Abstract
A fuel-based solution combustion method was used to synthesize Sr-substituted copper ferrites [Cu1−xSrxFe2O4 (x = 0.00, 0.25, 0.50, 0.75, 1.00)]. X-ray diffraction (XRD) pattern demonstrates that CuFe2O4 has a tetragonal structure and SrFe2O4 a cubic structure and the formation of particles of spherical and needle-like structures was observed using scanning electron microscopy (SEM). The magnetic behavior was investigated through a vibrating sample magnetometer (VSM) at room temperature under an applied magnetic field ranging from − 20 kOe to + 20 kOe. Based on the M–H hysteresis loop, the soft-ferrite nature of the samples was observed. The synthesized samples were investigated for adsorption and photocatalysis in the degradation of textile dyes malachite green, crystal violet, and congo red. Compared to other compositions, SrFe2O4 demonstrated comparatively better adsorption and photocatalytic efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes are frequently used in textile, pulp and paper, paint, food, and other industries (Affat 2021). Dyes and other industrial pollutants are highly toxic, carcinogenic, and cause environmental degradation due to their non-degradable nature (Fatima et al. 2022; Lellis et al. 2019). Pollutant reduces the reoxygenation capacity of the receiving wavelength in waterbodies due to the cutoff of sunlight that negatively influences the environment (Berradi et al. 2019). Water pollution caused by textile dyes and heavy metal ions is one of the major concerns (Ali and Gupta 2006) because they are the prime cause of dreadful diseases, and dysfunctioning of organs such as liver, brain, and kidney failure. The need for the degradation of toxic dyes from polluted water has gained appreciable attention (Jiad and Abbar 2023), particularly to develop materials that could degrade pollutants into harmless substances (Akarslan et al. 2015; Pereira et al. 2012). Depending on the nature of the contaminant, different methods are used for detoxification (Slama et al. 2021). Converting organic pollutants into harmless end products such as CO2, water and inorganic ions through heterogeneous semiconductor oxide has become a promising technology (Rafiq et al. 2021). Several methods are used to eliminate these pollutants, e.g., adsorption, solar photo fenton method (Khaleel et al. 2023), ultrafiltration, chlorination and photocatalysis (Sharma and Kaur 2018). Every method has pros and cons; therefore, a balanced approach is needed to select a material for degrading organic pollutants. Among all, photocatalysis appears to be the most suitable method for degrading textile dyes because it is eco-friendly, provides complete degradation of pollutants, is highly efficient, and leftover products have a very low level of toxicity (Rueda-Marquez et al. 2020).
In photocatalysis, semiconducting materials are used as catalysts for dye degradation in the presence of sun-light (Mills et al. 1997). However, commonly used photocatalysts, such as TiO2, ZnO, ZnS and MoS2 (Khan et al. 2023), are expansive and can’t be separated from the water after degradation (Jacinto et al. 2020). Filtration and centrifugation processes are commonly used to separate the photocatalyst after the reaction. Nevertheless, these are expensive, less efficient, and time-consuming methods for separating the photocatalyst. Meanwhile, the industrial application of photocatalysts is highly restricted due to the above-mentioned problems. Therefore, photocatalyst, which possesses magnetic and photocatalytic properties, has gained significant attention (Das 2019). Consequently, spinel ferrite nanoparticles (SFNPs) have been considered a potential candidate as a photocatalyst in dye degradation to overcome the above-mentioned limitations (Ma and Liu 2021). Along with excellent photodegradation properties, these materials can easily be separated from the solution after the completion of the reaction with the help of an external magnet. Different types of SFNPs (MFe2O4 where M—transition metal, e.g., Co, Ni, Cu, Mn) have been extensively used as a photocatalyst due to their unique magnetic, optical, and electric properties along with thermal and chemical stability (Nie et al. 2019).
In the family of spinel ferrites, copper ferrites are extensively used in sensors, catalysis, electronics, gas sensing, hydrogen production, ceramics, and memory devices (Amiri et al. 2019). Copper ferrites possess phase transitions, interesting magnetic and electrical properties, and are non-toxic in nature (Roman et al. 2019) and it has significant potential and possesses promising outcomes in photocatalysis, reported in the previous literature (Masunga et al. 2019). The photodegradation of rhodamine B was studied using pure and Ce-doped copper ferrites (Keerthana et al. 2021). CuFe2O4@biochar composite prepared by one-step sol–gel pyrolysis method to degrade malachite green dye (Huang et al. 2021). Hydrothermally synthesized copper ferrites nanocomposite with graphene oxide was used for the malachite green degradation (Yadav et al. 2021). Photodegradation activity of TiO2-doped copper ferrites studied toward the degradation of azo dyes (Masoumi et al. 2016). Green engineered synthesis of Ag doped copper ferrites and its catalytic activity toward malachite green degradation were investigated (Surendra 2018).
CuFe2O4 has an inverse spinel structure in which 8 Cu+2 ions occupy the octahedral B-site, and 16 Fe+3 ions occupy equal tetrahedral A-site and octahedral B-site in the unit cell (Sharma et al. 2020). This configuration can be written as (Fe3+)A[Cu2+Fe3+]BO42−. Copper ferrites exist in two phases; cubic (stable at higher temperatures) and tetragonal (due to the Jahn–Teller distortion) (Calvo-De La Rosa and Segarra Rubí 2020). Different synthetic routes are employed to synthesize copper ferrites, such as co-precipitation, hydrothermal, solid-state, mechanical milling, electrochemical advance oxidation methods, (Jiad and Abbar 2023) thermal decomposition, microwave-assisted, and sol–gel auto combustion (Dippong et al. 2021). In the present study, the synthetic chemical route (self-propagating-combustion) was adopted due to its ease, scalability and viability in synthesizing Cu(1−x)SrxFe2O4 nanoparticles. Citric acid monohydrate was used as a chelating agent. Furthermore, this method provides other merits, such as no post-annealing is required, high yield and good chemical homogeneity of the samples.
To the best of our knowledge, no studies till now have been carried out on the application of Sr-substituted copper ferrites for photocatalytic dye degradation and adsorption. The large ionic radius of Sr+2 ions as compared to Cu+2 ions leads to lattice distortion and hence significant alteration in material’s characteristics. In a prior investigation, the addition of Sr+2 to cobalt ferrites resulted in a comparable effect. The study also investigates the sample’s structural, optical, morphological, and magnetic characteristics and then this synthesized samples are used for the photocatalytic activity on cationic and ionic dyes as well as adsorption study are also carried out.
