Abstract
Investment in green chemistry to convert agro-industrial residues into value-added products could be economically feasible. The present study proved the utility of sugarcane bagasse as an inexpensive and easily available raw material for large-scale production of extracellular laccase from a newfound bacterial strain Lysinibacillus macroides LSO isolated from Alexandria paper and pulp industry effluents. To maximize the efficiency of laccases, a sequential optimization strategy focused on computational experimental designs accompanied by a bench-scale bioreactor batch cultivation strategy was adopted. Among the twelve variables analysed, sugarcane bagasse, NH4Cl, CuSO4·5H2O, and MnSO4·H2O were chosen based on their high positive significant impact on laccase productivity via the 2-level Plackett–Burman design (PBD). Rotatable Central Composite Design (RCCD) was exploited to create a polynomial quadratic model correlating the relationship between the four variables and the productivity of laccase. The highest extracellular laccase productivity and specific growth rate (µ) were achieved at the early period of the incubation time through cultivation Lysinibacillus macroides LSO in a 10-L stirred tank bioreactor under batch operation conditions. Laccase yield 7653.2 U L−1 min−1 and µ = 0.024 h−1, respectively, were obtained after 28 h that increased more than 70.861-, 4.181-, and 1.934-fold compared to basal media, PBD, and RCCD, respectively.
Graphic abstract
Utilization of bio-waste for scaling up the production of extracellular bacterial laccase from Lysinibacillus macroides strain LSO via bioreactor scale

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane industry is among the oldest industries in the Egyptian economy, and it is regarded as the most important and vital industry in Egypt. The sugarcane industry is considered as an open industrial system, in which the material and energy are utilized to produce the product, by-product, and waste streams. Agro-industries’ waste is generated as a result of disposal concerns that lead to environmental and economic concerns. The sugarcane industry has to close its production process throughout its lifecycle, in order to be a sustainable industry or a green industry; consequently, its wastes, residues, and by-products should be exploited as raw materials for other industries rather than burned or disposed of. A considerable amount of waste and by-products are generated through the sugarcane industry. Sugarcane bagasse is one of the most valuable bio-wastes; it was reported that the three million tons of bagasse are produced annually during the sugar manufacturing cycle in the mill. The bagasse is the fibrous material leftover after chopping and crushing cane stalks for the juice extraction (Nakhla and Haggar 2014).
In recent decades, there has been a growing search for the use of agro-industrial waste due to incessant demand for agricultural activities. The deposition of these residues causes environmental degradation and financial damage (Pandey et al. 2000). The green chemistry strategies are developed to utilize these materials, transforming them into chemical compounds and microbial products with high added value such as protein-rich animal feed, enzymes, organic acids, amino acids, and vitamins. The use of sugarcane bagasse residue in bioprocesses is a rational alternative substrate production and an aid in solving the problem of environmental pollution. Sugarcane bagasse is a renewable, reliable, and low-cost source of carbon because it has a high carbohydrate content and relatively low lignin content. It consists of approximately 50% cellulose, 25% hemicellulose, and 25% lignin (Ferreira et al. 2018). Thus, the use of sugarcane bagasse for enzymatic production is an alternative for biotechnology industries to obtain hydrolytic and oxidative enzymes at a lower cost than compared to the enzymes on the market. The major obstacle for the utilization of agro-industrial waste in fermentation technology through bioprocess strategies is the transformation of the complex polysaccharides into ready-to-use saccharides, which can be assimilated by micro-organisms, and this challenge could be handled via the application of microbial enzyme technology. In various agro-industries, lignocellulolytic enzymes have a major prospective application. Over the past century, laccases have acknowledged its potential for the bio-depolymerization of lignin and oxidization of various phenols, polyphenols, methoxy-substituted phenols, aromatic amines, and highly recalcitrant environmental pollutants combined with the reduction of molecular oxygen to water. Laccases (benzenediol: oxygen oxidoreductases, p-diphenol oxidase; EC 1.10.3.2) are the members of the most significant subgroup of the protein subfamily of blue multi-copper oxidases and contain binding domains of histidine-rich copper. Furthermore, in contrast to other members of oxidoreductases, laccases do not require co-factoring such as NADP (H) and do not generate toxic intermediate peroxides unlike peroxidases; these unique features of laccases put them as potential industrial oxidizing enzymes (Rajeswari and Bhuvaneswari 2016).
