTFE decreased to 102.7° ± 2.1°. The decreased surface hydrophobicity of acidified ePTFE makes it easier to be coated with ECM. Our results also showed that ECM coating caused the surface of ePTFE to become hydrophilic, as the ECM-ePTFE had a contact angle of 88.3° ± 1.3°. When ECM-ePTFE was immobilized with CD34 mAb, the water contact angle decreased to 79.1° ± 2.0°. These results confirmed the success of our method in the coating and immobilization of ECM and antibody on the ePTFE surface.
Water contact angle measurement of the hydrophilicity of the modified ePTFE surfaces. The water contact angle measurements of the bare ePTFE graft and after each stage of treatment are shown in A. The trends of the decreasing water contact angle are shown in B. Acid-treatment and ECM/CD34Ab coating reduced the water contact angle of ePTFE graft to as low as 79.1°. ***Significance compared to each previous procedure, p < 0.05
The curve-fitting analysis was applied to combine with the deconvolution technique to study the structural changes in the FTIR spectra of these samples after each step of modification. The peaks at 1204 cm−1 and 1150 cm−1 refer to typical CF2 groups of the ePTFE materials (Fig. 2A). The results of the bare and acidified ePTFE samples showed no characteristic amide I (1600–1700 cm−1) and amide II (1450–1600 cm−1) [Fig. 2B (line 0 and line 1)]. After coated with ECM or CD34 mAb-conjugated ECM, three additional peaks were observed at 1405 cm−1, 1550 cm−1 and 1650 cm−1 (Fig. 2B), which implied the aromatics C–C stretch and the N–H bending/C=O stretching of amino acid in material. Therefore, the transmittance of ePTFE samples changed after ECM [Fig. 2B (line 2)] and CD34 mAb coating [Fig. 2B (line 3)], indicating ECM and antibodies were successfully immobilized on the material surface [28].
Analysis of the structures of the modified ePTFE with FTIR spectra. The FTIR spectra of bare ePTFE (0), acidified ePTFE (1), ECM-ePTFE (2), and ECM/CD34 mAb-ePTFE (3). The distinctive peaks of ePTFE and amide were labeled as indicated. A amide I (1650 cm−1) and amide II (1550 cm−1) of amino acid, CF2 groups (1204 and 1150 cm−1) of ePTFE. B C–C stretch (1405 cm−1) and N–H bending/C=O stretching (1550 and 1650 cm−1) of amino acid
The morphologies of these modified ePTFE samples were analyzed with a scanning electron microscope. The surface of the bare ePTFE material exhibited a polyporous netty morphology (Fig. 3A). This typical node-fibril microstructure was not affected after acidified glycerol treatment. Also, SEM analysis of the acidified ePTFE showed that the ePTFE material kept white membranes visually after acid treatment (Fig. 3B). Following the Matigel coating, the meshwork of fibrils was filled with ECM proteins, although the nodes were visible clearly (Fig. 3C). The immobilization of the CD34 mAb on the ECM-coated ePTFE did not cause the ECM scaffolds to flake off. Conversely, the Matigel coating caused the ePTFE material to become flatter and more compact (Fig. 3D).
3.2 Reliable biocompatibility and hemocompatibility of ECM- and ECM/CD34 mAb-ePTFE
The possible cytotoxicities of the ECM- and ECM/CD34 mAb-ePTFE grafts were assayed using the cell counting kit-8 assay (CCK-8) kit. The viability of the bare ePTFE group was 97.2% compared with the negative control. The leaching solutions of the ECM-ePTFE and ECM/CD34 mAb-ePTFE showed no cytotoxicities as the cell viabilities of the ECM-ePTFE and ECM/CD34 mAb-ePTFE groups were found to be comparable to that of the negative control (P = 0.166 and P = 0.655, respectively; Fig. 4).
Evaluation of the cytotoxicity of ECM-CD34Ab modified ePTFE graft. The possible cytotoxicity of the ECM- and CD34 mAb-immobilized ePTFE grafts were assayed using the CCK-8 kit. DMEM media containing 0.64% phenol were used as the positive control. ***p < 0.05 indicates statistical significance compared to the negative control (DMEM medium). “ns” represents no cytotoxic effect, p > 0.05
The blood compatibility of ECM- and ECM/CD34 mAb-ePTFE grafts were further evaluated by hemolysis test. The results showed that the hemolysis rates of the bare ePTFE, acidified ePTFE, ECM-ePTFE, and ECM/CD34 mAb-ePTFE were 0.52, 1.45, 0.89 and 0.91%, respectively (Fig. 5). According to ISO 10993, a hemolysis rate lower than 5% is permissible for biomaterials. In the hemolysis test, the rate of hemolysis (direct contact method) of ECM-CD34Ab ePTFE was 0.89%, under the standard criteria, suggesting that the procedure of surface modification did not promote hemolysis and that our modification method can endue the ePTFE vascular grafts with good hemocompatibility.
