Abstract
Ground beef is risky by foodborne pathogens such as E. coli O157:H7 due to the cross-contamination during grinding. The objective of this study was to evaluate the antagonistic activities of Leuconostoc species isolated from ground beef product in order to limit the growth of E. coli O157:H7. While Leuconostoc has been known as spoilage bacteria, the Leuconostoc isolates showed antimicrobial activity on foodborne pathogens such as E. coli O157:H7, Salmonella, Staphylococcus aureus, Listeria monocytogenes, and meat-spoilage bacteria Brochothrix thermosphacta. Antimicrobial activity of cell-free supernatant (CFS) was evaluated by heat, enzyme, and pH adjustment and antagonistic activity by cell competitive growth. In addition, CFS concentration was screened for the presence of organic acids for the antimicrobial properties. Regulation of virulence genes was investigated by inhibition of locus of enterocyte effacement expression by CFS. The result showed that Leuconostoc isolates inhibited the growth of E. coli O157:H7 by active antimicrobial agent including organic acids and the growth of background microorganisms, and E. coli O157:H7 in ground beef was inhibited with the supplement of the CFS.
Similar content being viewed by others
Introduction
Pathogenic Escherichia coli has been the most frequent cause of foodborne illness in Korea since 2003 (MFDS 2015). While enterohemorrhagic E. coli (EHEC) is not the most reported pathogenic E. coli in Korea, it can cause significant disease such as hemolytic uremic syndrome. E. coli O157:H7 is one of the most frequent EHEC by about 30 % of infection in Korea and it was first isolated from a patient in 1998 in Korea (Jo et al. 2004). Raw meat and other meat products contaminated with animal feces have been studied as the major sources of E. coli O157:H7. Especially, ground meat product which is one of the most consumed types of meat, was ranked as the highest risk of contamination by foodborne pathogens (Mintel Group Ltd 2010). In addition, intervention technology on E. coli O157:H7 control should be continuous regardless of the seasons, since the prevalence was not affected by the seasons (Williams et al. 2010). While food safety regulations and HACCP programs reduced the risk of foodborne illness associated with contaminated ground beef, there are still pre-consumer interventions that are necessary to minimize the risk (Taylor et al. 2012). Although the efforts seemed to control the contamination, there are continuous outbreaks and recalls reported. In addition, there are increasing demands on no additives or chemical residues, minimal processing, convenient preparation, safe, and economic production by consumers.
Various types of natural antimicrobial materials have been tested in ground meat to reduce foodborne pathogens by researchers. Essential oils have been a great approach by their antimicrobial efficiency such as clove and lemongrass in bovine ground meat to reduce L. monocytogenes (de Oliveira et al. 2013) and cinnamon and olive extract to reduce S. Typhimurium in ground pork (Chen et al. 2013). However, essential oils have a strong and unique flavor that can limit the practical application in market. Cranberry concentrates in ground beef reduced E. coli O157:H7 growth. Other chemical agents such as lauric arginate with carvacrol showed synergistic effect in ground turkey by reducing Salmonella (Oladunjoye et al. 2013). Mustard leaf kimchi extract was added to raw ground pork and the total microbial count decreased significantly (Lee et al. 2010). Physical treatments that have been applied to meat products for the bacterial inactivation are temperature control, radiation, high pressure processing, pulsed electric fields and others, or packaging with modified atmosphere and/or active packaging with modified packaging materials (Mor-Mur and Yuste, 2010). Bacteriophages are another novel antimicrobial agent due to the high specificity and the examples are L. monocytogenes-specific phage (LMP-102) and E. coli O157:H7-specific bacteriophage in ground meat (Abuladze et al. 2008). Lactic acid bacteria (LAB) can be used in the food products in the form of fermented food, food biopreservatives, and functional food. This application is primarily based on their antimicrobial activities and their safe and health benefits to human (Suskovic et al. 2010).
