Abstract
As one of the most consumed beverages worldwide, green tea has been extensively studied for its wide array of health benefits. In this study, the composition of active compounds and antioxidative activity was analyzed using various antioxidant assay systems and compared between organic green tea (OGT) and conventionally grown green tea (CGT). OGT extract was prepared in the same way as CGT with the exception of fertilizer and insecticide, which were used only for CGT preparation. There were no significant differences in total polyphenol content and antioxidant activity tested against 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radicals and 1,1-diphenyl-2-picrylhydrazyl radicals. In addition, the antioxidant activities shown with both assays were highly correlated with the polyphenol contents (r 2 > 0.99). No significant differences were observed in the cellular antioxidant activity assay using HepG2 cells (P > 0.05). However, substantially higher concentrations of catechins, especially epigallocatechin-3-gallate, were found in the OGT (7.64 vs 5.54/100 mg extract). Thus, OGT could be considered as a potent and safe antioxidative resource, being free of both fertilizer and herbicide uses. However, the advantages of OGT should be further optimized to provide a wide range of physiological effects, considering the price of production and parallel amounts of the active ingredients in CGT.
Similar content being viewed by others
Introduction
Green tea (a product from the plant Camellia sinensis), one of the most widely consumed beverages in the world, has gained a great deal of attention in many scientific studies. Many studies have reported that green tea and its extract have excellent beneficial effects on human health, including anticarcinogenic (Farabegoli et al. 2007), anticariogenic (Linke and LeGeros 2003), hypocholesterolemic (Kim et al. 2012), and antioxidative activities (Skrzydlewska et al. 2002). The antioxidant properties of green tea extract have been mainly attributed to polyphenols, which are secondary metabolites with many positive effects on human health, including the anti-inflammatory and anticarcinogenic properties (Linke and LeGeros 2003). The major compounds in green tea are phenolic compounds, mainly catechins. The naturally occurring types of catechin in green tea are epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), and epicatechin (EC). Moreover, gallocatechin gallate (GCG), gallocatechin (GC), catechin gallate (CG), and catechin (C) are produced during the manufacturing process by epimerization (Wang and Helliwell 2000; Chen et al. 2001). In addition to catechin, tea leaves contain many other components such as tannin, theanine, polysaccharides, and flavonoids.
Although green tea has various health-promoting effects, it is not certain whether the active components and their consequent effects have full activity in the body, since many unexpected events could occur during digestion and absorption, such as epimerization, degradation, or coupling of the active compounds with other components. Zhu et al. (2000) reported low bioavailability of catechin as a result of a first pass effect, wide tissue distribution, and incomplete absorption after oral administration in rats. It was observed in an in vivo study that limited trans-epithelial absorption related to the multi-drug resistance protein (MRP) caused an efflux of green tea catechin from the Caco-2 cell line (Zhang et al. 2004). Moreover, the binding properties of catechin with proteins or iron were shown to lower the bioavailability of the proteins, iron, or catechin itself (Haslam 1996; Disler et al. 1975).
Major trends in current food consumption have been focused on making healthier choices, causing interest in organic foods to increase greatly in many industrialized countries during the past decades. Growth without pesticides and better functional values of organic foods is the major reasons why organic food sources are preferred by many over conventionally grown food sources. However, a parallel comparison of the functionality has not been thoroughly performed. In organically cultivated fields, the synthetic pesticide input is approximately 96.5 % lower than the conventional ones (Mader et al. 2002). Consequently, some studies have shown higher nutrient levels in fruits and vegetables grown organically compared to those grown conventionally (Bourn and Prescott 2002). However, there are controversial opinions regarding the superiority of organic agricultural products over conventionally grown products in terms of the physiological activity and health benefits, which have attracted attention when it comes to the price of the products. In the present study, the antioxidant effects and the profiles of active components were compared between extracts of organic green tea (OGT) and conventional green tea (CGT).