The platelet adhesion of the modified ePTFE grafts were assayed by SEM. The adhesions of platelets on the surfaces of pristine ePTFE, acidified ePTFE, ECM-ePTFE, and ECM/CD34 mAb-ePTFE were tested by contacting and incubating the materials with platelet-rich plasma (PRP) for 60 min. The results showed that the platelets on the surfaces of ECM-ePTFE and ECM/CD34 mAb-ePTFE were less than those on the pristine ePTFE and acidified ePTFE surfaces (Fig. 6). The lactate dehydrogenase (LDH) assay was also performed to conform the results of SEM-based assay and to quantify the number of adherent platelets on the ePTFE surfaces. The numbers of platelets on pristine ePTFE and acidified ePTFE were 7.8 × 106 ± 1.4 × 106 cells/cm2 and 9.1 × 106 ± 0.9 × 106 cells/cm2, respectively. Importantly, the numbers of adhered platelets were significantly less on the ECM-ePTFE (4.9 × 106 ± 1.0 × 106 cells/cm2) and ECM/CD34 mAb-ePTFE (4.2 × 106 ± 1.0 × 106 cells/cm2) than on the pristine ePTFE (ps < 0.001 for both, Fig. 6E). Interestingly, no aggregation was observed on the coated ECM/CD34 mAb-ePTFE by scanning electron microscopy,
Analysis of platelet adhesion on the modified ePTFE grafts with SEM. The representative SEM images of platelet-rich plasma (PRP) incubated on A bare ePTFE, B acidified ePTFE, C ECM-ePTFE and D ECM/CD34 mAb-ePTFE. Original magnification: ×1000. E The numbers of adherent platelets on the surfaces of modified ePTFE samples assayed with the lactate dehydrogenase (LDH) assay. ***Statistical significance, p < 0.05. Bar 10 μm
3.3 Cell adhesion to biomaterials
The adhesion of EPCs and HUVECs on the modified graft surfaces were assayed to study whether ePTFE modifications by ECM/CD34 mAb with our method could facilitate the adhesion of endothelial cells. The results showed that the pristine ePTFE surfaces allowed the adhesion of EPCs (1.4 × 103 ± 0.2 × 103 cells/cm2; Fig. 7A) and HUVECs (4.1 × 103 ± 0.5 × 103 cells/cm2; Fig. 7B). However, the numbers of adherent cells from both cell types on the pristine ePTFE surface were very negligible compared to those on the tissue culture polystyrene surfaces (Fig. 7A, B). Thus, the bare ePTFE surface was not conducive to EPCs and HUVECs adhesion. The numbers of EPCs and HUVECs on ePTFE disks after modification with ECM and CD34 mAb were significantly increased (both p < 0.001). The numbers of EPCs and HUVECs adhered on the surface of ECM/CD34 mAb-ePTFE were 4.8 × 103 ± 0.6 × 103 cells/cm2 and 2.0 × 104 ± 5 × 103 cells/cm2, respectively (Fig. 7A, B). These results showed that ePTFE coated with ECM/CD34 mAb by our method improved the adhesion of endothelial cells on the ePTFE grafts.
Analysis of endothelial progenitor cells retention on the surfaces of modified ePTFE samples. The static adhesion assays were performed using EPCs A and HUVECs B. ***Statistical significance compared to the data of pristine ePTFE, p < 0.05. C, D The diagram of the in vitro perfusion system C used to apply shear stress to the modified ePTFE grafts D. The system consists of the holders for ePTFE graft, vented media reservoir in a 37 °C incubator with 5% CO2, and a peristaltic pump. HUVECs suspension of DMEM was used as the perfusate in the system. E, F The representative SEM images of HUVECs retention on the surfaces of bare ePTFE graft E and ECM/CD34 mAb-ePTFE graft F. The difference in the cell affinity for HUVECs between the bare ePTFE graft and the ECM/CD34 mAb-ePTFE graft is evident. TCP tissue culture polystyrene
By combining the ePTFE graft with an in vitro perfusion system (Fig. 7C, D), we were able to mimic the shear stress during blood circulation that would be experienced in the ePTFE graft in vivo. After 24 h of 8 dynes/cm2 shear stress, the number of HUVECs on ECM/CD34 mAb-ePTFE grafts (n = 3) was significantly higher than that of the pristine ePTFE grafts (Fig. 7E, F).