Leuconostoc spp. has been known as spoilage bacteria, while it is also known to have antimicrobial activity by producing bacteriocin such as leucocin and mesentericin. These bacteriocins were reported to be produced only by Leu. mesenteroides, Leu. gelidum, Leu. carnosum, and Leu. pseudomesenteroides (Pujato et al. 2014). Lactic acid bacteria produce antimicrobial substances, and bacteriocin has been most studied. Bacteriocin is a small cationic peptide that can kill target bacteria by pore-forming the cytoplasmic membrane. Nisin (Nisaplin®), pediocin (ALTA 2341), and enterocin (AS-48, CCM4231, and EJ97) are the commercial bacteriocins that can be used in food products (Yang et al. 2014). However, the range of antimicrobial target of bacteriocins tends to be limited predominantly by gram-positive bacteria, and bacteriocin resistance has been an emerging concern that other types of antimicrobials are in demand (Hartmann et al. 2011). Not only bacteriocins but also other antimicrobial substances are produced by LAB, such as organic acids, biosurfactants, hydrogen peroxide, and low molecular mass compounds. Therefore, crude bacterial fermentate application is another type of antimicrobials to show synergistic effects to the food product (Hartmann et al. 2011). The purpose of this study was to isolate antagonistic bacteria such as Leuconostoc spp. from food to inhibit the growth of E. coli O157:H7 and to investigate the antimicrobial properties.
Materials and methods
Bacteria
Staphylococcus aureus ATCC 6538, ATCC 25923, and KCCM 40881; Salmonella Typhimurium PTU 302, PT 10, and ATCC 14028; Listeria monocytogenes ATCC 15315, ATCC 19114, and ATCC 19115; Escherichia coli O157:H7 ATCC 43895, ATCC 43890, and NCCP 11091; and Brochothrix thermosphacta KCTC 3402 were used in this study. All strains were from the culture collection at the Korea Food Research Institute. The cultures were maintained in 15 % glycerol at −80 °C. S. aureus, S. Typhimurium, and E. coli O157:H7 were incubated in tryptic soy broth (TSB, Merck& Co., USA). L. monocytogenes was incubated in TSB supplemented with 0.6 % yeast extract (TSB-YE) (BD, USA), and B. thermosphacta was incubated in brain heart infusion broth (BD). They were incubated at 37 °C for 18–20 h, except B. thermosphacta which was incubated at 25 °C before starting the experiment.
Isolation and identification of antagonistic bacteria
Ground beef was purchased from local grocery stores in Gyeonggi-do, Korea, and the products were kept under refrigeration until use. Purchased ground beef was used within 24 h of purchase. In order to isolate antagonistic bacteria from the food product, 25 g of ground meat was homogenized with 225 mL of 0.1 % peptone water for 2 min using stomacher (Interscience, France). Then, the homogenized sample was plated on MRS agar and plate count agar. Once the colonies were grown, TSA 0.8 % agar was poured over the plates with 0.1 % of overnight cultured E. coli O157:H7 ATCC 43895. Any colonies with a clear zone were isolated for the further identification. Any colonies showing antimicrobial activity were selected, and the genomic DNA was extracted and its 16s rRNA was sequenced and identified using the EzTaxon server (http://www.ezbiocloud.net/eztaxon; Kim et al. 2012).
Antimicrobial activity analysis
Double layer assay was performed by spot-inoculation of colonies of isolates on MRS and incubated at 30 °C for 48 h. Cocktails of the overnight culture of S. aureus, S. Typhimurium, L. monocytogenes, E. coli, and B. thermosphacta, respectively, were prepared with equal concentration and inoculated in TSA 0.8 % agar with total concentration of 107 CFU/mL, and the soft agar was poured onto the MRS. The double layer plate was incubated at 37 °C for 24 h, and the diameters of inhibition zone were measured.
Based on double layer assay, isolated Leuconostoc spp. were selected and their antimicrobial activity was investigated with the culture supernatant and the cells. Overnight culture of Leuconostoc isolates was subcultured at 30 °C for 24 h. The culture was centrifuged at 5,300×g for 10 min, and the supernatant was sterilized using 0.45 μm filter. The pellet was further used for the competitive growth study. The culture supernatant was treated with three different methods. First, the pH of each supernatant was measured (Table 2) and neutralized to the pH of 7.0 ± 0.1 using NaOH (1 mol/L) and the adjusted supernatant was sterilized using 0.45 μm filter. Secondly, the supernatant was heat treated at 100 °C for 1 h then cooled in ice. Last treatment was using pepsin 1 mg/mL at 37 °C for 1 h and the enzyme was inactivated at 65 °C for 30 min. Overnight culture of E. coli O157:H7 ATCC 43895 was inoculated in all treated supernatants and incubated at 37 °C for 18 h. Negative control was fresh TSB, overnight culture of TSB, and the positive control was fresh MRS. The absorbance at 600 nm of E. coli O157:H7 ATCC 43895 inoculated supernatant was measured after the incubation.