Materials and methods
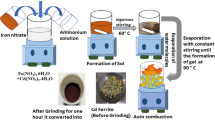
The sol–gel auto combustion method synthesizes Cu1−xSrxFe2O4 with x varies to 0, 0.25, 0.50, 0.75, and 1.00. Cu(NO3)2·6H2O, Sr(NO3)2·6H2O and Fe(NO3)2·6H2O of high purity (99.5%), purchased from Sigma-Aldrich, were used for sample preparation. Citric acid monohydrate was used as fuel to prepare the nanopowder. Metal nitrates and citric acid (C6H8O7⋅H2O) were used without any further purification. Nitrate precursors were weighed stoichiometrically and mixed in 50 mL of distilled water. Subsequently, 5 g citric acid was added into the precursor’s solution under continuous stirring for 15 min to mix the solution and the temperature was increased up to 90 °C, the ratio of fuel to oxidizer was 1:1. The pH of the solution was adjusted to 7 by using an ammonia solution after that solution color turns green. The ignition temperature was raised to 150 °C, and the solution covert into slurry gel under vigorous and constant stirring. The gel afterwards formed powder after the auto combustion process, and it was calcined at 700 °C, as shown in Fig. 1.
Adsorption and photocatalytic test on malachite green, crystal violet dye and congo red
Adsorption studies were carried out before the photocatalysis experiment. 20 mg of catalyst is mixed with 100 ml of dye solution. At fixed time intervals (0, 5, 10, 15, 20, 30, and 60 min), the adsorption kinetics were examined by taking 2.0 mL of the sample solution and observing for 1 h. Pseudo-first- and pseudo-second-order adsorption models were employed to study the degradation of textile dyes via adsorption. The linearized form of the kinetic model (Huang and Shih 2021) is shown in Eqs. (1 and 2).
where K1 and K2 are the reaction rate constant for the first order and second order, respectively. The adsorption capacity (Qt) can be determined from Eq. (3).
where Qt and Qe represent adsorption capacity at time (t) and equilibrium adsorption capacity, respectively, whereas C0 and Ct denote the initial dye concentration and dye concentration at time (t), respectively; V denotes the volume of dye solution; m stands for the mass of adsorbent.
Adsorption and photocatalysis efficiency were evaluated from Eq. (4)
The photocatalytic and adsorption experiment was performed at room temperature that was around 45 °C for all the prepared samples however, we reported here for the best three catalysts (CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4). The adsorption and photocatalytic experiment performed individually. Initially, the adsorption experiment was performed for 60 min. Only MG was degraded in adsorption phenomenon whereas other dyes’ degradation efficiency was quite less. The photocatalytic performance of CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 was evaluated separately for 30 min in the degradation of textile dyes (MG, CV, and CR). The stock solution of dye was prepared, and an aliquot of stock solution was taken for photocatalysis reaction. Catalyst (CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4), 20 mg, was added to 100 mL of aqueous solution of dye. All the prepared solutions were exposed to sunlight for 30 min in photocatalytic reaction. At fixed time intervals, 5 ml of dye solution was pipetted out until a complete degradation of dyes. This procedure is the same for both adsorption and photocatalysis. Finally, the degradation spectra of the pollutant were observed using a UV–Vis spectrophotometer by using Eq. 4.
Characterizations
Powder XRD patterns were recorded using Rigaku Ultima IV X-ray diffractometer with a Cu Kα source (λ = 1.54 Å) in the 2θ range 10°–80° at a rate of 2°/min). The surface morphology was examined using a high-resolution (SEM; Quanta 3D FEG). UV–Visible spectrophotometer (Agilent Technologies) was used to measure the absorption spectra at room temperature in the 200–450 nm range. The functional group of the samples has been studied from Fourier Transform infrared radiation (FTIR), Bruker tensor 37 in the range (500–4000) cm−1 and the Raman spectroscopy of the prepared was measured by using Invia Renishaw at 532 nm. Zeta potential measurements were done using a Malvern Zetasizer Nano ZS90, Mott–Schottky measurements were performed using potentiostat/galvanostat Autolab PGSTAT 204 (Metrohm, The Netherlands). The charge carriers’ recombination times were examined by recording time-resolved photoluminescence (TRPL) using a Horiba (DeltaFlex01-DD) spectrophotometer at 280 nm excitation wavelength. The magnetic measurements were carried out at room temperature in the range of applied field − 20 kOe to + 20 kOe from Cryogenic Limited UK VSM.
Results and discussion
Phase analysis using X-ray diffraction
The powder XRD was used to examine the crystallinity, phase purity and crystallite size. XRD patterns of CuxSr(1−x)Fe2O4 (x = 0.00, 0.25, 0.50, 0.75, and 1.00) are shown in Fig. 2. We observed the tetragonal phase of CuFe2O4 (JCPDS No. 34-0425) and cubic phase of SrFe2O4 (JCPDS No. (001-1027) in Cu(1−x)SrxFe2O4 when x = 0.00 and 1.00, respectively. SrFe2O4 secondary phase was observed in Cu(1−x)SrxFe2O4 for x = 0.25, 0.50, and 0.75. It confirms the substitution of Sr atoms in CuFe2O4 systems.
The presence of the SrFe2O4 phase is possibly due to phase segregation. Since the excess amount of Sr atoms occupy both substitutional and interstitial sites (tetrahedral and octahedral) in the host lattice and increases the Sr concentrations; consequently, the interaction probability increases with other atoms of the host lattice and forces them to leave their sites. With the higher temperature treatment given to the samples and increased Sr atoms incorporation, Gibb’s free energy stabilizes and produces the secondary phase (Jelonek et al. 2020). The sharp XRD peaks indicate a high crystallinity of the as-synthesized nanocomposites.
The lattice parameters (‘a’ & ‘c’) are calculated using the interplanar spacing (dhkl) given in Eq. 5.
The compositional variation of the lattice parameters is shown in Fig. 3A. Lattice parameters (‘a’ & ‘c’) are calculated taking the 2θ (hkl): 35.86° (211) and 2θ (hkl): 62.15° (224) miller plane values. The nonlinear behavior of lattice parameters indicates the contribution of both compositional and structural disorder, where the structural disorder can be attributed due to point defects. Crystallite size and the lattice strain were calculated from the line broadening of diffraction peaks of XRD using the Williamson–Hall method (Vishwaroop and Mathad 2020) and also shown in Fig. 3B. Crystallite size is also calculated using the Debye–Scherrer formula given by \(D\left( {{\text{nm}}} \right) = 0.9{\uplambda }/\beta {\text{cos}}\left( \theta \right)\), where λ represents X-ray wavelength (1.5406 Å), \(\theta\) is diffraction angle, and β is full-width half maximum (FWHM) (broadening of the peak) (Rabiei et al. 2020).
According to the W–H method:
ε is the strain produced in the lattice.
A plot between β cosθ and 4 sinθ is shown in Fig. 4A–E. From the linear fit to the data, the strain (ε) was calculated from the slope, and the crystalline size was calculated from the y-intercept of the plot. The negative slope signifies the compressive strain in the lattice (Dey et al. 2020). The crystallite size decreases with increasing Sr concentration due to the produced lattice strain by the lattice mismatch (as given in Table 1).