4 Discussion
Rapid in situ endothelialization of vascular grafts was thought to be a promising approach to improve the long-term patency rate of small diameter grafts [29]. Many strategies based on this principle have been developed. The ePTFE graft surface coating with ECM proteins can mimic native vessel architecture to the greatest extent. Moreover, ECM proteins can stimulate the adhesion and proliferation of the circulating EPCs, and thus achieve enhanced endothelialization [18, 29]. In adult blood, EPCs have lost expression of CD133, but remain positive for CD34 and VEGFR-2 [14]. Therefore, anti-CD34 antibody is a promising capture molecule for EPCs to aid in the endothelialization of small diameter vascular grafts [30]. In this study, we developed a method to activate ePTFE surfaces by the co-immobilization of ECM and CD34 mAb with the physisorption and crosslinking technique. Our results indicated that ePTFE grafts modified with a combinatorial coating of ECM and CD34 mAb significantly improved the hemocompatibility of ePTFE graft and its affinity for endothelial cells.
For ePTFE graft coating, the multiple ECM components have the advantages over single ECM protein in retaining the endothelial cells [31]. The Matrigel matrix, which composed of laminin, collagen IV, and entactin, can be used for the culture of endothelial cells. This combination of ECM components can provide better cell adhesion ability for biomaterials. Physisorption method provided a convenient procedure to attach matrix onto synthetic ePTFE surface. With such a method, the synthetic ePTFE surface can possess the activity of ECM proteins while not involving any chemical linkers between the proteins and polymers [31, 32]. Since ePTFE surfaces are highly hydrophobic, this retards the coating of ECM proteins. Ranjan et al. applied acidified glycerol treatment to modify ePTFE tubing to make the surface switching from being hydrophobic to being hydrophilic [31]. We also found that this technique could facilitate the immobilization of ECM proteins on ePTFE. Various techniques have been developed for the immobilization of antibodies on ePTFE polymers, such as physisorption [33], electrostatic interactions [34], and covalent attachment [10, 35]. Comparing with the other techniques, ECM and antibodies cross-linking on ePTFE polymers via covalent linkage is more stable and thus will not be broken easily by physical force [36]. EDC is a water-soluble cross-linking agent that used to crosslink proteins via amines and carboxylic acids. In addition, the excess reagents and by-products can be removed easily by washing with water [37, 38]. Thus, we used EDC to immobilize CD34 mAb on ECM-ePTFE grafts.
The characteristics of the modified ePTFE grafts were assayed by water contact angle, infrared spectroscopy, and SEM analyses. The hydrophilicity of ePTFE was greatly improved after CD34 mAb immobilization as the water contact angle decreased from 125.5° ± 2.9° to 79.1° ± 2.0°. The bare ePTFE surfaces are too hydrophobic for the adhesion of cells directly, thus the reduction in surface hydrophobicity can improve its biocompatibility for being used as an artificial blood vessel [39, 40]. Further analysis using FTIR spectroscopy was carried out to assess the changes in the modified ePTFE surfaces. The existence of ECM proteins on ePTFE grafts was verified by the peaks of C–C stretching in ring, C=O stretching vibration and N–H bending vibration at 1650 cm−1 and 1550 cm−1, respectively. These arise from the amide bonds that link with the amino acids. Also, the evidence of crosslinking reaction of CD34 mAb with ePTFE grafts was conformed to the decrease in peaks at 1650 cm−1, 1550 cm−1, and 1405 cm−1. Although the peptide formation may intensify the bending vibration of N–H bond, the reduction in the primary amine groups will cause a decrease in the peak area of amide II. Moreover, along with the reduction of hydrogen bond, the attenuated stretching vibration will cause a decrease in the peak area at 1650 cm−1 and 1405 cm−1. The surface of the modified ePTFE grafts were visualized with SEM analyses. The results showed that an obvious membranous film was formed on the ECM/CD34 mAb-ePTFE grafts after the process of our method without changing the structure of the pristine ePTFE grafts.