Antimicrobial activity by the competitive growth was evaluated under the co-culture condition. Preincubated E. coli O157:H7 culture was centrifuged at 7500 rpm for 10 min and washed with 0.9 % saline water. The pellet that was prepared from the supernatant preparation was also washed with 0.9 % saline water and co-inoculated with E. coli O157:H7 with the concentration of 109 CFU/mL at 37 °C for 24 h. Thereafter, the number of survived bacterial concentration was quantified on MacConkey agar with sorbitol supplemented with cefixime and tellurite (CT-SMAC, Oxoid, UK).
Analysis of organic acid
The analysis of lactic acid, acetic acid, succinic acid, malic acid, citric acid, and oxalic acid was performed using a HPLC system (Agilent 1260 infinity, Agilent, USA). Organic acids that are produced by LAB and have potential antimicrobial activities were evaluated using filter-sterilized overnight cell-free supernatant (CFS). Separation was achieved on Zorbax SB-AQ column (4.6 × 250 mm, 5 μm, Agilent) equipped with guard column (Zorbax SB-AQ guard column, 4.6 × 12.5 mm, 5 μm, Agilent) at 35 °C. The elution was performed with 20 mM phosphate buffer using a flow rate of 1.0 mL/min. Detection was carried out in a UV detector, using 210 nm. Samples were filtered with 0.45 μm syringe filter and 10 μL of each sample was injected. The organic acids were quantified by comparison of the area of their peaks with calibration curves obtained from commercial standards of each compound.
The analysis of derivative organic compounds including phenyllactic acid and hydroxyphenyllactic acid, which have shown the antimicrobial activities by Leuconostoc, was carried out using the filter-sterilized overnight CFS in a UPLC system (Agilent 1290 infinity, Agilent). The system was coupled with an accurate-mass quadrupole-time-of-flight instrument (Agilent 6520 with Jet Stream Technology, Agilent) with an ACE Excel UHPLC column (EXL-111-1546U, C18, 4.6 × 150 mm, 3 μm, ACE, UK) at 30 °C. The mobile phase was in gradient mode with 0.1 % formic acid in DW solution (A) and 0.1 % formic acid in ACN solution (B), with a flow rate of 0.9 mL/min within 10 min. An injection volume was 1 μL. The Q-TOF–MS instrument was operated with an electrospray ion source (Agilent Jet Stream Technology) in negative mode. It was set at 350 °C with drying gas at a flow rate of 12 L/mL, a nebulizer pressure of 30 psi, skimmer voltage of 65 V, and fragmentor voltage of 75 V. MS spectra were processed with a Mass Hunter workstation software (ver. B.06.00, Agilent) generating peak lists suitable for standard compounds.
Measurement of cellular luminescence
The polymerase chain reaction product of the LEE regulatory region in LEE3 (P LEE3 , 420-bp lengths) was digested with SpeI and BamHI and inserted into pBBR-lux that had been digested with the same enzymes. The latter plasmid carries a promoterless luxCDABE operon (Lenz et al. 2004). The ler ORF was amplified using primers that incorporated upstream NcoI and downstream SmaI restriction sites. The fragment was digested with NcoI and SmaI and cloned into an arabinose-inducible expression vector, pKS1101 (Kim et al. 2013). These two plasmids were co-transformed into E. coli DH5α cells (KS02). An overnight culture of KS02 was diluted 100-fold into fresh LB medium containing 0.0001 % l-(+)arabinose. Aliquots of 200 μL were transferred to wells of 96-well microtiter plates containing different concentrations of freeze-dried culture supernatant of DGB-1040. The cellular luminescence of the cultures was measured with a luminometer (Bio-Tek Instruments, Inc., VT) and expressed in arbitrary relative light units, as described previously (Jeong et al. 2010).
