Abstract
Wastewater discharge from the leather manufacturing process involves various operations such as beam house, tanning and post-tanning. The conventional treatment system does not efficiently treat these kinds of wastewaters without the generation of any secondary pollutant. Mostly vast quantities of chemicals are added to the wastewaters to adjust pH to favour the treatment units that results in a huge amount of primary chemical sludge. This paper reports a new treatment approach by mixing various tannery operational wastewaters in the appropriate treatment units to adjust pH instead of adding chemicals. It also designed a treatment sequence to simultaneously treat all tannery operational wastewaters in such a way to suit their biodegradability and to avoid sludge to a greater extent. The treated water resulted in biochemical oxygen demand, 58 ± 39 mg L−1; chemical oxygen demand, 305 ± 35 mg L−1; total organic carbon, 94.93 ± 45.46 mg L−1; total nitrogen, 295 ± 226 mg L−1; ammoniacal nitrogen, 146 ± 75 mg L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The leather manufacturing process involves various operations such as beam house, tanning and post-tanning. The beam house operations consist of the soaking process, liming process and de-liming process for the removal of adhered common salt, animal hair and calcium, respectively. The tanning operations comprised of the pickling process for protonating the collagen matrix using NaCl/H2SO4 and chrome tanning process to prepare leather for microbial resistance and thermal resistance using basic chromium sulphate. The post-tanning operations involve neutralization, re-tanning, dyeing and fatliquoring for imparting desired colour and flexibility to the leather using oil and dyes. The wastewater discharged from the leather industry was characterized by the high chemical oxygen demand (COD), biochemical oxygen demand (BOD), total organic carbon (TOC), ammoniacal nitrogen (NH4+-N), total nitrogen (TN), total suspended solids (TSS) and total dissolved solids (TDS). The wastewaters generated from each operation got different characteristics including vast changes in the pH (1.4–13), organics in terms of COD (2720–8694 mg L−1) and salt content of about 3–5% w/v as NaCl (Saxena et al. 2017). Commonly vast quantities of chemicals such as sulphuric acid, coagulant and flocculant are added in tannery wastewater treatment. The concentrated sulphuric acid up to 9.5 ml L−1 is added in the various operational wastewater system to drop pH from 7 to 3 which causes an increase in operational cost and acid handling difficulties (Korpe et al. 2019). The coagulants and flocculants such as lime 200–700 mg L−1, alum 100–600 mg L−1 and polyelectrolyte 0.4–1.4 mg L−1 are added, which results in a huge primary chemical sludge of 5.27 ± 0.15 kg m−3 generation (Mahesh et al. 2020). Divyalakshmi et al. (2015) reported that 50–60 kg of primary chemical sludge and 15–20 kg of secondary biological sludge were generated in the wastewater treatment for 1 ton of processed hide. The major problem in the current practicing wastewater treatment process is the presence of residual COD in the treated wastewater and the generation of sludge.
The advanced oxidation processes (AOPs) of tannery wastewater treatment were reported by electrochemical treatment (Naumczyk and Kucharska 2017), ozone treatment (Schrank et al. 2017), cavitation using ultrasonic wave (Korpe et al. 2019), electrocoagulation combined with photoreactor (Moradi and Moussavi 2019), photocatalytic degradation (Kuppusamy et al. 2017), electrocoagulation combined with electro Fenton (Varank et al. 2016), membrane bioreactor (Alighardashi et al. 2017), a combination of up-flow anaerobic sludge blanket (UASB) and electrochemical oxidation (Liu et al. 2017) processes.
The above-mentioned various AOPs have the disadvantage of a high amount of chemical utilization, i.e. up to 10 g L−1 of hydrogen peroxide for the reduction of 60.29% of COD, high footprint area required and sludge generation (Meenachi and Kandasamy 2017; Korpe et al. 2019). The limitation of using electrochemical, ozone and ultrasonic treatment methods is because of its high treatment cost. Moreover, these methods have operations and handling difficulties at a large industrial-scale level (Saxena et al. 2017; Crini and Lichtfouse 2018). The electrochemical methods for treating raw tannery wastewater can produce a huge amount of sludge 0.9 and 0.875 kg m−3 while using electrolytes KCl and NaCl, respectively, which may further lead to sludge disposal difficulties (Lofrano et al. 2013; Maha Lakshmi and Sivashanmugam 2013). The photocatalytic degradation and electrocoagulation methods cannot completely degrade the organic pollutants present in the tannery wastewater, which further results in more toxic secondary pollutants than parent compounds (Natarajan et al. 2013; Rueda-Marquez et al. 2020). The major disadvantage of photocatalytic treatment is that it greatly depends on the photosensitivity of the organic compounds, but all the compounds in tannery wastewater do not have photosensitivity (Lofrano et al. 2013). The membrane bioreactor has the difficulty of membrane fouling due to the contamination in a short time which increases the treatment cost (Aslam et al. 2017). The UASB process has many disadvantages like sulphide inhibition, sulphide gas emission and also maintaining many biological conditions such as pH, organic load and high hydraulic retention time (HRT) (Midha and Dey 2008).
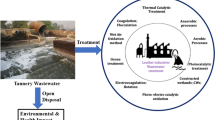
Thus, there is still a need for novel effective treatment to simultaneously treat various tannery operational wastewaters in focus with minimum residual COD and avoidance of primary chemical sludge. Hence, the focal theme of the present investigation is to apply various tannery operational wastewaters in the appropriate places to adjust pH instead of adding chemicals and to simultaneously treat all the tannery operational wastewater. The proposed treatment study will sustain the leather industry in terms of pollution removal and control. The reactors used in the manuscript scheme to favour the industry from the disadvantages of current technologies in terms of minimizing the sludge generation, avoiding the addition of chemicals for pH adjustment, avoiding the generation of primary chemical sludge and minimum COD in treated water further reduces the treatment of operational cost. This research work was carried out in Environmental Science Lab, CSIR—Central Leather Research Institute (CLRI), Chennai, India, from June 2019 to April 2020 throughout the study.