Most microbial laccases have been defined to date by fungal origin, particularly white-rot fungi, very little reporting on bacterial laccases, are available. Nevertheless, manufacturing and commercialization of fungal laccases are still not being achieved for bio-ligninolysis because of the slow growth rate of fungal cells, which extended the fermentation period, massive spore formation, and low laccase titre, and the acidic culture conditions are necessary for fungal growth (Chauhan et al. 2017). Bacterial laccases have been increasing quickly in the latest years, owing to their numerous notable characteristics compared with fungal laccases in view of industrial points such as its activation and stabilization under alkaline conditions and high temperatures with great stability against different inhibitors. Furthermore, due to their economical use in industrial applications, bacterial laccases have some extra advantages, including their short-term production of enzymes and ease of cloning and expressing in the host with appropriate manipulation appropriate (Zhu et al. 2020). Nevertheless, the industrial exploitation of bacterial laccases is hindered because most bacterial laccases reported are intracellular or spore-bound, which makes it extremely difficult to produce and purify them in large scale (Sondhi et al. 2015), because their environmental eco-friendliness and sustainability have made the pursuit of innovative high influential bacterial strains producing laccases a legitimate objective (Unuofin et al. 2019).
The main goal of the current study is the exploitation of green chemistry sustainable strategies for the ultimate benefit of bio-waste sugarcane bagasse for scaling-up production of extracellular bacterial laccase from Lysinibacillus macroides LSO on bench top-scale production, where the scale-up of bacterial laccase production on large-scale production has received a few attention to date. According to those mentioned above, the present study was focused on finding an innovative step towards scaling up of newfound laccase production. Moreover, the present study was a highlight on the exploitation of mathematical models for optimizing the conditions of growth, which give the highest laccase productivity from a newfound Lysinibacillus macroides LSO. To the best of our knowledge, the lag phase bacterial laccase production has not yet been reported in the literature; the finding of the current study represents the first report for lag phase, and bench-scale laccase production uses sugarcane bagasse as agro-industrial waste residual.
Materials and methods
Sample collection and isolate sources
The bacterial strain was isolated from sludge samples, which collected aseptically from different discharged sites of Alexandria paper and pulp manufactories. The samples were transported to the Bioprocess Development laboratory and stored in the refrigerator at 4 °C until further processing.
Enrichment and isolation of laccase-producing bacteria
The laccase-producing bacteria were enriched by liquid Luria–Bertani (LB) (yeast extract [Biobasic], 5; peptone [Merck], 10; NaCl [Adwic], 10 g/l) medium-supplemented with 0.2 mM CuSO4. 5H2O. One gram of sludge sample was suspended in 250-ml Erlenmeyer flasks contained 50-ml sterile LB medium supplemented with 0.2 mM CuSO4·5H2O, pH 7.0 and followed by incubation in a reciprocal shaker at 30 °C, 200 rpm for overnight (Rajeswari and Bhuvaneswari 2016). Then, a standard serial dilution (10–1–10–9) was prepared using sterile water saline solution (0.85% NaCl, 0.1% peptone). 0.1 ml aliquots of serially diluted samples were pipette out into LB medium agar plate and incubated at 30 °C, for overnight. Pure isolates were subcultured, kept on LB slants, stored at 4.0 °C, and subcultured regularly.
Qualitative screening for laccase-producing bacteria
The qualitative screening was carried out by streaking of diluted LB agar supplemented with 5.0 mM guaiacol [Sigma-Aldrich, Germany] as an indicator substrate (Mongkolthanaruk et al. 2012) with single colonies from each isolate and incubated overnight at 30 °C. The appearance of reddish-brown colonies indicated a positive laccase production, which confirmed by the quantitative procedure.