Morphology study
The morphology of the synthesized Cu1−xSrxFe2O4 nanoparticles for the selected composition was explored using SEM analysis and is shown in Fig. 5A–E. A mixed morphology, i.e., needle-like structure and spherical shape of the particles, were observed along with the large number of agglomerations suggesting the inhomogeneous synthesis process. The reason behind agglomeration is magnetic nature and primary particles union apprehended together via feeble surface interface such as van der Waals forces (Dhir et al. 2014). The uneven distribution of particles size could be due to the synthesis method.
UV–visible study
The absorbance spectra of undoped and Sr-doped CuFe2O4 are illustrated in Fig. 6A. The optical bandgap was estimated using Tauc’s equation given by \(\left(\alpha h\upsilon \right)=A{\left(h\upsilon -{E}_{{\text{g}}}\right)}^{n}\) where α is absorption coefficient, \(h\upsilon\) is incident photon energy in eV, Eg is optical bandgap (eV), and n indicates the type of transition. For direct transition (direct bandgap), n = 2 is used, and for indirect transition (indirect bandgap), n = 1/2 is used for the bandgap estimation (Arifin et al. 2019). The Eg was estimated through the intercept, and its relationship with the compositional variation is shown in Fig. 6B. The band gap of Cu(1−x)SrxFe2O4 was found to be decreased from 3.7 to 2.3 eV for increasing x from 0 to 1. The Eg shows a linear dependence on Sr composition x given by \({E}_{{\text{g}}}=2.59+1.51x\) and follows ideal Vegard’s law (Kempter 1966). A red shift is observed in the bandgap as the Sr composition increases, where additional sub-energy levels are introduced in the band structure due to lattice strain or point defects (Manikandan et al. 2013; Liu et al. 2004). Therefore, Sr-substituted copper ferrites can absorb more photons and generate more electrons and holes, which favors enhancing photocatalytic efficiency.
Raman and FTIR spectroscopy
According to the factor group analysis, cubic spinel has 5 Raman active mode that can be represented by (A1g + 3T2g + Eg) and in tetragonal spinel there are 10 Raman active mode represented by (2A1g + 3B1g + B2g + 4Eg) (shown in Fig. 7A). According to the literature, the modes beyond 600 cm−1 (A1g) frequently indicates the symmetric stretching of an oxygen atom with the metal ion at A-site. The presence of metal ions in the B-sites is responsible for the other low frequency symmetric and asymmetric bending and stretching modes (T2g, B1g, B2g, and Eg). (Channagoudra et al. 2021).
The FTIR spectra recorded in the range (500–4000) cm−1 to investigate the functional group and to determine the bonding between the atoms shown in Fig. 7B, C. Two metal oxygen bonds obtained in the range (200–800) cm−1 one is observed at 790 cm−1 for metal–oxygen bond. The other one in the range (200–400) cm−1 was not observed because of instrument limit. The wavenumber at 3386 cm−1 due to the bonding of O–H. The peak that are observed in the range (3200–3700) cm−1 appears due to the stretching vibrations of O–H (Dhyani et al. 2022).
VSM (Vibrating sample magnetometer) measurement
The Magnetic properties of undoped and Sr-doped copper ferrites were investigated from vibrating sample magnetometer (VSM) in a magnetic field of 20 kOe. The M–H curve (Hysteresis loop) describes the material’s magnetic behavior as shown in Fig. 8A–C. We calculated the saturation magnetization (Ms), Remanent magnetization (Mr), coercivity (Hc) and the squareness ratio (Mr/Ms) from the S-shaped hysteresis loop. The calculated values of Ms, Mr, Hc and squareness ratio are reported in Table 2. The value of magnetic parameters obtained from the hysteresis loop was observed to decrease with an increase in Sr-doping concentrations.
The magnetic response of the spinel ferrites depends on various parameters such as crystallite size, cation distribution in tetrahedral sites (A) and octahedral sites (B), oxygen deficiency, exchange interactions and ionic radii of alkaline earth metal (Ateia et al. 2020). In principle, there are three types of interactions in spinel ferrites, namely AOA, BOB and AOB. Interaction corresponding to the coupling of electrons with metal ions, AOB, is the strongest among them (Odio et al. 2017). CuFe2O4 belongs to the inverse spinel category, therefore, the divalent ion occupies the octahedral site, and trivalent is distributed in tetrahedral as well as octahedral sites, similar to the strontium ferrites (Ghahfarokhi and Shobegar 2020). The ionic radius of Sr+2 ion is larger than the Fe+2 ion; therefore, due to the incorporation of rare earth metals, Fe+2 ions migrate from the octahedral to the tetrahedral site. Consequently, AOB super exchange interaction decreases, which leads to the lower value of the saturation magnetization (Abdel Maksoud et al. 2020). Another possibility for the decrement in saturation magnetization is the non-magnetic nature of Sr+2 ions. The synthesized samples were found to be soft ferrites due to low coercivity (see Table 2). The squareness ratio (SQR) value was observed in the range of 0.53–0.21. The materials with squareness less than 0.5 belong to the single-domain category, and those with squareness greater than 0.5 belong to the multi-domain class (Gore et al. 2022). VSM results indicate durability and easy magnetic material recovery in the applied external magnetic field.
Adsorption study
An adsorption study was performed for MG, CV and CR dyes; however, only MG was degraded significantly and the rest of the dyes degraded below 55%. The possible reason for the difference in efficiencies is due to the small structural change in MG and CV. There are different factors such as charge density, electron distribution, substitutions present or steric factors which may responsible for this difference in efficiencies in cationic and anionic dyes (Liu et al. 2004; Zhuo et al. 2011). The adsorption experiment was done in the dark for one hour, and MG was observed to be degraded maximum from the Uv–Vis spectrometer. C/C0 vs time plotted for dark degradation as shown in Fig. 9A. Efficiency was calculated and obtained to be 68% in copper ferrites (SF1), 82% in copper strontium ferrites (SF2) and 92% in strontium ferrites (SF3) (shown in Fig. 9B).
Adsorption kinetics
To investigate the reaction mechanism of the adsorption of dye on the surface of the catalyst, two kinetic models, first and second order, were considered. Adsorption plots for first- and second-order kinetics are shown in Fig. 10A, B. The values of rate constants K1 and K2, corresponding linear regression coefficient (R2), were calculated and shown in Table 3. A lower R2 value for first-order reaction, reveals that the first-order model does not predict the rate kinetics of adsorption of MG dye on the nanocomposite surface. However, the R2 value corresponding to second-order kinetics is close to 1 and indicates that second-order model is suitable for the adsorption of MG onto the catalyst surface. Therefore, we conclude that the adsorption of MG follows pseudo-second-order kinetic model and chemisorption is paramount in the adsorption reaction (Sahoo et al. 2018; Vergis et al. 2018).
Adsorption mechanism
In principle, the adsorption mechanism contains three main steps (Alaqarbeh 2021).
-
1.
Film diffusion, where adsorbate ions or molecules transfer from the bulk solution to the external surface of the adsorbent’s particles due to the boundary layer.
-
2.