Materials and methods
The beam house wastewater (BHWW), pickle wastewater and post-tanning wastewater (PTWW) were collected from a tannery located in Tamil Nadu, India, in three batches with the capacity of 100L each. The wastewater was characterized for various parameters per American Public Health Association (APHA) methods (APHA 2017). Chemicals used in the treatability studies such as ferrous sulphate (FeSO4.7H2O), hydrogen peroxide (H2O2) and sulphuric acid were in analytical grade. A pH meter (Systronics 620) and oxidation reduction potential (ORP) meter (Thermo Orion 920A plus meter) were used to determine the pH and ORP of the wastewater. The TOC and TN were determined using TOC/TN analyser (Shimadzu). The wastewater samples were dried using nitrogen at 60 °C to find the functional groups using Fourier transform infrared (FTIR) spectrophotometer (Thermo Nicolet, PerkinElmer) in the range of 400—4,000 cm−1. Ultraviolet-visible spectroscopy (UV–Vis) (Varian Cary 100) was used to record the concentration of the organic compounds present in the wastewater at each stage of treatment. The liquid chromatography (LC) and liquid chromatography with tandem mass spectrometry (LC–MS) (Waters, Xevo TQD2000) were used to determine the profile of the organic compounds present in the tannery effluent.
Heterogeneous catalytic oxidation of acidic PTWW was carried using Fenton oxidation with ferrous sulphate (FeSO4.7H2O) and hydrogen peroxide (H2O2) in heterogeneous fluidized bed reactor (HFBR), packed bed reactor (PBR) and then the microbial oxidation of fragmented organics in the composite stream by heterogeneous fluidized bio-bed reactor (HFBBR).
Synthesis of carbon silica matrix
The carbon silica matrix (CSM) was prepared from the agricultural solid waste of rice husk according to the procedure followed by Swarnalatha et al. (2009). Rice husk was pre-carbonized at 400 °C for 4 h and followed by chemical activation with H3PO4 (85%) at 700 °C for 1 h. The activated CSM was used in all the three heterogeneous reactors of HFBR, PBR and HFBBR.
Description of reactors used in the treatment process
Description of the heterogeneous fluidized bed reactor
The schematic diagram of reactors is shown in Fig. 1. The HFBR reactor was designed and fabricated using a poly-acrylic sheet with a volume of 2.6L (Maharaja et al. 2016). It has four major zones in the reactor. They are the bottom zone which has an inverted cone shape to facilitate the better fluidization of CSM with the up-flow velocity of 5 m min−1. The air required for the oxidation of organics was injected through the perforated pipes provided in the bottom zone. The central zone of the reactor with a triangular septum was used to separate the solid CSM catalyst, wastewater and the unspent air. The third zone was an inverted cone shape to settle down the catalyst and biomass. Provision was made at the top of the reactor to collect the treated wastewater through a perforated tube covered with a nylon cloth to avoid the escape of CSM particles.
Description of the packed bed reactor
The total volume of 590-mL laboratory-scale PBR with a working volume of 390 mL having 5 cm diameter and 30 cm height was designed. PBR was packed with 5-mm quartz coarse aggregate to a height of 2 cm followed by 3-mm fine quartz aggregate to the height of 2 cm. Then a layer of quartz aggregate of diameter 1 mm filled to the height of 1 cm followed by packing of 600-μm CSM to the height of 20 cm. The total bed height was 25 cm from the bottom of the reactor. The treated water was collected through a perforated tube of diameter 5 mm provided at the height of 5 cm from the bottom of the reactor. The provision for injecting the air was made at 8 cm, 12 cm and 16 cm height from the bottom of the reactor for the oxidation of organics in the wastewater. The top portion of the PBR reactor was not closed to facilitate the unspent air to escape without any resistance (Ramani and Sekaran 2006).
Description of the heterogeneous fluidized bio-bed rector
The design of the HFBBR reactor is similar to that of the HFBR reactor, but they differ in working function, and the HFBBR reactor was added with immobilized CSM. The composite tannery wastewater (CTWW) was treated in an HFBBR reactor which was obtained by the mixture of catalytically oxidized PTWW neutralized with BHWW.
Tannery wastewater treatment scheme and sequence of reactors usage
The sequence of reactors used for the treatment was followed by HFBR-PBR-HFBBR as shown in Fig. 1. The pH of PTWW was 4.05 ± 0.78 which adjusted to lower pH using pickling wastewater of pH 1.64 ± 0.21 in the ratio of 12:1 instead of adding acids and then fed into the HFBR. The HFBR treated outlet was fed into PBR. The PBR outlet water with pH 4.38 ± 0.43 was mixed with BHWW (pH 10.58 ± 0.07) in the ratio of 1:2 to get CTWW of neutral pH, which favours the biological oxidation condition in HFBBR. The experiment was carried out continuously using three collected wastewater batches, and the analysis was carried out for the outlet of all the reactors. The average results of the three batches were plotted with the standard deviation.
Statistical analysis of data
SPSS software (version 19) was used to determine descriptive statistics. The Design-Expert 7.0.0 Software was used for the analysis of variance (ANOVA) in the optimization study of HFBR. The MS Excel spreadsheet 2019 software was used for data tabulation and graph drawing.
Results and discussion
Characteristics of post-tanning wastewater and beam house wastewater
The PTWW was obtained after the operations such as neutralization, re-chroming, re-tanning, dyeing and fatliquoring, whereas the BHWW was obtained after the operations such as liming, de-liming and bating. The characteristics of PTWW and BHWW are given in Table 1. The characteristics of PTWW suggest that it is less amenable to biodegradation as indicated by the low BOD/COD ratio (0.1986–0.2703). So, the chemical oxidation method was applied for PTWW treatment. The BOD/COD ratio of BHWW was showed as 0.3338–0.5172.