Materials and methods
Materials
Spray-dried hot water extract of OGT and CGT was provided by Dae Han Tea Produce Co. (Boseong, Korea). Ascorbic acid (vitamin C), gallic acid, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), caffeine, dichlorofluorescein diacetate (DCFH-DA), and catechin isomers including EGCG, EGC, ECG., and EC were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Wako Pure Chemical (Osaka, Japan). All other chemicals were analytical reagent grade.
Green tea sample preparation
The OGT, CGT samples were provided by Bosung Tea Farm (Dae Han Tea Produce Co., Jeonnam, Korea). The OGT was produced without the use of any insecticides or chemical fertilizers for 3 years, and was certified by the National Agricultural Products Quality Management Service (Korea). Five grams of tea sample among 10 kg each green tea sample was submerged in 1 L of methanol (>99 %) and underwent shaking extraction for 12 h at room temperature. The extracts were filtered through Whatman No. 41 filter paper (Whatman Int. Ltd., Maidstone, Kent, UK), evaporated at 40 °C with an evaporator, and freeze dried using a programmable freeze dryer (Ilshin Lab Co., Yangju, Korea) (Kim et al. 2010).
Determination of total polyphenol contents
The total polyphenol contents of the green tea extracts were determined using the Folin–Ciocalteu’s colorimetric method with slight modifications (Kim et al. 2010). Briefly, 200 μL of Folin–Ciocalteu’s phenol reagent was added to 2.8 mL aliquots of the diluted green tea extracts or standard solutions of gallic acid. After reacting in the dark for 7 min, 2 mL of 7 % Na2CO3 solution was added with mixing. After 90 min at room temperature, the absorbance was read against a blank at 750 nm. Total polyphenol contents were expressed as mg of gallic acid equivalent (GAE) per 100 mg of green tea extract.
Measurement of antioxidant capacity
DPPH radical scavenging activity
DPPH• (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity was evaluated using the method reported by Brand-Williams et al. (1995) with slight modifications. Briefly, 50 μM of DPPH• was dissolved in ethanol and adjusted to have an absorbance of 0.7 ± 0.02 at 517 nm. Next, 100 μL of sample solution was added to 2.9 mL of DPPH• solution and vortexed. The absorbance at 517 nm was measured 20 min after the reaction was initiated. Ethanol was used as the blank, and DPPH• solution (2.9 mL) plus water (0.1 mL) were used as the control.
ABTS radical scavenging activity
Antioxidant activity was measured using the modified ABTS•− assay described by van den Berg et al. (1999). First, 1.0 mM of AAPH and 2.5 mM of ABTS were dissolved in 100 mM of potassium phosphate-buffered solution (PBS, pH 7.4). The ABTS•− radical solution was heated at 70 °C for 30 min and then cooled down to room temperature. After filtration through a 0.45 μm syringe filter, the ABTS•− solution was diluted to obtain an absorbance of 0.65 ± 0.02 at 734 nm. Next, 0.98 mL of ABTS•− solution was mixed with 0.02 mL of the sample solution, and the absorbance at 734 nm was measured 20 min later. The PBS solution was used as the blank, and the control consisted of 0.98 mL of ABTS•− radical solution mixed with 0.02 mL of water.
The antioxidant capacity was evaluated by measuring the free radical scavenging activity of the green tea extracts, and the absorbance was recorded on a UV/VIS spectrophotometer, model UV 2100 (Shimadzu Co., Tokyo, Japan). Each sample stock solution and vitamin C was diluted in distilled water. The fresh radical solution was prepared before the experiment. The percent of free radical scavenging activity of the green tea extracts and vitamin C, used as a positive control, was calculated as follows, and calibration curves were obtained
where A c is the absorbance of the control and A s is the absorbance of the sample.
The antioxidant capacity was expressed as VCEAC (Vitamin C Equivalent Antioxidant Capacity) in mg/100 mL sample solution, which was calculated using the vitamin C standard curves (Kim et al. 2002).