It is well-known that the biocompatibility and hemocompatibility are the most important factors for biomaterials being implanted into the human body. Thus, we also evaluated the biocompatibility and hemocompatibility of the modified ePTFE grafts in the present study. Cell cytotoxicity of the graft samples were tested using a cell viability assay. We observed that the ECM/CD34 mAb-ePTFE had the similar viability rate compared with that of the pristine ePTFE. Thus, the modification procedure of our method would not cause cytotoxicity of the modified ePTFE. Furthermore, the hemolytic activity of ECM/CD34 mAb-ePTFE graft was lower than 5%, demonstrating that our method would not induce hemolysis according to ISO 10993, the international standards for evaluating the biocompatibility of medical devices. Also, the results of the blood platelet adhesion assay revealed that ePTFE graft coating with ECM/CD34Ab by our method could inhibit platelet adhesion as verified by SEM imaging and LDH activity measurements. Although different modifications to the surfaces of diverse materials can lower the platelet adhesion significantly [41,42,43,44], we did not find a significant threshold for the occurrence of thrombosis and the number of adhered platelets in our study, likely for the following reasons. Thrombus formation is a complex and multifactorial process involving the interaction of blood cells, platelets, and clotting factors [45]. The recruitment of platelets, especially after their adherence to abnormal vessel walls and their consequent activation, plays a key role in thrombus formation [46]. Therefore, this study focused on platelets while the interference from heterocellular interactions was excluded by using the platelet adhesion test. Platelet-rich plasma used in the adhesion assay is composed of a relatively high concentration of platelets in a static state [42] and might result in a quantity of adhered platelets up to 4.2 × 106 cells/cm2, as observed on our ePTFE-based surface. Nevertheless, we still used SEM to investigate the inhibitory effect of our surface on platelet adhesion: no severe platelet adhesions were observed under SEM. However, it is not certain that the modified ECM/CD34 mAb-ePTFE surface will have no incidence of thrombosis. Lamichhane et al. recently demonstrated that the topography of the PTFE affected the responses of endothelial cells, platelets and smooth muscle cells [47]. This principle may in part contribute to the benefits observed in our study with the coating of ePTFE because the surfaces became flatter and more compact.
The number of adhered EPCs was less than that of adhered platelets in our study, and this phenomenon may result from the fact that EPCs were isolated from peripheral blood mononuclear cells (MNCs) and cultured. These cultured EPC cells were derived from the same batch of blood, required a longer culture period (e.g., 2–3 weeks), and were limited in quantity. As a result, during the preparation of the EPC cell suspensions with a guaranteed volume, the EPC seeding density in each well had to be lower and the platelet density in the PRP had to be higher in order to provide maximal antithrombotic properties. Many reports have revealed that early-outgrowth and late-outgrowth EPCs exist. Late-outgrowth EPCs have been defined as ‘true’ EPCs because they are capable of generating ECs that express typical endothelial surface markers and exhibit typical EC function [48,49,50]. Thus, we selected EPCs that had undergone 2–3 weeks of amplification for the cell adhesion experiments. Also, the number of EPCs in peripheral blood is less than the number of platelets. Hence, that’s why many technical approaches are developed and used to obtain more colonized EPCs for vascular grafts.
As we mentioned above, the hydrophilicity of the graft was dramatically improved after ECM coating and CD34 mAb immobilization. It is well-known that the hydrophilicity of a polymeric material is a key point in determining whether the polymer possesses good blood compatibility [51, 52]. Indeed, studies have reported and proved that matrix protein coating can change the character of the vascular graft surface and results in the inhibition of platelet adhesion and activation [43, 53]. The findings in our study are consistent with these prior reports.