The UV–Vis spectrum of PTWW and BHWW is shown in Fig. 2a, b; PTWW shows two peaks at the visible region due to the presence of functional dyes at 570 nm and 417 nm. The peak at 570 nm is due to the presence of azo dye Acid Blue 113 used in the tannery dyeing process (Senthilvelan et al. 2014). The peaks in the UV region at 252 nm, 239 nm and 219 nm are due to the transition of π → π* and n → σ* of polycyclic unsaturated aromatic compounds such as naphthalene syntans and phenolic syntans. The UV-Vis spectrum of BHWW contains peaks at 271 nm and 240 nm, which may be due to the n → π* transition of protein molecules, and peaks at 231 nm and 214 nm are due to the π → π* of aliphatic and aromatic chain molecules.
The FTIR spectrum of PTWW is presented in Fig. 3a. The peak at 3445 cm−1 is due to the stretching of –OH functional groups, the peak at 1635 cm−1 is due to the stretching vibrations of –C=C– of naphthalene, the peak at 1384 cm−1 may be attributed to alkyl C–H stretching. The peaks at 1091 and 661 cm−1 are ascribed to the sulphur trioxide ion (SO3−) present in the unsaturated aromatic compound of naphthalene sulphonic acid, a peak at 1200 cm−1 due to C–OH stretching of the aromatic group (Silverstein et al. 2005; Basavaraja et al. 2012).
The PTWW was characterized by LC–MS which is shown in Fig. 4a, b. The chromatogram showed many peaks at various retention time (RT) of 15.3, 13.56, 12.78, 11.83, 9.72, 9.34, 8.69, 5.97, 3.99 and 0.49, indicating the complexity of the wastewater. The RT at 3.99 might correspond to 4-N-Octylphenol, which is used as a wetting agent in the post-tanning operation to wetback the tanned leather (Black et al. 2013). It was confirmed in MS (Fig. 4b) that the molecular ion peak at m/z 205 and base peak at m/z 163.07 due to the removal of propane group followed by the removal of butane group gives m/z at 107.05. The peak at RT 9.38 might be attributed to polymeric substituted products of dibutyl phthalate present in PTWW (Quintão et al. 2018), which was confirmed using MS. The fragmented masses of m/z 301, 279, 205 and 149 confirm the presence of dibutyl phthalate molecule (Bharagava et al. 2018). The peak at RT 12.78 could be due to the presence of Neohesperidin dihydrochalcone flavonoids which is used as a vegetable oil in the tanning process; the m/z peaks at 594.3, 397.24, 301.16 confirm its presence (Brodowska 2017; Johnson et al. 2018). The mass fragmentation of the peak at RT 11.83 is due to the presence of polycondensate naphthalene sulphonic acid which is used as a re-tanning agent.
The fluorescence spectrum of the PTWW is shown in Fig. S1a. It shows the excitation peak at 300 nm and emission peak at 368 nm due to the presence of fluorescent active organic compounds. The stokes shift with the difference between excitation and emission peak observed as 68 nm. The high stokes shift value because of the loss in energy of the excited molecule by resonance owing to a high population of molecules, which is the typical characteristic feature of the aromatic or hyper conjugated molecules. The resonance caused by the presence of sulphonic acid group in naphthalene molecules and also by the presence of chromophores fluorescent dyes was used to impart the desired colour to the finished leathers (Oliveira et al. 2018). It confirms the presence of sulphonated aromatics such as sulphonated naphthalene in PTWW which was used as a re-tanning agent in post-tanning operations.
Treatment of post-tanning wastewater using heterogeneous fluidized bed reactor
The heterogeneous Fenton oxidation method was followed in HFBR in which the hydroxyl radical produced by the reaction of ferrous sulphate and hydrogen peroxide with CSM used for the oxidation of organic pollutants present in the PTWW. So, the treatment efficiency of HFBR majorly depends upon the process conditions such as pH, HRT, the dosage of hydrogen peroxide, the dosage of ferrous sulphate and the mass of CSM. Thus, the effect of various process conditions studied and discussed is as follows.
Effect of pH in heterogeneous fluidized bed reactor
The optimum pH for the Fenton oxidation was determined by carrying out the reaction at different pH (2.5, 3, 3.5 and 4) of the PTWW. The pH was adjusted by slowly adding the pickling wastewater with constant stirring; the PTWW consumed pickling wastewater in the ratio 12:1 to adjust from pH 4.05 ± 0.78 to pH 3.5 ± 0.02. The characteristic of pH 3.5 adjusted water is shown in Table 2. The oxidation reaction at different pH water was carried out by keeping the dosage of hydrogen peroxide, 13 mmol L−1, ferrous sulphate; 0.72 mmol L−1 mass of CSM 30 mg L−1 and HRT 12 h as a constant. The COD removal as a function of different pH was plotted and is shown in supplementary information Fig. S2a. It shows that the COD removal was increased up to pH 3.5 after which the decrease in COD at pH 2.5 was observed due to the precipitation of ferrous ion as ferric hydroxide sludge. Thus, the removal efficiency was retarded at pH < 3.5 > (Bulut et al. 2008). There is a strong scavenging effect of hydroxyl radical by H+ at a very low pH < 2.5, as shown in Eq. (1).
It was also reported by other researchers that the pH 3.5 favoured Fenton oxidation of organic pollutants (Hartmann et al. 2010; Borba et al. 2018). Based on the COD removal, pH 3.5 was selected as the optimum pH for the treatment of tannery wastewater.