Quantitative screening
The extracellular cell-free supernatant is used as crude enzyme for the quantitative determination of laccase activity. It was obtained through the submerged culture of selected isolate via inoculating 50 ml of laccase production media (w/v%): yeast extract; 0.896; (NH4)2SO4, 0.035; CuSO4·5H2O, 0.02; FeSO4·7H2O, 0.000133; glucose, 0.0943, pH 7.0, as described by Abdelgalil et al. (2018), dispensed in 250-ml Erlenmeyer flask with one ml of 20-h, old suspension of the tested isolate. The inoculated flasks were incubated at 30 °C for 24 h using a reciprocal shaker (200 rpm). The cell-free supernatant was obtained via centrifugation at 6000 rpm for 30 min. at 4.0 °C. Laccase activity has been monitored calorimetrically using 2,6-dimethoxyphenol (DMP) as a substrate with an extinction coefficient (ε) 470 = 35,645 M−1 cm−1 at 470 nm (Chen et al. 2013). The reaction mixture contained appropriately diluted enzyme solution in 0.25 ml of 0.1 M sodium phosphate buffer pH 8.0; 0.6 ml of 0.1 M sodium phosphate buffer pH 8.0; and 0.25 ml of DMP reagent (4.0 mM) dissolved in 10% ethanol; the reaction mixture was incubated after thorough mixing at 60 °C for 10 min. The blank contained all the constitutes except the active enzyme. As a result of the oxidation of DMP, the increase of the absorbance was monitored at 470 nm (ε = 35,645 M−1 cm−1) in a UV–visible spectrophotometer. One unit of enzyme activity has been expressed as an international unit (IU); it represents as the amount of enzyme that catalyses the oxidization 1.0 μmol of DMP per minute under standard assay conditions; and the activity was expressed in U L−1 min−1.
Amplification of the 16S rDNA gene, sequencing, and similarity
The bacterial isolate that shows the highest laccase activity was selected for further molecular identification. The salting-out method (Marahatta et al. 2006) was followed to extract the genomic DNA from the isolate under investigation. Thereafter, amplification of the 16S rRNA gene from the genome of the interested isolate was carried out via the polymerase chain reaction technique, using universal forward primer 5′-AGAGTTTGATCMTGGCTCAG-3′ (corresponded to position 8 of Escherichia coli 16S rRNA) and reverse primer 3′-TACGGYACCTTGTTACGACTT-5′ (corresponded to position 1514 of Escherichia coli 16S rRNA). The PCR mixture consisted of (0.0015 ml) 10 pM for each primer, 0.1 μg of chromosomal DNA, (0.005 ml) 2 mM dNTPs, (0.0004 ml) 2U of Taq polymerase, (0.005 ml) 10 × polymerase buffer (Fermentas, Germany) and 0.0368 ml of nuclease-free water for a 0.05 ml reaction. PCR amplification was performed using a thermal cycler (MultiGene OptiMax, Labnet International, Inc.) under the following conditions: 4.0 min at 95 °C, then 30 cycles at 94 °C for 1.0 min, 55 °C for 1.0 min and 72 °C for 2.0 min, followed by an additional 10 min at 72 °C as a final extension step. The amplification product was analysed via a 1.0% agarose gel electrophoresis and then purified for further use. The purified PCR fragments were subject to automated DNA sequencing according to the protocol recommended by the manufacturer. The obtained sequence was submitted to the BLAST network (www.ncbi.nlm.gov/blast) to assess the DNA homologous. Subsequently, the sequence has been deposited in the GenBank to get an accession number. Multiple sequence alignment was carried out via ClustalW2, and a phylogenetic tree was constructed using the MEGA software (version 7.0) by the neighbour-joining method (Kumar et al. 2016).
Effects of different lignocellulosic residues on laccase production
To obtain the ultimate benefit from lignocellulosic wastes, 1% (w/v) from sugarcane bagasse, corn stove, molasses, black liquor and 1 mM from kraft lignin and humic acids (Arora and Gill 2000) has been exploited to enhance the Lysinibacillus macroides LSO (MN216323) laccase productivity. The sugarcane bagasse and corn stove were collected from the fruit opened market and Agriculture Institute Research-Alexandria, respectively, and then chopped into small chips with scissors. The chips were sun-dried, oven-dried (50 °C) to constant weight, ground to 40-mm mesh size electric blender, and stored in the airtight plastic jars to keep the material moisture-free, while black liquor was obtained from pulp and paper factory, Alexandria, Egypt. The laccase production medium (w/v%): yeast extract; 0.896; (NH4)2SO4, 0.035; CuSO4·5H2O, 0.02; FeSO4·7H2O, 0.000133; glucose, 0.0943, pH 7.0 (Abdelgalil et al. 2018) supplemented with the above lignocellulosic wastes separately has been inoculated with an aliquot (20%) of activated culture suspension and incubated in a reciprocal shaker incubator at 30 °C and 200 rpm for 20 h. Aliquots (2.0 ml) were withdrawn for laccase activity determination.