Intraparticle diffusion, where the adsorbate transports into adsorbent either due to the internal pores (pore diffusion) or through the internal surface (surface diffusion).
-
3.
Energetic interaction between the final adsorbate and internal adsorption active sites (Worch 2012; Han et al. 2017).
The adsorption process rate, in general, is controlled by the second and third steps, whereas the first step occurs rapidly.
The adsorbent initially encounters the solution of adsorbate material then the pores of the adsorbent’s material are filled with water (pores water). In the first step of adsorption process, the metal ion transforms from a bulk solution to a thin film solution and surrounds the adsorbent by a layer which is known as the boundary layer. The concentration of the adsorbate (dye molecules) in the solution decreases because the adsorbent ion (catalyst) ensnared into the boundary layer. This film diffusion process is the rate-governing mechanism in the adsorption process until the adsorbate concentration equilibrates in the bulk and boundary layer solutions.
More reduction of the adsorbate concentration in the bulk solution depends on intraparticle diffusion of adsorbate ions/molecules through pore diffusion and/or surface diffusion (Han et al. 2017). This reduction in concentration can be explained by Weber-Morris intraparticle diffusion model.
where q refers to the solid phase concentration (mg/g), i.e., quantity of adsorbate on the adsorbent, t is the adsorption time (minutes), k is the rate constant (mg/g–h0.5) for film or intraparticle diffusion, and c is a proportionality constant for the boundary layer thickness (mg/g). If the intraparticle diffusion is the solitary rate-controlling mechanism, then the q versus t0.5 gives a straight line that passes through the origin. A non-zero intercept means another mechanism is also involved in the adsorption process (Singh et al. 2012).
Two intraparticle diffusion rate constants have been calculated from the linear fit data. The adsorption mechanism can be explained based on the above explanation. The steep slope K1 represents the rapid adsorption process due to the electrostatic interaction between the adsorbate and adsorbent. K1 was found to be maximum for SrFe2O4 and correlated with maximum degradation efficiency. The value of the first slope is larger than the second one, see Fig. 10C. The second slope K2 is more gradual, representing a slow diffusing process (Mortazavian et al. 2019).
The adsorption efficiency is maximum for SrFe2O4, and adsorption isotherms at different concentrations (1 mg/100 ml, 2 mg/100 ml, 3 mg/100 ml and 4 mg/100 ml) were studied for SrFe2O4. The Langmuir and Freundlich adsorption isotherms were used to investigate the adsorption capability of MG at different equilibrium concentrations.
The amount of dye adsorbed at the equilibrium concentration is qe, Ce is the concentration of solute at equilibrium, maximum adsorption capacity is Qm and KL is the equilibrium constant of Langmuir isotherms. Kf and 1/n are the Freundlich constants related to adsorption intensity and adsorption capacity, respectively (Edet and Ifelebuegu 2020; Chen 2015). Figure 10D, E shows the fitting results for the isotherms Langmuir and Freundlich adsorption model. Isotherm constants obtained from the fitted data are given in Table 4. We obtained Langmuir isotherm adsorption model is better fit for adsorption equilibrium of Malachite Green on CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4. In Langmuir model, one dye molecule is adsorbed on an individual (one) adsorption site, and a monolayer is formed on the surface of the adsorbent, resulting decrease in the adsorption sites for further layer (Alaqarbeh 2021). The half life of the photocatalyst was also calculated from the below expression (Mahmoud et al. 2009)
We have calculated the half life of the photocatalyst from rate constant. The minimum decrement in half life is obtained in diffusion model and second-order rate kinetics. We have calculated the half life of the photocatalyst from rate constant. The minimum decrement in half life is obtained in diffusion model and second-order rate kinetics (as given in Table 3).
The feasibility and favorability of adsorption are determined by Langmuir dimensionless separation factor RL shown in Eq. (10).
RL value lies in the range (0–1) favors the adsorption process, whereas RL > 1 indicates the unfavorable adsorption (Ayawei et al. 2017). RL value obtained from Langmuir isotherms is 0.92 and confirms the favorable adsorption (as given in Table 5).
Adsorption isotherms was studied for Cu0.5Sr0.5Fe2O4 and found that it is also follows Langmuir isotherms model as the SrFe2O4 followed.
Effect of pH and temperature on degradation of dye
The effect of temperature has been studied at three different temperature 30 °C, 50 °C and 70 °C from SrFe2O4 (SF3) for 30 min in case of adsorption. Increase in temperature leads to improved dye adhesion on catalyst surfaces compared to lower temperatures. As indicated in Fig. 11A, the adsorption efficiency varies with temperature. When the temperature increased from 303 to 343 K, there was an initial increase in adsorption efficiency from 75 to 95% for a 1 mg/100 ml concentration at pH 7.0. This observation suggests that the adsorption reaction is endothermic in nature. (Karthikeyan et al. 2005) The enhanced adsorption efficiency at higher temperatures may be attributed to chemical interactions between adsorbates and the adsorbent, potentially resulting in the creation of additional adsorption sites or an accelerated rate of pollutant ion diffusion into the pores of the adsorbent.
Effect of pH Usually, the pH of the solution plays a very crucial role in wastewater treatment, and it is a very important factor that influence the photocatalytic degradation process of organic compounds. The effect of pH on the degradation of MG was studied at pH values of 4, 7 and 9 and it was found that efficiency increases as pH vary from (4–9), i.e., the photodegradation efficiency increases from acidic to alkaline medium (Rahman et al. 2021) (shown in Fig. 11 B. This result indicates that the photodegradation process occurs at the catalyst surface and not in bulk solution. In addition, one would expect that the interaction between the photogenerated holes and hydroxide ions (OH) to generate hydroxyl radicals (OH) is enhanced in alkaline pH, which in turn would facilitate the photocatalytic degradation of MG. The maximum degradation efficiency was obtained 95.04% at pH 9. The increased proton concentration in acidic solutions slows down the dye’s photodegradation, which lowers the degradation efficiency. In a basic Conversely, the existence of the acidic end products are neutralized by hydroxyl ions once they are generated by the process of photodegradation.
Photocatalytic degradation of textile dyes (malachite green, crystal violet, congo red)
The photocatalytic activities of CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 nanocomposite were evaluated by degrading malachite green, crystal violet and congo red dyes. We found that complete degradation of MG occurred in 30 min. The catalyst SrFe2O4 displayed the highest photodegradation efficiency due to uniform structure and negative surface charge. The photo-decolorization of MG was found to 93.20% for copper ferrite, 94.50% for Cu–Sr ferrites and 97.00% for strontium ferrites. For CV, 16.92% efficiency was observed in the presence of CuFe2O4, and 26.15% dye was degraded by Cu0.5Sr0.5Fe2O4 photocatalyst, while 41.67% degradation was observed for SrFe2O4. Notably, the surface charge of SrFe2O4 is negative and supports cation dye degradation. Therefore, from Fig. 12A, B, it can be concluded that SrFe2O4 exhibits relatively better photocatalytic activity compared to pure copper ferrites and their composites.