Effect of hydraulic retention time in heterogeneous fluidized bed reactor
The HRT in HFBR was optimized by analysing samples with different time intervals up to 18 h at optimized pH 3.5, hydrogen peroxide dosage, 13 mmol L−1, ferrous sulphate, 0.72 mmol L−1 and CSM 30 mg L−1. The results are presented in the supplementary information Fig. S2b. It showed that the removal of COD was observed up to 12 h and then a steady state was attained with a marginal increase in the COD removal. It was due to the lowering of driving force on catalyst site by lowering in concentration potential, so it was fixed as the optimum HRT.
Effect of hydrogen peroxide dosage in heterogeneous fluidized bed reactor
The dosage of hydrogen peroxide was optimized by experimenting with different dosages of hydrogen peroxide (6.50, 9.75, 13.00 and 16.25 mmol L−1) by keeping ferrous sulphate, 0.72 mmol L−1, HRT 12 h and CSM 30 mg L−1 as a constant (Fig. S3a). The almost maximum removal of COD was observed with an optimum dosage of hydrogen peroxide of 13.00 mmol L−1 at 12 h. Thereafter no significant removal in COD was observed with an increase in dosage of hydrogen peroxide so, it was fixed as optimum.
Effect of Ferrous sulphate dosage in heterogeneous fluidized bed reactor
In the Fenton oxidation process, the dosage of ferrous sulphate plays an important role in the generation of hydroxyl radical along with hydrogen peroxide. The optimum dosage of ferrous sulphate was determined by experimenting it with different doses of 0.36, 0.54, 0.72 and 0.9 mmol L−1 by keeping the hydrogen peroxide dosage, 13.0 mmol L−1, HRT, 12 h and CSM 30 mg L−1 as a constant. Figure S3b shows that the removal of COD increased with the increase in dosage of ferrous sulphate up to 0.72 mmol L−1 at 12 h and only then a marginal increase in COD removal was observed. It was due to the generation of hydroxyl radicals by 0.72 mmol L−1, which was considered to be enough or used entirely for the oxidation of pollutants in wastewater. The excess Fe2+ might increase the formation of Fe3+ and hydroxyl ion (Eq. 2), and it would deactivate the hydroxyl radicals (Eq. 3). Thus, the COD removal was not observed for the excess dosage of ferrous sulphate (Boonrattanakij et al. 2011).
And also, the excess Fe2+ would lead to the formation of hydroperoxyl radicals, having a very low oxidation potential than hydroxy radical and precipitation of Fe3+ ions as ferric hydroxide. It resulted in lowering the rate of generation of hydroxyl radicals; thus the COD removal decreased by increasing the dosage of Fe2+ (Eq. 4 & 5). Hence, the optimum dosage of ferrous sulphate was fixed as 0.72 mmol L−1.
Effect of mass of carbon silica matrix on heterogeneous fluidized bed reactor
The optimum dosage of CSM was determined by experimenting with different dosages of CSM (20, 25, 30 and 40 g L−1) by keeping the optimized dosage of hydrogen peroxide (13 mmol L−1), ferrous sulphate (0.72 mmol L−1), HRT (12 h) and pH (3.5). The COD removal varied with the dose of CSM as shown in Fig. S4. It showed that the removal of COD was the maximum at the dose of CSM, 30 g L−1 and only a marginal reduction in COD observed at higher doses of CSM, 40 g L−1. The number of active sites that serves as a source for the generation of hydroxyl radicals increased with the mass of CSM. The availability of active sites was less enough to generate hydroxyl radicals at a low dosage of CSM for the concentration of organics in the wastewater to be oxidized. As the dosage of CSM was increased, the active sites also increased proportionally with the mass of CSM contributing to the generation of hydroxyl radicals for the oxidation of organics in wastewater. However, the reduction in COD was not significant beyond the optimum dosage of 30 g L−1 because the active sites became abundant for the available concentration of organics and resulted in adsorption of organic pollutant. Moreover, the active sites also serve to adsorb the organics in tannery wastewater and then oxidize. The dangling bonds in the carbon silica matrix adsorbed the energy released in the partial oxidation of organics and produce energetic active centres. The energized dangling bonds adsorb the molecular oxygen to generate OH● radicals and further react with organics in wastewater. The process continued ultimately, the proportion of active centre reached the maximum value or saturation value. Further, the increase in the dose of CSM increased the active centre to retard the reaction due to overcrowding of molecules or steric hindrance of the adsorbed molecules. Thus, the optimized conditions to run the HFBR reactor are pH 3.5, ferrous sulphate, 0.72 mmol L−1; hydrogen peroxide, 13.00 mmol L−1; CSM, 30 g L−1 and HRT, 12 h. It is also confirmed with ANOVA which is discussed in supplementary information Sect. S1.
The characteristics of heterogeneous fluidized bed reactor treated water
The PTWW was treated in HFBR at the above-mentioned optimized conditions, and the characteristics of HFBR treated water (PTWW1) are presented in Table 2. The percentage removal of COD, TOC, TN, BOD and NH4+-N in PTWW1 was observed as 27.1 ± 4.5%, 29.0 ± 5.45%, 9.56 ± 5.29%, 10.62 ± 13.75% and 2 ± 7.09%, respectively. The PTWW1 was subjected to instrumental analysis such as UV–Vis spectroscopy, FTIR and LC–MS. The UV–Vis spectrum of PTWW1 (Fig. 2c) showed the shifting of peaks to 248 nm from 252 and 211 nm from 218 nm of PTWW due to the removal of organic pollutants (hypsochromic shift); functional dyes peaks at the visible region of PTWW were also eliminated. The FTIR spectrum of PTWW1 is shown in Fig. 3b. The shifting of peaks due to breaking down of organic pollutant was observed. The fluorescence spectrum of PTWW1 showed (Fig. S1b) excitation peak at λ302 nm and emission peak at λ342nm with a lower emission difference of 40 nm indicating the cleavage of fluorescent active organic molecules in the HFBR process. The LC spectrum of PTWW1 is shown in Fig. 4a, and the chromatogram showing the disappearance of the peaks at RT 12.78, 5.97, 9.34 was observed. The formation of new peaks with a very low intensity at 8.69, 9.72 due to intermediates confirms the oxidation of the organic compounds in PTWW.