Statistical optimization for laccase production
Plackett–Burman design
A fractional factorial PBD was employed in the current study to select the variables that control laccase production notably from an array of factors, and insignificant ones were uninvolved to obtain the smaller, more controllable set of factors with lesser experimentations (Chhaya and Modi 2013). The experimental design for Plackett–Burman was built to screen for twelve parameters; each was evaluated at two levels: (+) at a high level and (−) at a low level as coded to describe each independent variable's linear influence on the performance objectives (laccase activity) irrespective of the interactions (Plackett and Burman 1946). The numbers of positive and negative signs per trial are (n+1)/2 and (n−1)/2, respectively. Table 1 shows both the independently examined variables, the levels of each variable in the experimental design and the design matrix of the 12 selected variables screened with a central point in 22 combination trials with the corresponding laccases yield response.
For the data interpretation, determination of the coefficients and the polynomial model reduction, the Plackett–Burman experiment was subject to multiple regression analysis with the aid of Excel software program. The mean effect chart was used in the statistical study to elucidate the importance of variables that depend on their nature and positive or negative results in the production process as Al Fattah explained (2011). Through measuring the p value by the standard regression analysis, the significance of variables was calculated. When their p value is less than 0.05, these variables are considered meaningful (Abdel-Fattah et al. 2009). Plackett–Burman factorial design followed the first-order polynomial model:
where Y is the response (laccase activity U L−1 min−1), βo is the model intercepts, βi is the linear coefficient, and Xi is the level of the independent variable. From the Pareto chart, the factors showing the highest positive effects were selected for further optimization using RCCD (El-Sersy et al. 2010). A confirmation test was carried out in which the predicted optimum levels of the independent variables were examined and compared to the basal conditions, and then, the average of enzyme activity was calculated.
Rotatable Central Composite Design
Response surface methodology (RSM) is the empirical simulation methodology used to maximize the concentration of critical screened variables to boost the laccase productivity. Using RCCD was adopted to find the optimum levels of the significant variables (sugarcane bagasse, NH4Cl, CuSO4·5H2O, and MnSO4·H2O) and the effects of their mutual interactions on enzyme production with reverence to enzyme activity (U L−1 min−1) as a response. Each independent variable was studied at five different levels denominated as (− 2, − 1, 0, + 1 and + 2, respectively) (Box and Behnken 1960) along with 27 trials. For the error calculation, the middle point of the pattern was repeated three times. Table 3 shows the coded and real levels of every variable and the layout of the experimental design matrix used in this study.
Experimental design, data processing, and quadratic model construction were included in the free download of available programmes. The observed performance (laccase yield) was taken as the experimental values of the variable or response (Y), while quadratic model-fitting techniques were used to obtain the intended response values. With the resolution of the obtained polynomial equation, optimum values of the four parameters were achieved. Furthermore, three-dimensional plots were constructed using a STATISTICA 7.0 software with a vertical axis that reflects enzyme yield and two horizontal axis representing five levels of two explanatory nutrients, while maintaining other two variables at zero level for visual observation of the maximal response pattern and the interactive influence of the significant variables on the response. A second-order model was used to correlate the response with the different parameters. In the following quadratic predictive polynomial equation, the correlation between the four parameters and the response (laccase activity) is defined.
where Y is the predicted response (laccase activity U L−1 min−1); β0 is the model intercept; X1, X2, X3, and X4 are the independent variables, β1, β2, β3, and β4 are linear coefficients; β12, β13, β14, β23, β24, and β34 are cross-product coefficients; and β11, β22, β33, and β44 are the quadratic coefficients. In order to identify the relationship of the variables and the responses, the data were analysed through multiple linear regressions like a variance analysis (ANOVA) using the free available download software. The fitness of the studied model was shown by a determination coefficient, R2. The validity of the quadratic model picked was verified by further experimental validation of the mathematical model obtained by means of RSM. Every experiment was performed three times, and the actual data obtained experimentally were correlated with the anticipated data.