Evaluation of cellular antioxidant activity
This protocol was established with practical modifications from the DCFH-DA assay previously published (Wolfe and Liu 2007). In brief, HepG2 cells were incubated in a 96-well plate at a density of 6.0 × 104 cells/well for 24 h. The cells were subsequently washed with PBS, and each well was treated with 100 μL of fresh medium containing quercetin (positive control) or green tea sample, along with 25 μM DCFH-DA, for 1 h. After washing with PBS, 600 μM ABAP in HBSS was added to the wells, and the fluorescence was measured every 5 min for 1 h at 37 °C using a Victor 3 Multilabel Counter (Perkin Elmer, Waltham, MA, USA). The emission and excitation wavelengths for DCF formation were 538 and 485 nm, respectively. The AUC for fluorescence versus time was calculated. Each plate included triplicate control and blank wells: control wells contained cells treated with DCFH-DA and ABAP as an oxidant; blank wells contained cells treated with dye and HBSS without the oxidant. The cellular antioxidant capacity of each sample was calculated as follows:
Reverse-phase HPLC analysis of tea catechins and caffeine
The amount of caffeine and catechins in the OGT and CGT samples was determined by HPLC analysis using a Waters 600E system controller. The Waters 484 tunable absorbance detector was used to detect constituents of the tea at 280 nm, and all peaks were plotted and integrated using the Waters 745 data module. The green tea extracts were filtered through 0.45 μm filter disks and then injected into the column (Cosmosil 5C18-MS packed column; 5 μm, 4.6 mm × 250 mm, code 379-72). The concentrations of the working solutions of caffeine and catechins were 100 μg/mL. Five hundred nanograms of each standard compound (caffeine, C, EC, ECG, EGC, and EGCG) was injected. The mobile phase was composed of methanol/doubly distilled water/formic acid (19.5:82.5:0.3, v/v/v), and was degassed by sonication (Branson 5200) and run by an isocratic elution at a flow rate of 1.0 mL/min.
Statistical analysis
Values were reported as mean ± SD from experiments conducted at least in triplicate. One-way analysis of variance (ANOVA) was used for comparisons between the groups, and the differences were considered to be statistically significant when the p values were less than 0.05.
Results and discussion
Total polyphenol contents and antioxidant capacity
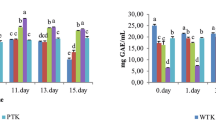
Various parameters such as catechin, polyphenol contents, and antioxidant activity were compared between the OGT and CGT samples. The OGT and CGT extracts contained 179.01 ± 4.15 and 187.48 ± 7.24 μmol of polyphenols, respectively, expressed as GAE/100 mg of extract, demonstrating no significant difference (P > 0.05) (Fig. 1).
The antioxidant activity was measured by both the DPPH and ABTS methods, which allow convenient measurement of the free radical scavenging capacity with high sensitivity. The absorbance decreased when DPPH• was scavenged by the antioxidants, donating hydrogen to form a stable DPPH molecule. After a 20 min reaction of the antioxidant content with the DPPH• radical solution, the DPPH• radical scavenging activities of OGT, CGT, and vitamin C (Vit-C) measured (Fig. 2). The OGT and CGT extracts (10 mg/100 mL) showed 48.9 and 45.9 % antioxidant activity compared with Vit-C. There was no significant difference between the antioxidant activities of the OGT and CGT extracts evaluated by the DPPH assay.
The antioxidant potential of the extracts and Vit-C used in the DPPH assay was also evaluated against the ABTS•− radical. ABTS was oxidized to the ABTS•− radical anion by AAPH, a thermolabile water-soluble radical generator, during heating at 70 °C before the addition of test samples. The reduction of the ABTS•− radical chromogen by antioxidants decreases its absorbance at 734 nm. The OGT and CGT extracts (10 mg/100 mL) demonstrated radical scavenging activities of 85 and 83.6 % compared with Vit-C, which were lower than an equal concentration of Vit-C (Fig. 2). The ABTS assay also revealed no significant differences in the antioxidant activities of the OGT and CGT extracts.