Treatment of heterogeneous fluidized bed reactor treated post-tanning wastewater in packed bed reactor
The PTWW1 still contains organic pollutants and a low BOD/COD ratio of 0.1861 ± 0.1317. To increase the biodegradability and to remove the organic pollutant present in PTWW1, the second Fenton oxidation method of PBR was used, so the PTWW1 was applied to the PBR. The dosage of Fenton reagents such as H2O2, 13.00 mmol L−1 and ferrous sulphate, 0.72 mmol L−1 were fixed based on the optimization studies in the HFBR process. The optimum HRT of the PBR process optimized is as follows.
Effect of hydraulic retention time in packed bed reactor
The PTWW1 was treated in PBR with H2O2, 13.00 mmol L−1 and ferrous sulphate, 0.72 mmol L−1. The effect of HRT was studied by analysing the samples in different time intervals, and the removal of COD is shown in supplementary information Fig. S5. It was observed that there was a decrease in COD up to 60 min after which there was only a marginal reduction. Therefore, 60 min was considered as an optimum HRT for the PBR treatment process.
Characteristics of packed bed reactor treated water
The PBR treated water (PTWW2) at optimized condition was characterized and is presented in Table 3. It was characterized by pH, 4.38 ± 0.43; COD, 1920 ± 509.12; BOD, 238 ± 52.68; TOC, 422 ± 173.17; TN, 310.35 ± 136.54. The PBR has the removal efficiency of COD, TOC, TN, BOD and NH4+-N by 23.8 ± 7.09%, 15.67 ± 5.03%, 13.17 ± 3.7%, 62.32 ± 34.19% and 8.08 ± 17.11%, respectively. The PTWW2 was subjected to instrumental analysis such as UV–Vis, FTIR and LC.
The UV–visible spectrum of PTWW2 is shown in Fig. 2c. The spectrum shows that the disappearance of the peak at λ248 nm and λ211 nm and the appearance of a new peak with the hypochromic shift might be due to the intensive oxidation in the PBR reactor. The fluorescence spectrum of PTWW2 is shown in Fig. S1c, which shows the absence of fluorescent active compounds, indicating the intensive oxidation of organic compounds in the PBR reactor. In the FTIR spectrum of PTWW2 shown in Fig. 3b, it was observed that the shifting of peaks was similar to PTWW1.
The LC of PTWW2 is shown in Fig. 4a. It shows that the spectrum contains similar characteristic peaks of PTWW1 but with a decrease in intensity. A peak at 8.69 RT was observed as maximum intensity (100%) of PTWW, which was reduced around 25–30% in PTWW2. It confirms the high molecular weight compounds present in PTWW were degraded during the treatment in HFBR and PBR reactors, which the UV–Vis and FTIR spectra also supported the findings.
Characterisation of composite tannery wastewater
The PTWW2 has the pH of 4.38 ± 0.43 which was mixed with BHWW (pH 10.58 ± 0.07) in the ratio of 1:2 to neutralize it and to get the biological oxidation treatment condition. Thus, the neutralized water is termed as CTWW, and its characterization is presented in Table 4. It was characterized as COD, 4640 ± 1414.21 mg L−1; BOD, 1350 ± 296.98 mg L−1; BOD/COD, 0.2948 ± 0.0259, COD/TOC, 4.80 ± 0.0514 and pH 7.07 ± 0.06. The fluorescence spectrum of CTWW is shown in Fig. S1d, showing the excitation peak at λ366nm (low energy absorbed may be by conjugated π bonds) and emission peak at λ439nm. The PTWW2 has no fluorescent active compound but CTWW has a fluorescent active compound, which may be originated from BHWW.
Treatment of composite tannery wastewater in the heterogeneous fluidized bio-bed reactor
Table 4 suggests that the CTWW has an improved biodegradability index (0.2948 ± 0.0259) indicating the amenability for biodegradation. As the HFBBR is a biological oxidation reactor by immobilized CSM, so the CTWW was treated by HFBBR. The immobilized CSM was prepared by immobilization of bacterial culture of Bacillus sp. on the pores of the CSM. The culture was generated from the PTWW contaminated soil as per the procedure given by Mahesh et al. (2020). The generated culture was cultivated and allowed to immobilize on active sites of CSM in reactor startup of HFBBR. It was done by fluidization with CSM and culture using CTWW and sewage (10:90) diluted mixture. The CTWW concentration was raised to 100% by increasing it day by day in 15 days. Thus, the acclimatization of culture to CTWW takes place. The immobilized CSM can metabolize organic compounds present in the wastewater into CO2 and H2O by microbial oxidation. The optimum HRT in the HFBBR process studied is as follows.
Effect of hydraulic retention time for heterogeneous fluidized bio-bed reactor
The effect of HRT for the treatment of CTWW in the HFBBR reactor was determined by experimenting up to 30 h (Fig. S6) by keeping the dose of the same CSM optimized for the HFBR reactor. The removal of COD was increased with an increase in HRT up to 24 h after that only a marginal reduction in COD. So, 24 h was fixed as optimized HRT for the operation of the HFBBR reactor.
Characteristic of treated composite tannery wastewater
The CTWW was treated in the fluidized biological reactor HFBBR at the operating conditions of HRT, 24 h and Bacillus sp. immobilized CSM, 30 mg L−1. The characteristics of HFBBR treated water which is termed as treated composite tannery wastewater (CTWWt) are presented in Table 4. The CTWWt was characterized by pH,7.4 ± 0.07; BOD, 58 ± 39.6; COD, 305 ± 35.35; TOC, 94.4 ± 45.46; TN, 295 ± 226.7; NH4+-N, 146 ± 75.38. The HFBBR reactor has the removal efficiency of COD, TOC, TN, BOD and NH4+-N by 93.4 ± 0.92%, 90.17 ± 1.2%, 72.46 ± 0.6%, 95.64 ± 1.4% and 71.33 ± 15.77%, respectively.