Scale-up production of bacterial laccase
A process of scale-up for laccase provides more realistic information on the energy, and material demands to enable a comprehensive financial assessment of these products (Piccinno et al. 2016). In the current study, the final optimized conditions, which obtained from statistical optimization steps, were utilized for the scaling-up of laccase productivity from shake flask scale to bench-top scale. The primary goal of this study was to establish a large-scale fermentation system to evaluate the kinetics of microbial growth in a submerged cultivation system.
Shake-flask cultivation system
A 250-ml Erlenmeyer flask containing 50 ml of the optimized medium [(w/v%): sugarcane bagasse; 0.3; NH4Cl, 0.035; CuSO4·5H2O, 0.02; MnSO4·H2O, 0.1; pH, 7.0] was inoculated with 20% aliquot of activated culture suspension that pre-cultured in LB media for 17 h and then incubated at 30 °C in a shaker incubator (200 rpm) for 216 h. The samples were periodically withdrawn, and culture filtrate was obtained via centrifugation at 6000 rpm for 15 min, 4.0 °C. Thereafter, biomass dry weight, laccase activity, total carbohydrate concentration, and concentration of total soluble protein were monitored.
Stirred-bioreactor batch cultivation system
Batch fermentation was carried out in a working volume of 4 L in a 10-L bench-top bioreactor (Cleaver, Saratoga, USA), supplemented with three 6-bladed disc-turbine impeller and four baffles, and joined to a digital control unit. The bioreactor vessel that contained the statistically optimized medium was sterilized by autoclaving at 121 °C for 20 min, while CuSO4·5H2O and MnSO4·H2O solutions were sterilized separately by autoclaving at 121 °C for 20 min and then added under the aseptic condition to the bioreactor, in case of sucrose and vanillin acid solution, they were sterilized separately by syringe filter. Thereafter, the bioreactor was inoculated with prepared inoculum (20%). Temperature, agitation speed, aeration rate, and initial pH were adjusted at 30 °C, 200 rpm, 1.0 VVM and 7.0, respectively. The formation foam was suppressed by adding antifoam (Silicone oil, Sigma-Aldrich, 0.5:10 v/v) at a concentration of 1:100 (v/v) in distilled water at the beginning of the process. The fermentation process is performed under uncontrolled conditions. The samples (20 ml) were withdrawn periodically for estimation biomass, protein content, total carbohydrate consumption, and laccase productivity.
Analytical procedures
Morphological characterization of Lysinibacillus macroides strain LSO
The strain under investigation was undergone to the morphological investigation during its growth pattern via scanning electron microscopy (JSM 5300 JOEL, USA) and transmission electron microscope (100-CX JEOL, USA) at laboratory centre-A City of Scientific Research and Technological Applications.
Protein concentration assay
Protein content was carried out by the method described by Lowry et al. (1951). Bovine serum albumin (BSA, Sigma) was used for the standard curve.
Determination of total carbohydrates concentration
Anthrone-sulphuric acid method was applied to estimate total carbohydrate according to the method described by Morris (1948). Five millilitres of ice-cold 0.2% anthrone [Biobasic, Canada] reagent (0.2 g anthrone reagent in 100 ml of 96% H2SO4 fresh prepared) was added to a one mL of diluted cell-free supernatant sample. After that, the samples were boiled at 100 °C for 10 min and then kept it immediately on the ice bath for 5 min. The developed colour was measured at 620 nm against blank, which contained the same constitute except the sample replaced by distilled water. 0.1 mg/ml of sucrose solution stock was used for the standard curve.
Determination of lignin residual
Residual of lignin (Klason lignin) from biodegradation of sugarcane bagasse was estimated according to the method described by Templeton and Ehrman (1995). 1.0 oven-dried gram of sample was mixed with 20 ml of sulphuric acids (72%), and the mixture swirled at room temperature overnight. 540 ml distilled water was added and the sample was heated by condenser at 100 °C for 4.0 h. After cooling to room temperature, samples were filtrated through Whatman #1 filter paper, after that the filter paper that contained lignin residues was washed with hot water many times and dried at 105 oC for overnight and weighted thereafter, muffled at 450 °C for 30 min, and at 800 °C for 45 min for determining the ash content.