Food application study
Supernatant of selected Leuconostoc isolate, DGB-1040, was concentrated by freeze drying to minimize the increase of water content in ground beef and enhance the antimicrobial activity. After sterilization using 0.45 filter, the supernatant was freeze dried (OPERON, Korea) for 4 days and kept at −80 °C until use. Ten grams of ground beef was prepared for each treatment and each collection time point. Freeze-dried supernatant was inoculated in ground beef at the concentration of 2.65 mg/g of ground beef and massaged for 1 min for the homogeneous distribution in the food sample. Then, E. coli O157:H7 ATCC 43895 was inoculated for the final concentration of 1.27 × 103 CFU/g of ground beef and massaged for 1 min for the homogeneous distribution in the food sample. The inoculated ground beef samples were stored at 5 and 10 °C, and total aerobic bacteria and E. coli O157:H7 were enumerated on Petrifilm™ Aerobic count plates (3 M) and CT-SMAC at day 0, 1, 3, 7, and 10 with appropriate dilution. Negative control was ground beef without freeze-dried supernatant, without E. coli O157:H7 or without both.
Statistical analysis
Statistical analysis was carried out by comparing the averages of each growth point based on one-way analysis of variance ANOVA according to Ducan’s multiple range test at a significance level of 5 %, p < 0.05 using IBM SPSS Statistics (IBM Corporation, USA). Each experiment was performed at least three times.
Results and discussion
Putative LAB 157 strains which inhibited the growth of E. coli O157:H7 ATCC 43895 were isolated from ground meat products. Among the isolates, LAB 18 strains that had the strongest antimicrobial activity were identified as Lactobacillus, Leuconostoc, and Lactococcus species. Four isolates of Leuconostoc spp. that have been lesser known for their antimicrobial activity against E. coli O157:H7 were selected for further characterization.
Antimicrobial activity of Leuconostoc species
Four isolates were identified as Leu. citreum for DGB-810 and DGB-818, and Leu. mesenteroides subsp. mesenteroides for DGB-938 and DGB-1040. All isolates showed the best activity against meat-spoilage bacteria, B. thermosphacta followed by L. monocytogenes (Table 1). B. thermosphacta is a major spoilage bacterium in raw meat along with Pseudomonas which depends on oxygen availability so that a shelf life of food product can be extended by inhibition of the growth of B. thermosphacta (Pennacchia et al. 2011). While E. coli O157:H7 was not as well inhibited as other pathogens, the inhibition zone was detected. The activity was primarily strain dependent; however, Leu. mesenteroides subsp. mesenteroides tended to show slightly stronger activity than Leu. citreum. There have been few studies on the antimicrobial activity of Leuconostoc species against foodborne pathogens and their application in food products. Aronia fermented with Leu. mesenteroides showed antibacterial activity against L. monocytogenes (Hwang et al. 2014). Competitive growth of E. coli O157:H7 and Leu. mesenteroides and Lb. plantarum in cucumber ferment brines showed complete reduction to the undetectable level in 2–3 days of fermentation (Breidt and Caldwell 2011). Leu. mesenteroides isolated from tortellini and boiled sausages showed antimicrobial activity against L. monocytogenes, while no activity was observed on Brochothrix spp. (Maragkoudakis et al. 2009). Pujato et al. (2014) reported an antimicrobial activity of Leu. citreum cell-free supernatant against L. monocytogenes. Leu. citreum GJ7 reduced the growth of E. coli O157:H7, Salmonella Typhi, and Staphylococcus aureus during co-culture condition and by the culture supernatant (Chang and Chang 2011). As shown, while most studies were able to investigate the inhibition activity of Leuconostoc against foodborne pathogens, only limited studies have found the antimicrobial agents in Leuconostoc.