The UV–Vis spectrum of CTWWt shows cleavage of organic molecules in the treatment process. The hypochromic shift is due to the cleavage of unsaturated macromolecules present in the CTWW into simpler organic compounds, and the hypsochromic shift was due to the formation of small stable compounds from the unstable molecules present in the CTWW (Fig. 2c).
The FTIR spectrum of CTWWt is shown in Fig. 3b, a peak at 990 cm−1 asymmetric stretching of C–O–C of CTWW (Fig. 3a), which disappeared after the HFBBR process. A peak at 610 cm−1 regions may be due to the out of plane bending or deformation of C-H groups present in the alkyl chain which was formed from the cleavage of unsaturated aromatic organic compounds. The fluorescent spectrum is shown in (Fig. S1e), which shows no fluorescent active peaks in CTWWt because of the oxidation of fluorescent active compounds present in the CTWW (Fig. S1d). The LC chromatogram of final treated water showed the elimination of peaks recorded at RT 3.99 and 8.69 of CTWW (Fig. 4a) by integrated oxidation methods. This confirms the complete oxidation of organics present in the PTWW and CTWW at the end of the treatment process. It is also supported with the mass spectrum of the final treated CTWWt which has minimum organic compounds than the initial shown in (Fig. 4c). The UV–Vis, FTIR and fluorescence spectrum also support the mass spectrum findings.
Variations observed in physicochemical parameters of post-tanning and composite tannery wastewater
The pH of the wastewater was increased slowly from 3.5 (PTWW) under the treatment in HFBR and PBR reactors due to the removal of acidic organic pollutants. A little increase in pH was observed from CTWW to CTWWt due to oxidation (Fig. 5a) of organics in wastewater. The changes in ORP of tannery wastewater at different stages of treatment were measured in millivolts (mV) and is presented in Fig. 5b. ORP signifies the concentration of oxidizers and reducers present in the wastewater. The average ORP of PTWW was -97.05, suggesting the reduced state of unstable constituents present in the wastewater and observed an increase in positive ORP up to PBR treatment, indicating the oxidized stable form of the constituents. Then, the ORP was changed to negative, due to the addition of BHWW during neutralization which contains the reduced species. The ORP was shifted towards a positive side after treatment in the HFBBR process. It was confirmed that the complete conversion of the unstable reduced the organic compounds to an oxidized stable state. The BOD/COD of PTWW shows a very poor biodegradability of the wastewater and a substantial increase in the biodegradability was observed in PTWW2. The high biodegradability index indicates that the wastewater was amenable for the biological treatment process in HFBBR and conventional biological treatment process (Fig. 5c). The reduction in TOC from PTWW to PTWW2 and from CTWW to CTWWt was observed in all the process which is evident by the COD/TOC ratio. The changes in the COD/TOC ratio confirm the degradation of macromolecule in tannery wastewater (Fig. 5d).
The NH4+-N present in the PTWW was due to the usage of ammonium salts for neutralizing purpose in the re-tanning operation (Nalyanya et al. 2020) and might also be the usage of aniline based dyes in dyeing operations (Andaluz et al. 2017). The decrease in NH4+-N was observed in PTWW1 and PTWW2 which was due to the oxidation of NH4+-N into nitrite (NO2−) and nitrate (NO3−) and also oxidation of nitrogen-containing organic compounds by hydroxyl radical. The removal of nitrogen-containing azo dye Acid Blue 113 in PTWW1 from PTWW confirms the oxidation of the compounds (Fig. 2a, c). And also, the increase in ORP confirms its oxidation present in PTWW (Fig. 5b). A characteristic reduction of NH4+-N was observed in CTWWt water due to the oxidation of protein molecules. The removal of pollution parameters COD, TOC, BOD, TN and NH4+-N at different stages of treatment of tannery wastewater are presented in Fig. 6a–d.
The suggested treatment scheme consisting of HFBR, PBR and HFBBR processes and adjusting the pH for the required condition with the various wastewater itself without the addition of chemicals reduces pollution parameters without generation of primary chemical sludge and minimum generation of secondary biological sludge. The remaining pollution parameters such as salt content and pathogens can be eliminated in tertiary treatment using reverse osmosis (RO) and chlorination process etc. The economic feasibility of the proposed treatment for its implementation in a leather manufacturing industry was discussed in the supplementary information (section S2) for the production capacity of 5000 kg d−1. The proposed treatment technique had an operational cost of 0.6 USD m−3 wastewater to be treated.
Comparison of the proposed scheme with other technologies
The reported results using the suggested scheme are better than the ozone treatment of tannery wastewater reported by Schrank et al. (2017). They reported that COD, BOD and TOC removal efficiency were 85%, 83%, 62%, respectively, at a high ozone dosage of 500 mg O3 L−1 for 4 h. In addition to that, they are using H3PO4 and NaOH for pH adjustment which increases the cost of the treatment and sludge generation. Varank et al. (2016) reported the electrocoagulation and electro-Fenton with a removal efficiency of COD by 54.8 and 87.3% with the cost of the treatment 7.76 and 8.25USD m−3, respectively. The reported cost was 12–14 times higher than that of the proposed treatment cost. Sivagami et al. (2018) depicted AOPs of a three-step combined processes which include coagulation, aeration and ozonation for COD removal efficiency of 80–90% but their operational cost was much higher due to the high amount of chemical addition and it also possesses sludge disposal difficulties. The integrated approach of electrocoagulation and fungal treatment method for the removal of an organic contaminant from tannery wastewater was reported by Nguyen et al. (2020). They reported that the COD removal efficiency of 63.8% by electrocoagulation alone using Al and Fe electrodes and the combined technique gave 96% of removal efficiency with the total treatment operational cost of 1.73 USD m−3. The major disadvantages of using this treatment method in large industrial-scale applications are periodical electrodes corrosion, toxic gases generation, sludge generation, high operational cost and crucial working conditions of fungal treatment. Thus, among these treatment methods, the current proposed treatment approach stands as a sustainable way to treat tannery wastewater.