Based on the antioxidant activities, the OGT and CGT extracts showed VCEACs of 4.84 ± 0.2 /10 mg and 4.65 ± 0.1/10 mg in the DPPH assay, respectively, while values of 8.42 ± 0.3/10 mg and 8.17 ± 0.2/10 mg were obtained in the ABTS assay (Fig. 3). The ABTS assay overestimated the antioxidant capacity by about 42.5 % compared to the DPPH assay. This overestimation of VCEAC by assaying ABTS•− radicals may be due to the different solvent systems used in the ABTS and DPPH assays. The activity in the ABTS assay was higher than that shown in the DPPH assay because the buffer solvent in the ABTS assay holds less catechins available to the radicals in the reaction solution compared to the DPPH assay, which uses ethanol as solvent. Thus, the solvent used to dissolve the extracts could affect antioxidant activity of the compounds according to the fraction solubility of antioxidant available for different radicals. This difference in solvents may have caused differences in the antioxidant pattern between the two assays, which are in good agreement with our previous study which showed that the solvent and solubility of the active components affect the hydrogen donating ability of the tested antioxidants (Lee et al. 2009).
Cellular antioxidant activity assay
To evaluate the protective effects of the two green tea extracts against ROS-induced oxidative stress in a cellular system, the DCFH-DA assay was performed. Since endogenous oxidative stresses were present from the Hep G2 cells, which oxidize DCFH to the highly fluorescent compound DCF, the fluorescence intensity in the control group was adjusted to 100 %. The Hep G2 cells had a 50.7 % increase in the level of oxidative stress after exposure to H2O2. As shown in Fig. 2, the intracellular protective effects of the OGT and CGT extracts against the cellular oxidative stress induced by H2O2 were found to be proportional to the green tea concentration tested (Fig. 4a). When the concentration of green tea extract was more than 2 mg/mL, a significant decrease of the oxidative stress was observed. The oxidative stress dropped back down to 110.2 and 110.7 %, when the cells were treated with 5 mg/mL of OGT and CGT extracts, respectively. A significant difference in the inhibition of DCF formation by OGT and CGT extracts was not observed (Fig. 4b).
The intracellular protective effect of green tea extract on the DCF formation in Hep G2 cells (a) and the dose–response relationship of cellular antioxidant activity (b). a Means followed by different superscripts are statistically different (P < 0.05). *P < 0.05, versus H2O2 exposed group. NC negative control (b) filled circle OGT extract (r 2 = 0.994); inverted triangle CGT extract (r 2 = 0.995)
Catechin analysis by HPLC system
To compare the catechin and caffeine profiles of the OGT and CGT extracts, HPLC analysis was performed. The catechin and caffeine concentrations in the green tea extracts were determined by comparing the peak areas with that of each standard compound. Excluding catechin (C) and GCG, OGT had significantly higher amounts of the major catechins compared to CGT, including EGCG, EGC, ECG, and EC (Fig. 5). It was also shown that the OGT extract contained higher total catechin levels than the CGT extract. The decrease of epicatechin (EGCG, EC) and increase of epicatechin epimer (GCG, C) levels observed in the tea cultivated conventionally may suggest that the degree of epimerization depends on the cultivation conditions. It was reported that epicatechin epimers such as GCG and C are less effective than epicatechin, in both antioxidant activity and bioavailability (Xu et al. 2004). These results suggest that organic cultivation might be a more suitable method to produce green tea with higher concentrations of active compounds. In contrast to the major catechin contents, no significant difference in the CAF levels was observed between the OGT and CGT (P < 0.05). The catechin and caffeine contents in the OGT and CGT extracts are shown in Table 1.
Catechin and caffeine contents in organic and conventional green tea extracts. Catechin contents in 100 mg of organic/conventional green tea extract. Values represent the mean (n = 3) ± SD. Different letters indicate significant differences from the other tested group. P < 0.05. CAF caffeine, C catechin, EC epicatechin, EGCG epigallocatechin gallate, GCG gallocatechin gallate, ECG epicatechin gallate, EGC epigallocatechin
When total polyphenol contents, chemical and cellular antioxidant activities, and catechin contents were compared between OGT and CGT, only the catechin contents showed significant differences, being higher in the OGT extract. Although there were no significant differences in the other parameters, such as total polyphenol contents and resultant antioxidant activity, OGT, which contained more catechins, could be considered a better green tea resource for the production of functional food, due its advantages. OGT is free of fertilizer and herbicide use, although there were no significant differences in the antioxidant activity shown against some free radicals.