Inhibition of targeted pathogen, E. coli O157:H7, was tested with the cell-free supernatant of the isolates and the cells by competitive growth. The average pH of overnight culture supernatant was 4.50, 4.39, 4.53, and 4.64 for the isolates DGB-810, DGB-818, DGB-938, and DGB-1040, respectively. Cell-free supernatant (CFS) completely inhibited the growth of E. coli O157:H7 potentially due to several antimicrobial agents produced by LABs (Table 2). The pH of CFS was adjusted to neutral pH between 6.90 and 7.10 in order to limit the effect of low pH level and antimicrobial agents that are active at low pH. The antimicrobial activity was significantly reduced for all foodborne pathogens by pH adjustment showing the potential antimicrobial activity at low pH. With the pH adjustment, E. coli O157:H7 was able to grow in the LAB culture supernatant. On the other hand, the CFS activity of DGB-1040 was not completely diminished by pH adjustment in meat juice which shows the additional antimicrobial agent present to inhibit the growth of background microflora, while other isolates completely lost their activity in meat juice. When the supernatant was treated with heat or protease, it did not affect the growth of target pathogen as well as background microflora in ground meat which suggested that the inhibitory action was not due to proteins and/or peptides in the culture supernatant. These heat-stable and protease-stable cell-free filtrates may have lesser effect by bacteriocin such as leucocin and/or mesentericin that are known to present in Leuconostoc species. The competitive growth activity between E. coli O157:H7 and cells of isolates was determined by co-inoculation in TSB. E. coli O157:H7 concentration was significantly reduced by 68.13–94.49 % (p < 0.05), while concentration of LAB isolates except DGB-938 was not significantly reduced (Table 3).
Leu. mesenteroides and Leu. citreum are heterofermentative bacteria which produce not only lactic acid but also others such as acetic acid during carbohydrate fermentation process. These organic acids are the main products and the most common organic acids. Leuconostoc spp. are also known to produce phenyllactic acid and hydroxyphenyllactic acid to perform antimicrobial activity against microorganisms (Suskovic et al. 2010). Therefore, the organic acid contents were investigated by HPLC analysis for lactic acid, acetic acid, and others and by LC–Q–TOF–MS analysis for phenyllactic acid and hydroxyphenyllactic acid. The result showed significant correlation of antimicrobial activity by the organic acids in CFS which was supported by adjustment of pH to 7. In this study, acetic acid was produced by the highest amount (Table 4), while Tejero-Sarinena et al. (2012) found that Lactobacilli and Bifidobacterium produced more significant amount of lactic acid than acetic acid. Tejero-Sarinena et al. (2012) showed a strong correlation between lactic acid concentration, pH and the antimicrobial activity, while acetic acid showed a negative correlation. In case of Lb. rhamnosus GG, the bacterium had antimicrobial activity against S. Typhimurium, and the antimicrobial agent was heat-stable, non-proteinaceous, and active at low pH. It was identified that lactic acid was the major factor to show the inhibition activity against S. Typhimurium (de Keersmaeker et al. 2006). In this study, pH level of the CFS for each isolate was strongly related to the amount of lactic acid of each isolate. However, the antimicrobial activity was not depended on the lactic acid concentration. Low pH in a microbial environment can kill bacteria by reduction of intracellular pH, while the decrease in pH is affected by not only lactic acid but also other organic acids. For example, citric acid and malic acid were active against foodborne pathogens (Over et al. 2009). Phenyllactic acid is produced from phenylpyruvic acid by D-lactic dehydrogenase in Leuconostoc species and this organic acid showed a wide range of antimicrobial activity against gram-positive bacteria such as S. aureus, B. cereus, and L. monocytogenes, and gram-negative bacteria including Salmonella, Klebsiella, and E. coli, and also in yeast and molds. Only limited studies have been known for the mode of action by phenyllactic acid except it can cause damage on bacterial cell wall (Li et al. 2014).
Effects of DGB-1040 on the LEE expression
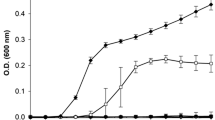
DGB-1040 which had the best antimicrobial activity among four isolates was selected for evaluation of reduction in pathogenicity and application in food products. A distinctive histopathology of E. coli O157:H7 infection in host intestinal cells is known as attaching and effacing (A/E) lesion, which is essential for the development of hemorrhagic colitis and hemolytic uremic syndrome in humans. The genes for the A/E lesion are encoded by the locus of enterocyte effacement (LEE) pathogenicity island (McDaniel et al. 1995). Transformant KS02 harboring two plasmids, P LEE3 inserted pBBR-lux and ler ORF inserted arabinose-inducible expression vector pKS1101, was incubated with or without lyophilized culture supernatant DGB-1040 to evaluate the inhibitory effect against LEE expression by the cellular luminescence. Expression of LEE-encoded regulator (Ler) was induced by addition of l-arabinose and Ler regulated the expression of LEE3 promoter for luminescence gene expression. Thus, if the CFS suppressed the LEE expression, the host can lost its pathogenicity. As shown in Fig. 1, transcription of the LEE operon was significantly suppressed by concentration-dependent manner. The cellular luminescence of KS02 treated with 2120 μg of lyophilized DGB-1040 supernatant for 6 h was 27-fold lower than that of KS02 without DGB-1040, indicating that DGB-1040 plays a role in inhibiting LEE expression. Therefore, inhibition of the transcription of LEE operon by CFS of DGB-1040 has shown the potential ability of DGB-1040 not only to kill target pathogen but also to block the infection route of pathogen.