The photocatalytic degradation efficiency of CR in the presence of CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 nanocomposite is shown in Fig. 12B. It was observed that after 30 min, about 83.64% of CR was degraded in the presence of CuFe2O4, and 73.03% was degraded by Cu0.5Sr0.5Fe2O4 photocatalyst, while 72.98% degradation was observed for SrFe2O4. Photocatalysis efficiency is minimal for SrFe2O4 due to the interaction between the anionic dye and the negatively charged surface of SrFe2O4. Moreover, photocatalytic efficiency was enhanced in light compared to dark in half time.
Zeta potential and TRPL (time-resolved photoluminescence) studies
Zeta potential study was performed to investigate the surface charge of CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4. The CuFe2O4 and Cu0.5Sr0.5Fe2O4 exhibited a positive zeta potential of + 5.49 mV and + 3.08 mV, respectively, while SrFe2O4 showed a negative zeta potential of − 13.8 mV (see Fig. 13A. These results are in good agreement with the previous literature (Kamel et al. 2020). We calculated the surface charge for Cu0.75Sr0.25Fe2O4 and Cu0.25Sr0.75Fe2O4 nanocomposites, and their values were obtained to be + 4.42 mV and − 1.21 mV, respectively. Sr-doped CuFe2O4 samples suggest the higher dispersion stability of nanoparticles in the solution. The opposite surface charge favors the adsorption of cationic dye (malachite green and crystal violet) on the SrFe2O4 photocatalyst due to the electrostatic force of attraction. Degradation efficiency is observed less for anionic dye (congo red) because of the opposite surface charge.
The enlarged portion of decay-associated spectra of Sr-substituted Cu ferrites is shown in Fig. 13B. However, the complete decay spectra of the samples are shown in the inset Fig. 13B. The calculation and fitting of the experimental data were implemented by triexponential function using Eq. 12 (Chen et al. 2012).
where B1, B2 and B3 are amplitudes, \({\tau }_{1}\), \({\tau }_{2}\), and \({\tau }_{3}\) correspond to the lifetime of components 1, 2 and 3, respectively. The average lifetime of the samples CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 were found to be 2.07 ns, 3.62 ns and 3.90 ns, respectively. The recombination time was obtained maximum for SrFe2O4, which can be correlated with the higher degradation efficiency. The χ2 value was obtained in the good fit data range. The TRPL results (see Table 7) of undoped and Sr-doped copper ferrites indicated high charge carrier recombination in Sr ferrite, which correlates with better photocatalytic performance (Table 6).
Mott Schottky measurement
Mott Schottky measurements were conducted in 0.1 M solution ofNa2SO4 with Ag/AgCl electrode for CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 nanocomposites. We calculated the flat band potential and the positions of the valance band (VB) and conduction band (CB) (see Fig. 14A-C). The negative slope demonstrates that CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 possess a p-type conductivity. These results are in good agreement with the previous literature (Fedailaine et al. 2016). The flat band potential value for CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4 nanocomposites are − 0.28 V, − 0.09 V and − 0.84 V, respectively (see Table 8).
Reported degradation efficiencies of textile dyes on photocatalysts
Here, we collected the reported degradation efficiencies of the textile dyes due to photocatalysts in Table 9. We compare our results with the reported data on the degradation of textile dyes using copper ferrites and their composites in Table 9. The comparison is made on the basis of the degradation efficiency and performance of the synthesized samples. The present work shows better degradation efficiency of MG, CV and CR dyes using Sr-substituted copper ferrites (97%, 41.67% and 72.98%, respectively) and consumed relatively lesser time to degrade the dyes (see Table 9).
Mechanism of textile dye de-colorization
The photocatalytic degradation mechanism of textile dyes is shown in Fig. 15. When the surface of photocatalyst (CuFe2O4, Cu0.5Sr0.5Fe2O4 and SrFe2O4) is irradiated by UV–vis light, electrons in the valance band get excited, as shown in Eq. 13. Furthermore, the complete mechanisms of photodegradation are shown in Eqs. 13–21. Electrons jump to the conduction band and create an equal number of holes in the valance band (Ajormal et al. 2020). The holes in the VB can directly react with dye or interact with water and form hydroxyl radicals (·OH), a powerful oxidant for the mineralization of dyes. Meanwhile, the photogenerated electron can be captured by absorbed reactive oxygen molecules to yield O2−. The generated O2− reacts with H+ to produce H2O2, which further reacts with the excited electrons and generates OH− radicals. All the reactive radicals react with the dye and lead to the formation of degradation products. Sr-substituted copper ferrites have a lower band gap than pure copper ferrites. CuFe2O4 band gap was determined from Tauc’s plot as 3.7 eV, which lies in the UV–Vis region, i.e., ~ 5% of the solar spectrum. However, the modified bandgap of Sr-substituted copper ferrites (2.3 eV) lies in the visible region (which is approx. 46% of the spectrum) that enhances the photocatalytic degradation efficiency of the synthesized material.
Scavenger test for malachite green (MG)
To investigate the role of the active species, including holes (h+), hydroxyl radicals (·OH) and superoxide radicals (·O2) which are responsible for the degradation of dyes, trapping experiments were performed in the representative dye MG. Different types of scavengers were used for trapping active species. Radicals benzoquinone (BQ) was used for the superoxide, KI for holes and hydroxyl radicals trapping; however, t-BuOH for hydroxyl radicals (Janani et al. 2021). As expected, it was observed that maximum photodegradation efficiency was obtained without a scavenger. However, photodegradation efficiency with the scavenger decreased due to the trapping of produced active species by the scavenger (see Fig. 16). The photocatalytic degradation efficiency for malachite green is reduced to 73.8% from 98.8% (blank, i.e., without scavenger) after the addition of BQ, implying that superoxide radicals are mainly responsible for the degradation of MG. The degradation efficiency was reduced to 96.8% with KI; however, the degradation efficiency remains unchanged for t-BuOH (see Fig. 16). Based on the scavenger test, it is observed that superoxide radicals (·O2) and hydroxyl radicals (·OH) are the major and minor active species for the degradation of MG, respectively. Similarly, the scavenger test can be performed for the other dyes as well. In addition, H2O2 was also used as scavenger in scavenger test. H2O2 generates super oxide and hydroxyl radical that are responsible to degrade the pollutant. The degradation efficiency is 91% in case of H2O2.
The reusability of the magnetic photocatalyst
The reusability of a catalyst plays a crucial role in using it for industrial applications. We tested reusability for the strontium ferrites, and 20 mg of the photocatalyst was used in 100 ml of dye solution. After the photodegradation process, the photocatalyst was magnetically extracted using a magnet. To remove the remnant dye molecule on strontium ferrite, the used strontium ferrites were washed thrice with 10 ml of ethanol followed by 10 ml of deionized water. The difference in colour of MG dye is shown in Fig 17A. The recyclability was tested five times to verify the reusability of strontium ferrite, and obtained efficiencies were 97%, 96%, 95%, 93% and 92%, respectively, shown in Fig. 17B. There is a slight change in degradation efficiency, which confirms the reusability of the photocatalyst.