The existence of a confluent monolayer of endothelial cells on the graft lumen is crucial for the outcome of an implanted graft. Although endothelial cells can migrate from the neighboring tissues to an implanted graft, the newly formed endothelium often only distributed nearby the anastomosis regions [54]. For this reason, the EPCs capturing strategy might be able to fill the gap [55, 56]. It was reported that the adhesion of EPCs might be a complex process, which involves EPC rolling and adhesion, followed by migration and differentiation [55]. Meanwhile, in situ endothelialization of vascular grafts usually depends on the stable attachment of EPCs on the surface of vascular grafts [56]. Nearly beginning from the time of implantation, besides EPCs, many other blood cells and plasma proteins also start to adhere to the surface of implanted graft. In this situation, immobilized anti-CD34 antibodies will be covered by the competing cells and proteins (i.e. other blood cells and plasma proteins) [57]. This will consequently create a microenvironment, which may lead to a failure in vascular grafts implantation. Rapid in situ endothelialization seems to have a time window of opportunity before the build-up of an adverse microenvironment in the implanted graft. Since ECM/CD34 mAb are essential for the attachment of endothelial cells, we further studied the adherence of HUVECs and EPCs on the modified grafts by static cell adhesion assay. Our results confirmed that the co-immobilization of ECM and CD34 mAb could improve the affinity of ePTFE graft for HUVECs and EPCs. Furthermore, the cell retention ability of modified grafts in this study was assayed under physiological shear stress by a self-designed in vitro perfusion system. The shear stress was adjusted to 8 dynes/cm2, which is half the maximum shear stress measured in in vivo bypass grafts [58]. Similarly, the number of the adhered HUVECs was significantly increased on the surface of ECM/CD34 mAb-ePTFE. Therefore, our modification technique might improve ePTFE vascular graft performance since it provides low cytotoxicity, low hemolysis rate, and limited platelet adhesion while simultaneously supporting endothelialization.
There were limitations to our study. Homogeneity is important in cell adhesion experiments. However, currently we are unable to generate enough EPCs from the same cell sources simultaneously. We will design a more miniaturized peristaltic cycle pump for refining this experiment. According to the literature regarding platelet adhesion, the static platelet adhesion assay is commonly used. Meanwhile, the in vitro perfusion system might provide better results. Our collected platelets were derived from healthy adult volunteers and do not provide sufficient PRP for the assays. Nevertheless, we will include an improved set of experiments with human platelet suspensions in the future.
5 Conclusions
Taken together, the co-immobilization of ECM and CD34 mAb could be a potential new option for the rapid in situ endothelialization of vascular graft. No matter what the really mechanisms between the immobilized antibody and HUVECs and EPCs are, the attachment of HUVECs and EPCs were greatly accelerated. The method could thus be used as a way to modify the surfaces of vascular grafts or be used in tissue engineering applications.
References
Abbott WM, Callow A, Moore W, Rutherford R, Veith F, Weinberg S. Evaluation and performance standards for arterial prostheses. J Vasc Surg. 1993;17:746–56.
Albers M, Battistella VM, Romiti M, Rodrigues AA, Pereira CA. Meta-analysis of polytetrafluoroethylene bypass grafts to infrapopliteal arteries. J Vasc Surg. 2003;37:1263–9.
Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regen Med. 2010;5:107–28.
Faries PL, Logerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080–90.
Larsen CC, Kligman F, Tang C, Kottke-Marchant K, Marchant RE. A biomimetic peptide fluorosurfactant polymer for endothelialization of ePTFE with limited platelet adhesion. Biomaterials. 2007;28:3537–48.
Goh ET, Wong E, Farhatnia Y, Tan A, Seifalian AM. Accelerating in situ endothelialisation of cardiovascular bypass grafts. Int J Mol Sci. 2014;16:597–627.
Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61.
Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12:329–41.
Avci-Adali M, Paul A, Ziemer G, Wendel HP. New strategies for in vivo tissue engineering by mimicry of homing factors for self-endothelialisation of blood contacting materials. Biomaterials. 2008;29:3936–45.
Rotmans JI, Heyligers JM, Verhagen HJ, Velema E, Nagtegaal MM, de Kleijn DP, et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112:12–8.
Tseng DY, Edelman ER. Effects of amide and amine plasma-treated ePTFE vascular grafts on endothelial cell lining in an artificial circulatory system. J Biomed Mater Res. 1998;42:188–98.
Catto V, Farè S, Freddi G, Tanzi1 MC. Vascular tissue engineering: recent advances in small diameter blood vessel regeneration. ISRN Vascular Medicine 2014. doi:10.1155/2014/923030.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7.
Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9.
Melchiorri AJ, Hibino N, Yi T, Lee YU, Sugiura T, Tara S, et al. Contrasting biofunctionalization strategies for the enhanced endothelialization of biodegradable vascular grafts. Biomacromolecules. 2015;16:437–46.
Walachova K, Svorcik V, Bacakova L, Hnatowicz V. Colonization of ion-modified polyethylene with vascular smooth muscle cells in vitro. Biomaterials. 2002;23:2989–96.