Conclusion
In this proposed treatment scheme, the non-biodegradable post-tanning wastewater containing complex organic compounds was catalytically oxidized by using various heterogeneous catalytic oxidation reactors. The high molecular weight complex organic molecules in PTWW were degraded into a low molecular weight organic compounds which were confirmed by LC–MS analysis. The HFBR treated PTWW was mixed with the beam house wastewater to get composite tannery wastewater which degraded further in the biological (HFBBR) reactor system at HRT of 24 h without sludge production. The pollution parameters of the treated tannery wastewater with proposed treatment were pH,7.4 ± 0.07; BOD, 58 ± 39.6; COD, 305 ± 35.35; TOC, 94.93 ± 45.46; TN, 295 ± 226.7; NH4+-N, 146 ± 75.38. The removal efficiency of parameters COD, TOC, TN, BOD and NH4+-N by 93.4 ± 0.92%, 90.17 ± 1.2%, 72.46 ± 0.6%, 95.64 ± 1.4% and 71.33 ± 15.77% was observed, respectively, without generation of primary chemical sludge and secondary biological sludge. The removal of the pollutants was confirmed with UV–Vis spectroscopy, FTIR spectroscopy, fluorescence spectroscopy and LC–MS spectroscopy.
References
Alighardashi A, Pakan M, Jamshidi S, Shariati FP (2017) Performance evaluation of membrane bioreactor (MBR) coupled with activated carbon on tannery wastewater treatment. Membr Water Treat 8:517–528. https://doi.org/10.12989/mwt.2017.8.6.517
Andaluz VH, Pazmiño AM, Pérez JA et al (2017) Training of tannery processes through virtual reality. Int Conf Augment Real Virtual Real Comput Graph. https://doi.org/10.1007/978-3-319-60922-5_6
APHA (2017) Standard methods for the examination of water and wastewater
Aslam M, Charfi A, Lesage G et al (2017) Membrane bioreactors for wastewater treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem Eng J 307:897–913
Basavaraja C, Kim WJ, Kim DG, Huh DS (2012) Microwave absorption studies of polyaniline nanocomposites encapsulating gold nanoparticles on the surface of reduced graphene oxide in the presence of 2-naphthalene sulfonic acid. Colloid Polym Sci 290:829–838. https://doi.org/10.1007/s00396-012-2596-z
Bharagava RN, Saxena G, Mulla SI, Patel DK (2018) Characterization and identification of recalcitrant organic pollutants (ROPs) in tannery wastewater and Its phytotoxicity evaluation for environmental safety. Arch Environ Contam Toxicol 75:259–272. https://doi.org/10.1007/s00244-017-0490-x
Black M, Canova M, Rydin S et al (2013) Best Available Techniques (BAT) Reference Document for the Tanning of Hides and Skins. Eur Comm Database 46:2013
Boonrattanakij N, Lu MC, Anotai J (2011) Iron crystallization in a fluidized-bed Fenton process. Water Res 45:3255–3262. https://doi.org/10.1016/j.watres.2011.03.045
Borba FH, Pellenz L, Bueno F et al (2018) Pollutant removal and biodegradation assessment of tannery effluent treated by conventional Fenton oxidation process. J Environ Chem Eng 6:7070–7079. https://doi.org/10.1016/j.jece.2018.11.005
Brodowska KM (2017) Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur J Biol Res 7:108–123. https://doi.org/10.5281/zenodo.545778
Bulut E, Özacar M, Şengil IA (2008) Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 115:234–246. https://doi.org/10.1016/j.micromeso.2008.01.039
Crini G, Lichtfouse E (2018) Wastewater treatment : an overview. Green Adsorbents Pollut Remov. https://doi.org/10.1007/978-3-319-92111-2_1
Divyalakshmi P, Murugan D, Sivarajan M et al (2015) In situ disruption approach on aerobic sludge biomass for excess sludge reduction in tannery effluent treatment plant. Chem Eng J 276:130–136. https://doi.org/10.1016/j.cej.2015.04.085
Hartmann M, Kullmann S, Keller H (2010) Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J Mater Chem 20:9002–9017. https://doi.org/10.1039/c0jm00577k
Johnson P, Loganathan C, Krishnan V et al (2018) Plant extract as environmental-friendly green catalyst for the reduction of hexavalent chromium in tannery effluent. Environ Technol (UK) 39:1376–1383. https://doi.org/10.1080/09593330.2017.1329355
Korpe S, Bethi B, Sonawane SH, Jayakumar KV (2019) Tannery wastewater treatment by cavitation combined with advanced oxidation process (AOP). Ultrason Sonochem 59:104723. https://doi.org/10.1016/j.ultsonch.2019.104723
Kuppusamy S, Jayaraman N, Jagannathan M et al (2017) Electrochemical decolorization and biodegradation of tannery effluent for reduction of chemical oxygen demand and hexavalent chromium. J Water Process Eng 20:22–28. https://doi.org/10.1016/j.jwpe.2017.09.008
Liu WH, Zhang CG, Gao PF et al (2017) Advanced treatment of tannery wastewater using the combination of UASB, SBR, electrochemical oxidation and BAF. J Chem Technol Biotechnol 92:588–597. https://doi.org/10.1002/jctb.5037
Lofrano G, Meriç S, Zengin GE, Orhon D (2013) Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ 461–462:265–281. https://doi.org/10.1016/j.scitotenv.2013.05.004
Maha Lakshmi P, Sivashanmugam P (2013) Treatment of oil tanning effluent by electrocoagulation: Influence of ultrasound and hybrid electrode on COD removal. Sep Purif Technol 116:378–384. https://doi.org/10.1016/j.seppur.2013.05.026
Maharaja P, Gokul E, Prabhakaran N et al (2016) Simultaneous removal of NH4+-N and refractory organics through sequential heterogeneous Fenton oxidation process and struvite precipitation: Kinetic study. RSC Adv 6:4250–4261. https://doi.org/10.1039/c5ra20492e