Conclusion
Cu1−xSrxFe2O4 (where x varies from 0 to 1) were synthesized using a fuel-based sol–gel auto-combustion method. The X-ray diffraction pattern indicated the formation of different structures, including a single-phase tetragonal spinel for CuFe2O4 (x = 0), a cubic spinel for SrFe2O4 (x = 1). The particles exhibited mixed morphologies, with both spherical and needle-like shapes observed. The optical bandgap decreased from 3.7 eV (x = 0) to 2.3 eV (x = 1). The adsorption and photocatalytic properties of the prepared samples were studied for dye degradation. Maximum adsorption was observed for MG, and the highest decolorization efficiency, reaching 97%, was achieved for SrFe2O4. Photocatalytic degradation efficiency improved under light compared to dark conditions in half time. The adsorption mechanism followed the second-order kinetic model and intra-particle diffusion model, consistent with Langmuir isotherms. For various dyes, the highest photocatalytic efficiency was achieved with strontium ferrites (97% for MG, 41.67% for CV, and 72.98% for CR), except for copper ferrites, which achieved 83.64% efficiency in CR degradation. The magnetic study showed that samples exhibited a ferromagnetic nature. This property allowed for easy recovery and magnetic separation of the nanocatalysts from the reaction mixture. Consequently, these highly efficient and reusable photocatalysts offer a promising solution for the degradation of industrial pollutants. The photocatalyst was found to be reusable for up to five cycles, especially in the case of SrFe2O4.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdel Maksoud MIA, El-Sayyad GS, Abd Elkodous M, Awed AS (2020) Controllable synthesis of Co1−xMxFe2O4 Nanoparticles (M=Zn, Cu, and Mn; x = 0.0 and 0.5) by cost-effective sol–gel approach: analysis of structure, elastic, thermal, and magnetic properties. J Mater Sci Mater Electron 31(12):9726–9741. https://doi.org/10.1007/s10854-020-03518-0
Abdulla Y, Abbar AH (2023) Applications of advanced oxidation processes (Electro-Fenton and sono-electro-Fenton) for COD removal from hospital wastewater: optimization using response surface methodology. Process Saf Environ Prot 169(2023):481–492. https://doi.org/10.1007/s13399-021-01753-x
Affat SS (2021) Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: a review. UTJ Sci 8(1):130–135
Ajormal F, Moradnia F, Taghavi Fardood S, Ramazani A (2020) Zinc ferrite nanoparticles in photo-degradation of dye: mini-review. J Chem Rev 2(2):90–102. https://doi.org/10.33945/sami/jcr.2020.2.2
Akarslan F, Demiralay H (2015) Effects of textile materials harmful to human health. Acta Phys Polonica A 128(2):407–408. https://doi.org/10.12693/APhysPolA.128.B-407
Alaqarbeh M (2021) Adsorption phenomena: definition, mechanisms, and adsorption types short review. RHAZES Green Appl Chem 13:43–51. https://doi.org/10.48419/IMIST.PRSM/rhazes-v13.28283
Ali I, Gupta VK (2006) Advances in water treatment by adsorption technology. Nature Protoc 1(6):2661–2667. https://doi.org/10.1038/nprot.2006.370
Amiri M, Eskandari K, Salavati-Niasari M (2019) Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv Coll Interface Sci 271:101982. https://doi.org/10.1016/j.cis.2019.07.003
Arifin MN, Karim KMR, Abdullah H, Khan MR (2019) Synthesis of titania doped copper ferrite photocatalyst and its photoactivity towards methylene blue degradation under visible light irradiation. Bull Chem React Eng Catal 14(1):219–227. https://doi.org/10.9767/bcrec.14.1.3616.219-227
Ateia EE, Abdelmaksoud MK, Arman MM, Shafaay AS (2020) Comparative study on the physical properties of rare-earth-substituted nano-sized CoFe2O4. Appl Phys A Mater Sci Process 126(2):1–10. https://doi.org/10.1007/s00339-020-3282-5
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem. https://doi.org/10.1155/2017/3039817
Berradi M, Hsissou R, Khudhair M, Assouag M, Cherkaoui O, El Bachiri A, El Harfi A (2019) Textile finishing dyes and their impact on aquatic environs. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e02711
Bo L, Yusen Hu, Zhang Z, Tong J (2019) Efficient photocatalytic degradation of rhodamine B catalyzed by SrFe2O4/g-C3N4 composite under visible light. Polyhedron 168:94–100. https://doi.org/10.1016/j.poly.2019.04.036
Calvo-de la Rosa J, Segarra Rubí M (2020) Influence of the synthesis route in obtaining the cubic or tetragonal copper ferrite phases. Inorg Chem 59(13):8775–8788. https://doi.org/10.1021/acs.inorgchem.0c00416
Channagoudra G, Saw AK, Dayal V (2021) Role of structure and cation distribution on magnetic and electrical properties in inverse spinel copper ferrite. J Phys Chem Solids 154:110086. https://doi.org/10.1016/j.jpcs.2021.110086
Chen X (2015) Modeling of experimental adsorption isotherm data. Information (switzerland) 6(1):14–22. https://doi.org/10.3390/info6010014
Chen YC, Pu YC, Hsu YJ (2012) Interfacial charge carrier dynamics of the three-component in 2O3–TiO2–Pt heterojunction system. J Phys Chem C 116(4):2967–2975. https://doi.org/10.1021/jp210033y
Das S (2016) Research progress on iron oxide-based magnetic materials: synthesis techniques and photocatalytic applications. Remote Sens Appl Soc Environ 42(1):9–34. https://doi.org/10.1016/j.rsase.2019.02.006
Dey PC, Sarkar S, Das R (2020) X-ray diffraction study of the elastic properties of jagged spherical CdS nanocrystals. Mater Sci Poland 38(2):271–278. https://doi.org/10.2478/msp-2020-0032
Dhir G, Uniyal P, Verma NK (2014) Effect of particle size on multiferroism of barium-doped bismuth ferrite nanoparticles. Mater Sci Semicond Process 27:611–618. https://doi.org/10.1016/j.mssp.2014.07.041
Dhiwahar AT, Maruthamuthu S, Marnadu R, Sundararajan M, Manthrammel MA, Shkir M, Sakthivel P, Reddy VRM (2021) Improved photocatalytic degradation of rhodamine B under visible light and magnetic properties using microwave combustion grown ni doped copper ferrite spinel nanoparticles. Solid State Sci 113:106542. https://doi.org/10.1016/j.solidstatesciences.2021.106542
Dhyani R et al (2022) Structural and elastic properties of tetragonal nano-structured copper ferrite. Int J Mater Res 113(10):884–892. https://doi.org/10.1515/ijmr
Dippong T, Levei EA, Cadar O (2021) Recent advances in synthesis and applications of MFe2O4 (M=Co, Cu, Mn, Ni, Zn) nanoparticles. Nanomaterials 11(6):3–8. https://doi.org/10.3390/nano11061560