Moby V, Boura C, Kerdjoudj H, Voegel JC, Marchal L, Dumas D, et al. Poly (styrenesulfonate)/poly(allylamine) multilayers: a route to favor endothelial cell growth on expanded poly(tetrafluoroethylene) vascular grafts. Biomacromolecules. 2007;8:2156–60.
Bu X, Yan Y, Zhang Z, Gu X, Wang M, Gong A, et al. Properties of extracellular matrix-like scaffolds for the growth and differentiation of endothelial progenitor cells. J Surg Res. 2010;164:50–7.
Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41.
Williams SK, Kleinert LB, Patula-Steinbrenner V. Accelerated neovascularization and endothelialization of vascular grafts promoted by covalently bound laminin type 1. J Biomed Mater Res A. 2011;99:67–73.
Caiado F, Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair. 2012;5:4.
Vartanian KB, Kirkpatrick SJ, McCarty OJ, Vu TQ, Hanson SR, Hinds MT. Distinct extracellular matrix microenvironments of progenitor and carotid endothelial cells. J Biomed Mater Res A. 2009;91:528–39.
Motlagh D, Allen J, Hoshi R, Yang J, Lui K, Ameer G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J Biomed Mater Res A. 2007;82:907–16.
Grunkemeier JM, Tsai WB, Horbett TA. Hemocompatibility of treated polystyrene substrates: contact activation, platelet adhesion, and procoagulant activity of adherent platelets. J Biomed Mater Res. 1998;41:657–70.
Raz O, Lev DL, Battler A, Lev EI. Pathways mediating the interaction between endothelial progenitor cells (EPCs) and platelets. PLoS ONE. 2014;9:e95156.
Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol 2008; Chapter 2: Unit 2C.1.
Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–57.
Mihaly J, Sterkel S, Ortner HM, Kocsis L, Hajba L, Furdyga E, et al. FTIR and FT-Raman spectroscopic study on polymer based high pressure digestion vessels. Croat Chem Acta. 2006;79:497–501.
de Mel A, Jell G, Stevens MM, Seifalian AM. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9:2969–79.
Yin M, Yuan Y, Liu C, Wang J. Combinatorial coating of adhesive polypeptide and anti-CD34 antibody for improved endothelial cell adhesion and proliferation. J Mater Sci Mater Med. 2009;20:1513–23.
Ranjan AK, Kumar U, Hardikar AA, Poddar P, Nair PD, Hardikar AA. Human blood vessel-derived endothelial progenitors for endothelialization of small diameter vascular prosthesis. PLoS ONE. 2009;4:e7718.
Bacakova L, Filova E, Rypacek F, Svorcik V, Stary V. Cell adhesion on artificial materials for tissue engineering. Physiol Res. 2004;53:S35–45.
Petersen S, Strohbach A, Busch R, Felix SB, Schmitz KP, Sternberg K. Site-selective immobilization of anti-CD34 antibodies to poly(l-lactide) for endovascular implant surfaces. J Biomed Mater Res B Appl Biomater. 2014;102:345–55.
Chen JL, Li QL, Chen JY, Huang N. Adsoption CD34 antibody onto the col/hep coating film on titanium to improve cytocompatibility of titanium. Adv Mater Res. 2008;47:1411–4.
Lin Q, Ding X, Qiu F, Song X, Fu G, Ji J. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayer. Biomaterials. 2010;31:4017–25.
Liu H, Li X, Niu X, Zhou G, Li P, Fan Y. Improved hemocompatibility and endothelialization of vascular grafts by covalent immobilization of sulfated silk fibroin on poly(lactic-co-glycolic acid) scaffolds. Biomacromolecules. 2011;12:2914–24.
Vashist SK, Dixit CK, MacCraith BD, O’Kennedy R. Effect of antibody immobilization strategies on the analytical performance of a surface plasmon resonance-based immunoassay. Analyst. 2011;136:4431–6.
Sohn YS, Lee YK. Site-directed immobilization of antibody using EDC-NHS-activated protein A on a bimetallic-based surface plasmon resonance chip. J Biomed Opt. 2014;19:051209.
Njatawidjaja E, Kodama M, Matsuzaki K, Yasuda K, Matsuda T, Kogoma M. Hydrophilic modification of expanded polytetrafluoroethylene (ePTFE) by atmospheric pressure glow discharge (APG) treatment. Surf Coat Technol. 2006;201:699–706.