Green tea contains many compounds, especially polyphenols, and epidemiological studies showed that polyphenolic compounds present in tea reduce the risk of a variety of disease. Catechins are the main compounds in green tea; they consist of (−)epicatechin, (−)epicatechin-3-gallate(ECG), (−)-epigallocatechin, and (−)-epigallocatechin-3-gallate (EGCG). Many in vitro and in vivo studies on catechins report mechanism consistent with protection against degenerative disease (Vanessa and Gary 2004).
In the result of our study, the catechin contents showed significantly higher in the OGT extract. The advantages of using OGT in functional food development could be optimized in a wide range of functional aspects, in terms of the parallel amount of active ingredients contained in CGT. This study was performed to investigate the differences in the functional ingredients and antioxidant activities of OGT compared to CGT. Although OGT showed no significant difference with CGT in the total polyphenol contents and antioxidant activities, significantly higher levels of green tea catechins, especially EGCG, were found in the OGT. This suggests that the utilization of OGT could be beneficial as a food resource with no pesticide residues and better functional values compared to CGT.
References
Bourn D, Prescott J (2002) A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit Rev Food Sci 42:1–34
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol 28:25–30
Chen ZY, Zhu QY, Tsang D, Huang Y (2001) Degradation of green tea catechins in tea drinks. J Agric Food Chem 49:477–482
Disler PB, Lynch SR, Torrance JD, Sayers MH, Bothwell TH, Charlton RW (1975) The mechanism of the inhibition of iron absorption by tea. S Afr J Med Sci 40:109–116
Farabegoli F, Barbi C, Lambertini E, Piva R (2007) (−)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev 31:499–504
Haslam E (1996) Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod 59:205–215
Kim DO, Lee KW, Lee HJ, Lee CY (2002) Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem 50:3713–3717
Kim JH, Pan JH, Heo W, Lee H, Kwon EG, Lee H, Shin DH, Liu RH, Kim YJ (2010) Effects of cellulase from Aspergillus Niger and solvent pretreatments on the extractability of organic green tea waste. J Agric Food Chem 58:10747–10751
Kim YJ, Houng SJ, Kim JH, Kim YR, Ji HG, Lee SJ (2012) Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J Nutr Biochem 23:186–191
Lee KW, Kim YJ, Kang NJ, Kim JH, Lee SJ, Kim DO, Lee CY, Lee HJ (2009) Improved assay for determining the total radical-scavenging capacity of antioxidants and foods. Int J Food Sci Nutr 60:12–20
Linke HAB, LeGeros RZ (2003) Black tea extract and dental caries formation in hamsters. Int J Food Sci Nutr 54:89–95
Mader P, Fliebbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K (2002) Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine 9:232–238
van den Berg R, Haenen GRMM, van den Berg H, Bast A (1999) Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements. Food Chem 66:511–517
Vanessa C, Gary W (2004) A review of the health effects of green tea catechins in In Vivo animal models. J Nutr 134:3431S–3440S
Wang H, Helliwell K (2000) Epimerisation of catechins in green tea infusions. Food Chem 70:337–344
Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55:8896–8907
Xu JZ, Yeung SYV, Chang Q, Huang Y, Chen ZY (2004) Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br J Nutr 91:873–881
Zhang L, Zheng Y, Chow MSS, Zuo Z (2004) Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int J Pharm 287:1–12
Zhu M, Chen Y, Li RC (2000) Oral absorption and bioavailability of tea catechins. Planta Med 66:444–447
Acknowledgment
This work was supported by Korea University Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.J., Kim, J.H., Kim, J.H. et al. Comparative studies on the antioxidant capacities and catechin profiles of conventional and organic green tea. J Korean Soc Appl Biol Chem 58, 475–480 (2015). https://doi.org/10.1007/s13765-015-0045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0045-7