Edet UA, Ifelebuegu AO (2020) Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 8(6):665. https://doi.org/10.3390/PR8060665
Fatima T, Husain S, Khanuja M (2022) Superior photocatalytic and electrochemical activity of novel WS2/PANI nanocomposite for the degradation and detection of pollutants: antibiotic, heavy metal ions, and dyes. Chem Eng J Adv 12(July):100373. https://doi.org/10.1016/j.ceja.2022.100373
Fedailaine M, Bellal B, Berkani S, Trari M, Abdi A (2016) Photo-electrochemical characterization of the spinel CuFe2O4: application to Ni2+ removal under solar light. Environ Process 3(2):387–396. https://doi.org/10.1007/s40710-016-0142-6
Ghahfarokhi SEM, Shobegar EM (2020) Influence of PH on the structural, magnetic and optical properties of SrFe2O4 nanoparticles. J Mater Res Technol 9(6):12177–12186. https://doi.org/10.1016/j.jmrt.2020.08.063
Gore SK, Tumberphale UB, Jadhav SS, Shaikh SF, Al-Enizi AM, Rana AUHS, Khule RN, Raut SD, Gore TS, Mane RS (2022) Grain and grain boundaries influenced magnetic and dielectric properties of lanthanum-doped copper cadmium ferrites. J Mater Sci Mater Electron 33(10):7636–7647. https://doi.org/10.1007/s10854-022-07912-8
Han S, Liu K, Linfeng Hu, Teng F, Pingping Yu, Zhu Y (2017) Superior adsorption and regenerable dye adsorbent based on flower-like molybdenum disulfide nanostructure. Sci Rep 7(January):1–11. https://doi.org/10.1038/srep43599
Huang Y-T, Shih M-C (2021) Application of the analysis toolpak for comparison of various linearized expressions of kinetics equations for sorption of methylene blue onto nitric acid-treated rice husk. Int J Sci Res Publ (IJSRP) 11(6):692–698. https://doi.org/10.29322/ijsrp.11.06.2021.p11490
Huang Q, Chen C, Zhao X, Bu X, Liao X, Fan H, Gao W, Hu H, Zhang Y, Huang Z (2021) Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: efficiency, stability and mechanism. J Environ Chem Eng 9(4):105800. https://doi.org/10.1016/j.jece.2021.105800
Jacinto MJ, Ferreira LF, Silva VC (2020) Magnetic materials for photocatalytic applications—a review. J Sol Gel Sci Technol 96(1):1–14. https://doi.org/10.1007/s10971-020-05333-9
Janani B, Syed A, Thomas AM, Al-Rashed S, Elgorban AM, Raju LL, Khan SS (2021) A simple approach for the synthesis of bi-functional p-n type ZnO@CuFe2O4 heterojunction nanocomposite for photocatalytic and antimicrobial application. Phys E Low Dimens Syst Nanostruct 130:114664. https://doi.org/10.1016/j.physe.2021.114664
Jelonek Z, Drobniak A, Mastalerz M, Jelonek I (2020) Environmental implications of the quality of charcoal briquettes and lump charcoal used for grilling. Sci Total Environ 747:141267. https://doi.org/10.1016/j.scitotenv.2020.141267
Jiad MM, Abbar AH (2023) Treatment of petroleum refinery wastewater by electrofenton process using a low cost porous graphite air-diffusion cathode with a novel design. Chem Eng Res Des 193:207–221
Kamel AH, Hassan AA, Amr AEGE, El-Shalakany HH, Al-Omar M (2020) Synthesis and characterization of CuFe2O4 nanoparticles modified with polythiophene: applications to mercuric ions removal. Nanomaterials 10(3):586. https://doi.org/10.3390/nano10030586
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium (VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater 124(1–3):192–199
Keerthana SP, Yuvakkumar R, Ravi G, Pavithra S, Thambidurai M, Dang C, Velauthapillai D (2021) Pure and Ce-doped spinel CuFe2O4 photocatalysts for efficient rhodamine B degradation. Environ Res. https://doi.org/10.1016/j.envres.2021.111528
Kempter CP (1966) Vegard’s ‘Law.’ Phys Status Solidi b 18(2):K117–K118. https://doi.org/10.1002/pssb.19660180251
Khaleel GF, Ismail I, Abbar AH (2023) Application of solar photo-electro-Fenton technology to petroleum refinery wastewater degradation: optimization of operational parameters. Heliyon 9(4):e15062
Khan M et al (2023) Investigation of the annealing temperature for few-layer MoS2 and ion-beam induced athermal annealing/purification behaviour by in-situ XRD. Appl Surface Sci 639:158106
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3(2):275–290. https://doi.org/10.1016/j.biori.2019.09.001
Liu W, Chao Y, Yang X, Bao H, Qian S (2004) Biodecolorization of Azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. J Ind Microbiol Biotechnol 31(3):127–132. https://doi.org/10.1007/s10295-004-0123-z
Ma H, Liu C (2021) A mini-review of ferrites-based photocatalyst on application of hydrogen production. Front Energy 15(3):621–630. https://doi.org/10.1007/s11708-021-0761-0
Mahmoud MA, Poncheri A, Badr Y, Abd El Wahed MG (2009) Photocatalytic degradation of methyl red dye. South Afr J Sci 105(7):299–303
Manikandan A, Judith Vijaya J, Sundararajan M (2013) Optical and magnetic properties of Mg-doped ZnFe2O4 nanoparticles prepared by rapid microwave combustion method. Superlattices Microstruct 64(1):118–131. https://doi.org/10.1016/j.spmi.2013.09.021
Masoumi S, Nabiyouni G, Ghanbari D (2016) Photo-degradation of azo dyes: photo catalyst and magnetic investigation of CuFe2O4–TiO2 nanoparticles and nanocomposites. J Mater Sci Mater Electron 27(9):9962–9975. https://doi.org/10.1007/s10854-016-5067-3
Masunga N, Mmelesi OK, Kebede KK, Mamba BB (2019) Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: review. J Environ Chem Eng 7(3):103179. https://doi.org/10.1016/j.jece.2019.103179
Mills A, Le Hunte S (1997) An overview of semiconductor photocatalysis. J Photochem Photobiol, A 108(1):1–35. https://doi.org/10.1016/S1010-6030(97)00118-4
Mortazavian S, Saber A, Hong J, Bae JH, Chun D, Wong N, Gerrity D, Batista J, Kim KJ, Moon J (2019) Synthesis, characterization, and kinetic study of activated carbon modified by polysulfide rubber coating for aqueous hexavalent chromium removal. J Ind Eng Chem 69:196–210. https://doi.org/10.1016/j.jiec.2018.09.028
Nie J, Sun Y, Zhou Y, Kumar M (2019) Bioremediation of water containing pesticides by microalgae: mechanisms, methods, and prospects for future research. Sci Total Environ 707:136080. https://doi.org/10.1016/j.scitotenv.2019.136080
Odio OF, Reguera E (2017). Nanostructured spinel ferrites: synthesis, functionalization, nanomagnetism and environmental applications. In: Magnetic spinels—synthesis, properties and applications, pp 185–216, https://doi.org/10.5772/67513