Mahesh SS, Ganesan S (2020) Treatment of post tanning wastewater with minimum sludge generation using sequential anoxic/oxic bioreactor and its microbial community profile. J Water Process Eng 35:101244. https://doi.org/10.1016/j.jwpe.2020.101244
Meenachi S, Kandasamy S (2017) Review on wastewater treatment methods in tannery waste water. Int J Adv Sci Eng Res 2(1):512–521
Midha V, Dey A (2008) Biological Treatment of Tannery Wastewater for Sulfide Removal. Int J Chem Sci 6:472–486
Moradi M, Moussavi G (2019) Enhanced treatment of tannery wastewater using the electrocoagulation process combined with UVC/VUV photoreactor: Parametric and mechanistic evaluation. Chem Eng J 358:1038–1046. https://doi.org/10.1016/j.cej.2018.10.069
Nalyanya KM, Rop RK, Onyuka AS et al (2020) Variation of elemental concentration in leather during post-tanning operation using energy dispersive X-ray fluorescence spectroscopy: principal component analysis approach. Int J Environ Anal Chem 00:1–13. https://doi.org/10.1080/03067319.2020.1746292
Natarajan TS, Natarajan K, Bajaj HC, Tayade RJ (2013) Study on identification of leather industry wastewater constituents and its photocatalytic treatment. Int J Environ Sci Technol 10:855–864. https://doi.org/10.1007/s13762-013-0200-9
Naumczyk JH, Kucharska MA (2017) Electrochemical treatment of tannery wastewater—Raw, coagulated, and pretreated by AOPs. J Environ Sci Heal Part A Toxic/hazardous Subst Environ Eng 52:649–664. https://doi.org/10.1080/10934529.2017.1297140
Nguyen TB, Bui XT, Vo TDH et al (2019) Anaerobic membrane bioreactors for industrial wastewater treatment. Current developments in biotechnology and bioengineering: advanced membrane separation processes for sustainable water and wastewater management - anaerobic membrane bioreactor processes and technologies. Elsevier, Amsterdam, pp 167–196
Oliveira E, Bértolo E, Núñez C et al (2018) Green and red fluorescent dyes for translational applications in imaging and sensing analytes: a dual-color flag. ChemistryOpen 7:9–52. https://doi.org/10.1002/open.201700135
Quintão TC, Rabelo LM, Alvarez TGS et al (2018) Precopulatory sexual behavior of male mice is changed by the exposure to tannery effluent. Chemosphere 195:312–324. https://doi.org/10.1016/j.chemosphere.2017.12.087
Ramani K, Sekaran G (2006) Fenton activated carbon catalytic oxidation (FACCO) system for the treatment of soak liquor for reuse application. IULTCS II, Istanbul, Turki, pp 1–26
Rueda-Marquez JJ, Levchuk I, Fernández Ibañez P, Sillanpää M (2020) A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.120694
Saxena G, Chandra R, Bharagava RN (2017) Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Reviews of environmental contamination and toxicology. Springer, New York LLC, pp 31–69
Schrank SG, Gebhardt W, José HJ et al (2017) Ozone treatment of tannery wastewater monitored by conventional and substance specific wastewater analyses. Ozone Sci Eng 39:159–187. https://doi.org/10.1080/01919512.2016.1273090
Senthilvelan T, Kanagaraj J, Panda RC (2014) Enzyme-mediated bacterial biodegradation of an Azo Dye (C.I. Acid Blue 113): reuse of treated dye wastewater in post-tanning Operations. Appl Biochem Biotechnol 174:2131–2152. https://doi.org/10.1007/s12010-014-1158-x
Silverstein RM, Webster X, Francis KD (2005) Spectrometric identification of organic compounds. John Wiley, New Jersey
Sivagami K, Sakthivel KPP, Nambi IM (2018) Advanced oxidation processes for the treatment of tannery wastewater. J Environ Chem Eng 6:3656–3663. https://doi.org/10.1016/j.jece.2017.06.004
Swarnalatha S, Kumar AG, Sekaran G et al (2009) Electron rich porous carbon/silica matrix from rice husk and its characterization. J Porous Mater 16:239–245. https://doi.org/10.1007/s10934-008-9192-0
Varank G, Yazici Guvenc S, Gurbuz G, Onkal Engin G (2016) Statistical optimization of process parameters for tannery wastewater treatment by electrocoagulation and electro-Fenton techniques. Desalin Water Treat 57:25460–25473. https://doi.org/10.1080/19443994.2016.1157042
Acknowledgements
The author N Prabhakaran is very much thankful to the Council of Scientific and Industrial Research (CSIR), India, for providing Senior Research Fellowship (CSIR-SRF) (grant No.31/06(0462)/2K19-EMR-I, dated 29-03-2019). The authors are thankful to Director, CSIR-Central Leather Research Institute (CLRI), India, for granting the permission to carry out this work in the laboratory and CLRI’s Centre for Analysis, Testing, Evaluation & Reporting Services (CATERS) for providing an LC-MS analysis facility to carry out this work. This research work was carried out as part of the PhD programme registered with the University of Madras of Mr Prabhakaran N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Josef Trögl.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prabhakaran, N., Patchai murugan, K., Jothieswari, M. et al. Tannery wastewater treatment process to minimize residual organics and generation of primary chemical sludge. Int. J. Environ. Sci. Technol. 19, 8857–8870 (2022). https://doi.org/10.1007/s13762-021-03634-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03634-2