Chong DS, Turner LA, Gadegaard N, Seifalian AM, Dalby MJ, Hamilton G. Nanotopography and plasma treatment: redesigning the surface for vascular graft endothelialisation. Eur J Vasc Endovasc Surg. 2015;49:335–43.
Jin G, Yao Q, Zhang S, Zhang L. Surface modifying of microporous PTFE capillary for bilirubin removing from human plasma and its blood compatibility. Mater Sci Eng C Mater Biol Appl. 2008;28:1480–8.
Hoshi RA, Van Lith R, Jen MC, Allen JB, Lapidos KA, Ameer G. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials. 2013;34:30–41.
Chandy T, Das GS, Wilson RF, Rao GH. Use of plasma glow for surface-engineering biomolecules to enhance bloodcompatibility of Dacron and PTFE vascular prosthesis. Biomaterials. 2000;21:699–712.
Zhou F, Jia X, Yang Y, Yang Q, Gao C, Zhao Y, et al. Peptide-modified PELCL electrospun membranes for regulation of vascular endothelial cells. Mater Sci Eng C Mater Biol Appl. 2016;68:623–31.
Nichols MD, Choudhary R, Kodali S, Reichert WM. Coagulation-induced resistance to fluid flow in small-diameter vascular grafts and graft mimics measured by purging pressure. J Biomed Mater Res B Appl Biomater. 2013;101:1367–76.
Freedman JE. Molecular regulation of platelet-dependent thrombosis. Circulation. 2005;112:2725–34.
Lamichhane S, Anderson JA, Remund T, Sun H, Larson MK, Kelly P, et al. Responses of endothelial cells, smooth muscle cells, and platelets dependent on the surface topography of polytetrafluoroethylene. J Biomed Mater Res A. 2016;104:2291–304.
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9.
Stroncek JD, Grant BS, Brown MA, Povsic TJ, Truskey GA, Reichert WM. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A. 2009;15:3473–86.
Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27.
Tanaka M, Mochizuki A, Ishii N, Motomura T, Hatakeyama T. Study of blood compatibility with poly(2-methoxyethyl acrylate). Relationship between water structure and platelet compatibility in poly(2-methoxyethylacrylate-co-2-hydroxyethylmethacrylate). Biomacromolecules. 2002;3:36–41.
Lee JH, Lee HB. Platelet adhesion onto wettability gradient surfaces in the absence and presence of plasma proteins. J Biomed Mater Res. 1998;41:304–11.
Wang YX, Robertson JL, Spillman WJ, Claus RO. Effects of the chemical structure and the surface properties of polymeric biomaterials on their biocompatibility. Pharm Res. 2004;21:1362–73.
Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–103.
Gong X, Li Y, Gao Q, Cheng B, Shen B, Yan Z, et al. Adhesion behavior of endothelial progenitor cells to endothelial cells in simple shear flow. Acta Mech Sin. 2011;27:1071–80.
Melchiorri AJ, Hibino N, Fisher JP. Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts. Tissue Eng Part B Rev. 2013;19:292–307.
Avci-Adali M, Ziemer G, Wendel HP. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization—a review of current strategies. Biotechnol Adv. 2010;28:119–29.
Shimizu T, Ito S, Kikuchi Y, Misaka M, Hirayama T, Ishimaru S, et al. Arterial conduit shear stress following bypass grafting for intermediate coronary artery stenosis: a comparative study with saphenous vein grafts. Eur J Cardiothorac Surg. 2004;25:578–84.
Acknowledgements
The authors thank all of the study participants and research staff of the Guangzhou Vascular Disease Center. This work was supported by Science Foundation of Guangdong Province (2010B080701034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical Statement
This study was approved by the Clinical Research and Experimental Animal Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University (IRB no. 2015_07) and institutional review board of Guangdong Provincial Health Bureau and Sun Yat-sen University.
Additional information
Lei Chen and Haipeng He are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1
The schematic diagram shows the four consecutive stages in the development of the coated ePTFE grafts (TIFF 1317 kb)
Rights and permissions
About this article
Cite this article
Chen, L., He, H., Wang, M. et al. Surface Coating of Polytetrafluoroethylene with Extracellular Matrix and Anti-CD34 Antibodies Facilitates Endothelialization and Inhibits Platelet Adhesion Under Sheer Stress. Tissue Eng Regen Med 14, 359–370 (2017). https://doi.org/10.1007/s13770-017-0044-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0044-3