Effects of DGB-1040 on the activity of P LEE ::luxCDABE. A Ler-dependent luciferase reporter plasmid was constructed by subcloning the LEE promoter region into pBBR-lux containing a promoterless luxCDABE. This was grown in medium supplemented with 0.0001 % l-arabinose and each concentration of DGB-1040
Antimicrobial application in food
DGB-1040 was also tested in ground meat where it was originally isolated from. The lyophilized CFS of DGB-1040 was supplemented in a ground beef product, and the growth of E. coli O157:H7 and background microflora were monitored. The concentration of total bacteria was reduced to 1.03 logCFU at day 7 at 5 °C and to 0.78 log CFU at day 3 at 10 °C. The storage time when the greatest reduction was monitored was the critical point when the total aerobic concentration was reached to the limit of storage time. Nychas et al. (2008) suggested that the quality control for ground meat is critical by controlling the microbial activity which is essential for meat spoilage. The reduction of E. coli O157:H7 was not as great as total aerobic count; however, a significant level of reduction was observed (Table 5). While significant reduction of total aerobic count as well as E. coli O157:H7 was observed, further investigation on optimization of the antimicrobial agent is necessary. Overall, this study has shown as a safe potential application of Leuconostoc species to inhibit the growth and infection of E. coli O157:H7 by isolation and application in ground meat.
References
Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A (2008) Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl Environ Microbiol 74(20):6230–6238
Breidt F, Caldwell JM (2011) Survival of Escherichia coli O157:H7 in cucumber fermentation brines. J Food Sci 76(3):M198–203
Chang JY, Chang HC (2011) Growth inhibition of foodborne pathogens by kimchi prepared with bacteriocin-producing starter culture. J Food Sci 76(1):M72–78
Chen CH, Ravishankar S, Marchello J, Friedman M (2013) Antimicrobial activity of plant compounds against Salmonella Typhimurium DT104 in ground pork and the influence of heat and storage on the antimicrobial activity. J Food Prot 76(7):1264–1269
de Keersmaeker SCJ, Verhoeven TLA, Desair J, Marchal K, Vanderleyden J, Nagy I (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG againstSalmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 259:89–96
de Oliveira TL, das Gracas Cardoso M, de Araujo Soares R, Ramos EM, Piccoli RH, Tebaldi VM (2013) Inhibitory activity of Syzygium aromaticum and Cymbopogon citratus (DC.) Stapf. essential oils against Listeria monocytogenes inoculated in bovine ground meat. Braz J Microbiol 44(2):357–365
Hartmann HA, Wilke T, Erdmann R (2011) Efficacy of bacteriocin-containing cell-free culture supernatants from lactic acid bacteria to control Listeria monocytogenes in food. Int J Food Microbiol 146:192–199
Hwang JH, Kang JH, Lee KH, Lee JH, Lee SM, Kim NH, Kim JY, Kim EJ (2014) Alteration in phenolic compounds and antioxidant activities of Aronia melanocarpa ethanol extracts following fermentation using different strains of Leuconostoc mesenteroides to develop natural antibiotic alternative. Kor J Organic Agric 22(4):825–839
Jeong HS, Kim SM, Lim MS, Kim KS, Choi SH (2010) Direct interaction between quorum-sensing regulator SmcR and RNA polymerase is mediated by integration host factor to activate vvpE encoding elastase in Vibrio vulnificus. J Biol Chem 285(13):9357–9366
Jo MY, Kim JH, Lim JH, Kang MY, Koh HB, Park YH, Yoon DY, Chae JS, Eo SK, Lee JH (2004) Prevalence and characteristics of Escherichia coli O157 frommajor food animals in Korea. Int J Food Microbiol 95:41–49