Results and discussion
Isolation, screening, and identification of laccase-producing bacteria
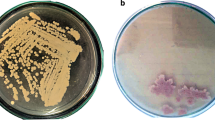
Laccase screening was carried out in two stages: primary qualitative screening program and secondary quantitative screening program. The qualitative screening of isolates which developed by enrichment isolation (20 isolates) for laccase production capabilities reaveled that approximately ten different isolates exhibit laccase activity by the formation of reddish-brown colonies as shown in Fig. 1a. Among the isolates subjected to the secondary quantitative screening program, only one of the potential isolate-coded LSO having a high level of laccase activity was picked out for further studies, which showed a preference growth at 30 °C and pH 7.0.
a Qualitative screening for alkaline phosphatase activity b Phylogenetic tree based on 16 S rDNA gene sequence analysis, showing the relationship of Lysinibacillus macroides strain LSO with reference strains (NCBI GenBank) which constructed by using the neighbour-joining method with the aid of MEGA 7.0 program. Sequence divergence is indicated by the scale bar. c Transmission Electron Microscope (TEM) micrograph of Lysinibacillus macroides strain LSO showing the morphology of the cell
The molecular identification of LSO isolate was carried out, and the Sanger’s dideoxynucleotide sequencing of the amplified 16S rRNA gene resulted in 1393bps nucleotide sequence. The primary sequence alignment carried out by NCBI nucleotide blast search revealed that the LSO isolate belongs to the Lysinibacillus. A neighbour-joining tree analysis illustrated the phylogenetic position of LSO isolate closest to Lysinibacillus macroides, with an identity 99.89% and query cover 100% (Fig. 1b). The nucleotide sequence was submitted to GenBank, NCBI, and was assigned the accession number MN216323, so it could be identified as Lysinibacillus macroides strain LSO (MN216323). Lysinibacillus macroides strain LSO is gram-positive and endospore-forming bacilli, endospores are spherical and lie terminally as shown in Fig. 1c. The cell size ranged from 1.05 μm in width and 2.78 μm in length.
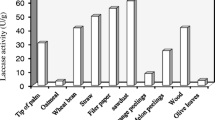
Effects of different lignocellulosic residues on laccase production
The obtained results showed that among the various agricultural wastes screened for laccase production, sugarcane bagasse was found to be the most suitable substrate as it supported the maximum laccase production (108 U L−1 min−1) in comparison with control trial (35.36 U L−1 min−1). Similarly, corn stove and molasses exerted a stimulatory effect on Lysinibacillus macroides strain LSO (MN216323) laccase production, compared to the control media. However, the media supplement with humic acid, black liquor, and kraft lignin characterized by tiny induction effect, whereas laccase productivity was slightly climbed up by 6.5, 5, and 4.3%, respectively, more than control trial (data not shown). Due to the difference in the chemical and nutritional composition of the designated substrates, a substantial variation in laccase production could be noted. Reports have been made in which the different agro-waste residues such as sugarcane bagasse, rice husk, and kraft lignin were exploited as a potential inducer of extracellular bacterial laccase production in the submerged cultivation system (Rajeswari and Bhuvaneswari 2017; Neelkant et al. 2019). Moreover, Reksohadiwinoto et al. (2019) pointed out that the maximum laccase activities (64, 54, and 202 U/L) were obtained from Lactobacillus plantarum 1, Lactobacillus plantarum 2, and Lactobacillus brevis, respectively, whilst Chen et al. (2013) recorded that 4.96 U/ml of laccase activity was achieved by the cultivation of Thermobifida fusca on sugarcane bagasse medium after 36 h of the incubation period.
Statistical optimization of laccase production by Lysinibacillus macroides LSO
Plackett–Burman design
The optimization of all cultivation conditions and ingredients of the processing media is extremely vital for an efficient laccases production, thereby rendering the economical construction of the large-scale fermentation method more facilitated. In the current study, there were two approaches to optimizing physicochemical variables for laccase production efficiency. In the first approach, a Plackett–Burman design was exploited to evaluate the relative significance of cultivation variables affecting Lysinibacillus macroides LSO (MN216323) laccase production. Table 1 displays the theoretical Plackett–Burman design matrices for the screening of relevant variables together with the related laccase efficiency and residuals. The obtained results in Table 1 indicate that there was a markedly vast variation in laccase productivity throughout the different trials ranging from 50 to 734 U L−1 min−1. The variations in the results show how important it is to use experimental design to optimize the media. Table 2 demonstrates the results evaluated by multiple regression statistical analysis and variance analysis (ANOVA), to determine the relation between laccase efficiency and the independent process variables. The estimated main effect of the variables under investigation was calculated using the formula ∑(+1)/n(+1) − ∑(−1)/n(−1) and is summarized graphically in Fig. 2a and Table 2. A Pareto diagram that indicates the significance of each variable estimate is shown in Fig. 2b. The twelve variables were evaluated through a linear multiple regression model, and from the formula (1 − p value) * 100 the confidence level was calculated. The lowest probability value (p value < 0.05) implies a significant influence of the process variable on the laccase efficiency. The findings illustrated that MnSO4·H2O (X11) with an extremely low probability value (0.000000207) was the most significant factor affecting laccase yield that produced by Lysinibacillus macroides strain LSO accompanied by sugarcane bagasse (X1) and then NH4Cl (X5) with p values of 0.0713 and 0.11, respectively. Studies also have shown that yeast extract (X3) is an insignificant factor with low effect (− 61.79689) on the production process.