Oliveira TP, Rodrigues SF, Marques GN, Viana Costa RC, Garçone Lopes CG et al (2022) Synthesis, characterization, and photocatalytic investigation of CuFe2O4 for the degradation of dyes under visible light. Catalysts 12:623
Pereira L, Alves M (2012) Dyes-environmental impact and remediation. Environ Prot Strateg Sust Dev. https://doi.org/10.1007/978-94-007-1591-2_4
Rabiei M, Palevicius A, Monshi A, Nasiri S, Vilkauskas A, Janusas G (2020) Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-Ray diffraction. Nanomaterials 10(9):1–21. https://doi.org/10.3390/nano10091627
Rafiq A, Muhammad Ikram S, Ali FN, Khan M, Khan Q, Maqbool M (2021) Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J Ind Eng Chem 97:111–128. https://doi.org/10.1016/j.jiec.2021.02.017
Rahman MU et al (2021) Solar driven photocatalytic degradation potential of novel graphitic carbon nitride based nano zero-valent iron doped bismuth ferrite ternary composite. Opt Mater 120:111408
Roman T, Pui A, Lukacs AV, Cimpoesu N, Lupescu S, Borhan AI, Kordatos K et al (2019) Structural changes of cerium doped copper ferrites during sintering process and magneto-electrical properties assessment. Ceram Int 45(14):17243–17251. https://doi.org/10.1016/j.ceramint.2019.05.280
Rueda-Marquez JJ, Levchuk I, Ibañez PF, Sillanpää M (2020) A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J Clean Prod 258:120694. https://doi.org/10.1016/j.jclepro.2020.120694
Sahoo JK, Kumar A, Rout L, Rath J, Dash P, Sahoo H (2018) An investigation of heavy metal adsorption by hexa-dentate ligand-modified magnetic nanocomposites. Sep Sci Technol 53(6):863–876. https://doi.org/10.1080/01496395.2017.1406950
Sharma M, Singh AK, Siddiqui AM (2020) Synthesis and characterization of copper ferrite nanoparticles. In: AIP conference proceedings, vol 2265, p 030170. https://doi.org/10.1088/1757-899X/928/7/072125
Sharma S, Kaur A (2018) Various methods for removal of dyes from industrial effluents—a review. Indian J Sci Technol 11(12):1–21. https://doi.org/10.17485/ijst/2018/v11i12/120847
Singh SK, Townsend TG, Mazyck D, Boyer TH (2012) Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res 46(2):491–499. https://doi.org/10.1016/j.watres.2011.11.007
Slama HB, Bouket AC, Pourhassan Z, Alenezi FN, Silini A, Cherif-Silini H, Oszako T, Luptakova L, Golińska P, Belbahri L (2021) Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl Sci (switzerland) 11(14):1–21. https://doi.org/10.3390/app11146255
Surendra BS (2018) Green engineered synthesis of ag-doped CuFe2O4: characterization, cyclic voltammetry and photocatalytic studies. J Sci Adv Mater Devices 3(1):44–50. https://doi.org/10.1016/j.jsamd.2018.01.005
Vergis BR, Hari Krishna R, Nagaraju Kottam BM, Nagabhushana RS, Darukaprasad B (2018) Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. J Nanostruct Chem 8(1):1–12. https://doi.org/10.1007/s40097-017-0249-y
Vijayaraghavana T, Suriyarajb SP, Selvakumarb R, Venkateswarana R, Ashoka A (2016) Rapid and efficient visible light photocatalytic dye degradation using AFe2O4 (A = Ba, Ca and Sr) complex oxides 3. Mater Sci Eng B 1(1):1–11. https://doi.org/10.1016/j.mseb.2016.04.005
Vishwaroop R, Mathad SN (2020) Synthesis, structural, W–H plot and size-strain analysis of nano cobalt doped Mgfe2o4 Ferrite. Sci Sinter 52(3):349–358. https://doi.org/10.2298/SOS2003349V
Worch E (2012) Adsorption technology in water treatment. De Gruyter, Berlin. https://doi.org/10.1515/9783110240238
Yadav P, Surolia PK, Vaya D (2021) Synthesis and application of copper ferrite-graphene oxide nanocomposite photocatalyst for the degradation of malachite green. Mater Today Proc 43:2949–2953. https://doi.org/10.1016/j.matpr.2021.01.301
Zhao Y, Lin C, Bi H, Liu Y, Yan Q (2017) Magnetically separable CuFe2O4/AgBr composite photocatalysts: preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl Surf Sci 392:701–707. https://doi.org/10.1016/j.apsusc.2016.09.099
Zhuo R, Ma Li, Fan F, Gong Y, Wan X, Jiang M, Zhang X, Yang Y (2011) Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp.En3 and cloning and functional analysis of its laccase gene. J Hazard Mater 192(2):855–873. https://doi.org/10.1016/j.jhazmat.2011.05.106
Acknowledgements
We acknowledge the Central Instrumentation Facility Centre and Center for nanoscience and nanotechnology, Jamia Millia Islamia (JMI) and AIRF (JNU) for their help with the characterization facilities. The presented work was supported by (Dr. Manika Khanuja), SERB, [ECR/2017/001222] Nanomission, (DST) [DST/NM/NB/2018/203(G)(JMI)].
Funding
Support of the Department of Science and Technology vide [DST/NM/NB/2018/203(G)(JMI)] is highly acknowledged.
Author information
Authors and Affiliations
Contributions
MS: data curation, formal analysis, methodology, software, writing—original draft, conceptualization, investigation, validation, visualization, writing—review & editing. NM: conceptualization, validation, visualization, writing—review & editing. SB: validation. AMS: supervision, validation, writing—review & editing. MK: funding acquisition, project administration, resources, supervision, conceptualization, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Ethical approval
This is an observational study, and the research ethics committee has confirmed that no ethical approval is required.
Consent to participate
All individual participants are included in the study. Please keep this consent form in the patient’s case files. The manuscript reporting this patient’s details should state that ‘Written informed consent for publication of their clinical details and/or clinical images' was obtained from the patient/parent/guardian/ relative of the patient. A copy of the consent form is available for review by the Editor of this journal.
Additional information
Editorial responsibility: F. ŞEN.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, M., Mishra, N., Bansal, S. et al. Efficient adsorption and photocatalytic degradation of textile dye from metal ion-substituted ferrite for environmental remediation. Int. J. Environ. Sci. Technol. 21, 6075–6092 (2024). https://doi.org/10.1007/s13762-023-05393-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05393-8