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Kim SM, Park JH, Lee HS, Kim WB, Ryu JM, Han HJ, Choi SH (2013) LuxR homologue SmcR is essential for Vibrio vulnificus pathogenesis and biofilm detachment, and its expression is induced by host cells. Infect Immun 81(10):3721–3730
Lee MA, Choi JH, Choi YS, Han DJ, Kim HY, Shim SY, Chung HK, Kim CJ (2010) The antioxidative properties of mustard leaf (Brassica juncea) kimchi extraction refrigerated raw ground pork meat against lipid oxidation. Meat Sci 84:498–504
Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL (2004) The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118(1):69–82
Li L, Shin SY, Lee KW, Han NS (2014) Production of natural antimicrobial compound D-phenyllactic acid using Leuconostoc mesenteroides ATCC 8293 whole cells involving highly active D-lactate dehydrogenase. Lett Appl Microbiol 59:404–411
Maragkoudakis PA, Mountzouris KC, Psyrras D, Cremonese S, Fischer J, Cantor MD, Tsakalidou E (2009) Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int J Food Microbiol 130(3):219–226
McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB (1995) A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA 92(5):1664–1668
Ministry of Food and Drug Safety (MFDS) (2015) Food poisoning statistical analysis system. http://www.mfds.go.kr/e-stat/index.do. Accessed 13 February 2015
Mintel Group Ltd (2010) Red Meat US Report. http://reports.mintel.com/display/482976/#. Accessed 1 Sept 2014
Mor-Mur M, Yuste J (2010) Emerging bacterial pathogens in meat and poultry: an overview. Food Bioprocess Technol 3(1):24–35
Nychas GE, Skandamis PN, Tassou CC, Koutsoumanis KP (2008) Meat spoilage during distribution. Meat Sci 78:77–89
Oladunjoye A, Soni KA, Nannapaneni R, Schilling MW, Silva JL, Mikel B, Bailey RH, Mahmoud BS, Sharma CS (2013) Synergistic activity between lauric arginate and carvacrol in reducing Salmonella in ground turkey. Poult Sci 92(5):1357–1365
Over KF, Hettiarachchy N, Johnson MG, Davis B (2009) Effect of organic acids and plant extracts on Escherichia coliO157:H7, Listeria monocytogenes, and Salmonella Typhimurium in broth culture model and chicken meat systems. J Food Sci 74(9):M515–M521
Pennacchia C, Ercolini D, Villani F (2011) Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiol 28:84–93
Pujato SA, del L Quiberoni A, Candioti MC, Reinheimer JA, Guglielmotii DM (2014) Leuconostoc citreum MB1 as biocontrol agent of Listeria monocytogenes in milk. J Dairy Res 81:137–145
Suskovic J, Kos B, Benganovic J, Pavunc AL, Habjanic K, Matosic S (2010) Antimicrobial activity: the most important property of probiotic and starter lactic acid bacteria. Food Technol Biotechnol 48(3):296–307
Taylor EV, Holt KG, Mahon BE, Ayers T, Norton D, Gould LH (2012) Ground beef consumption patterns in the United States, Food Net, 2006 through 2007. J Food Protect 75(2):341–346
Tejero-Sarinena S, Barlow J, Costabile A, Gibson GR, Rowland I (2012) In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe 18:530–538
Williams MS, Withee JL, Ebel ED, Bauer NE, Schlosser WD, Disney WT, Smith DR, Moxley RA (2010) Determining relationships between the seasonal occurrence of Escherichia coli O157:H7 in live cattle, ground beef, and humans. Foodborne Path Dis 7(10):1247–1254
Yang SC, Lin CH, Sung CT, Fang JY (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5(241):683
Acknowledgments
This research was funded by a research grant of Korea Food Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koo, O.K., Kim, S.M. & Kang, SH. Antimicrobial potential of Leuconostoc species against E. coli O157:H7 in ground meat. J Korean Soc Appl Biol Chem 58, 831–838 (2015). https://doi.org/10.1007/s13765-015-0112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0112-0