Plackett–Burman design obtained results. a Column chart showed the main of culture variables according to the results of Plackett–Burman design. b Pareto chart illustrates the order and significance of the variables affecting laccase production by Lysinibacillus macroides strain LSO using Plackett–Burman design; Ranks (%) values ranging from 1.607 to 51.439). c The normal probability plot of the residuals for laccase production determined by the first-order polynomial equation
Regression coefficient evaluation (Table 2) of the twelve process variables, namely sugarcane bagasse, sucrose, NH4Cl, CuSO4·5H2O, MgSO4·7H2O, NaCl, and MnSO4·H2O had shown a positive influence on the laccase activity. However, yeast extract, tryptone, KNO3, vanillic acid, and ZnSO4·7H2O were found to contribute negatively. It would be necessary to provide the polynomial model explaining the association between the twelve process variables and laccase productivity as follows:
Variance analysis was conducted with the ANOVA method, which gives Fisher’s F test of 17.92574, p = 0.000079606. It reveals that the relationship between the variables is statistically significant at 99.9992% confidence level. The determination coefficient values (R2) are normally utilized to evaluate the model quality and to calculate the variation in measurable response values, which can be clarified by experimental variables. In the present study, the statistical R-square shows that the fitted model explains 96% of the laccase activity variation. Additionally, the highest value of the adjusted R2 statistic (90%) (Table 2) indicates a strong level of model relevance and greater accuracy with respect to the relationship between the experimental variables and laccase efficiency. From the statistical analysis, the great attention was paid to the selected four variables: MnSO4·H2O, sugarcane bagasse, NH4Cl, and CuSO4·5H2O significantly affect the laccase production where they have confidence levels between 71 and 100%.
The verification experiment was performed to evaluate the Plackett–Burman design accuracy. The predicted ideal conditions to attain the maximum laccase productivity by Lysinibacillus macroides LSO (MN216323) were an initial medium composition as the following (g/L): sugarcane bagasse, 10; sucrose, 0.25; tryptone, 1; NH4Cl, 0.5; KNO3, 0.02; vanillic acid, 0.033; CuSO4·5H2O, 0.124; MgSO4·7H2O, 0.25; NaCl, 0.25; MnSO4·H2O, 0.125 and ZnSO4·7H2O, 0.001. The cultivation condition performed with agitation condition 200 rpm, at 30 °C and pH, 7 for 48 h. The maximum laccase productivity obtained under these optimum conditions was 1829.99 U L−1 min−1, which is higher than the laccase productivity that obtained prior to application of the Plackett–Burman design (108 U L−1 min−1) by approximately 16.944 times. Plackett–Burman Design enhanced the laccase activity (4.69 ± 0.39 U L−1) of Pichia pastoris after 168 h by 36.08-fold more than those obtained from the un-optimized medium (Morales-Álvarez et al. 2017), although Rajeswari and Bhuvaneswari (2017) reported that the application of Plackett–Burman design for optimization of laccase production from Bacillus sp. PK4 led to the improvement of laccase activity by 11.48 U ml−1.
It was found that the Plackett–Burman design was extremely helpful in determining the variables to optimize further, enabling consideration to be paid for many variables and avoid information loss. The present results obtained from Plackett–Burman are consistent with Sondhi et al. (2015) who found that MnSO4 had the highest influence on laccase productivity from Bacillus tequilensis SN4 among other variables and tryptone, and CuSO4 showed a significant effect on the productivity. On the contrary, MnSO4 had negatively affected on laccase productivity from Bacillus sp.MSK-01 (Sondhi and Saini 2019). On the other hand, Rajeswari and Bhuvaneswari (2017) reported that yeast extract suppressed the laccase productivity by Bacillus sp. PK4, and magnesium sulphate and copper sulphate had a positive significant effect on the productivity of laccase, while sodium chloride and zinc sulphate revealed negative and positive effects, respectively, on laccase productivity, which is not matching with the present study.
Response surface methodology (Rotatable Central Composite Design)
RSM is an efficient tool to determine the effects of interaction between variables in order to maximize the chosen variables for the optimal output. It additionally changes over bioprocessing partnerships into models of mathematics, predicting where the optimum might be found. These experimental designs are recommended to the microbial industry sponsors for maintaining high efficiency and profitable bioprocesses (Abdel-Fattah et al. 2005). In the current study, the findings of the study from the Plackett–Burman design shown that the most noteworthy components influencing laccase efficiency positively are sugarcane bagasse (X1), NH4Cl (X2), CuSO4·5H2O (X3), and MnSO4·H2O (X4) and therefore were chosen to further enhancement utilizing the RCCD. The four factors and their five levels (coded and actual) utilized in a design matrix are illustrated in Table 3. Insignificant variables, which have negatively impacted on laccase efficiency and other positively insignificant variables, were kept up in all trials at their low levels and zero levels of Placket–Burman design, respectively, for additional investigated via RCCD improvement. Table 3 displays the experimental results and the hypothetically results that anticipated laccase profitability alongside the residuals. It was noticed that the highest laccase productivity was obtained in the trial no. 6 (2332.64 U L−1 min−1) at a concentration of sugarcane bagasse, NH4Cl, CuSO4·5H2O, and MnSO4·H2O (0.9, 0.6, 0.112, and 1 g/L, respectively). While the minimum laccase activity was recorded in the trial no. 27 (1248 U L−1 min−1) at a concentration of sugarcane bagasse, NH4Cl, CuSO4·5H2O, and MnSO4·H2O (0.9, 0.6, 0.112, and 0.2 g/L, respectively). The predicted laccase productivity fit well with those of the experimentally obtained laccase productivity.
Multiple regression analysis has been used for an evaluation of the experimental RCCD results, and Table 4 shows the results of ANOVA. The existing regression model has an R2 value of 0.94, which implies the variance in laccase activity to be explained by the model with 94%, and about 6% of the total variations created by variables are not explaining laccase activity. Additionally, the adjusted R2 value (0.8621) was too high to validate the model's great significance (Table 4). The statistics of the quadratic model were determined by ANOVA results. This means that, as demonstrated in its high F-value (12.61161) and very small probability estimation (p) value of 0.0000439, the model is profoundly significant (Table 4). Seeing that the p value demonstrated in the ANOVA table is under 0.05, it is presumed that there is a statistically significant relationship between the variables tested at a level of confidence of 95% (p = 0.05).
A second-order polynomial function for predicting the optimal point within experimental constraints was adapted to evaluate laccase productivity results:
where X1, X2, X3, X4, and X5 are sugarcane bagasse, NH4Cl, CuSO4·5H2O, and MnSO4·H2O.
The interpretation of the experimental results in Table 4 relied on the signs of the variable coefficients and the p values to explain the interactions between the variables under investigation. Concerning the response, the interaction between the two variables can be either positive or negative. The negative sign has an adverse effect; however, the positive sign exhibits the response synergistically influence. In the current study, the interactions between X1 and X3 (sugarcane bagasse and CuSO4·5H2O), the interaction between X1 and X4 (sugarcane bagasse and MnSO4·H2O), and the interaction between X2 and X3 (NH4Cl and CuSO4·5H2O) had a significant effect with p values of 0.0142, 0.0058, and 0.025, respectively. Furthermore, the linear coefficients of X2 (NH4Cl) and X4 (MnSO4·H2O) had a very significant effect on laccase productivity with p values 0.0035 and 0.000000102, respectively.
The 3D response surface plots and 2D contour plots were drawn for the combinations of the four variables (X1X2, X1X3, X1X4, X2X3, X2X4, and X3X4) for the optimum levels of the variables under investigation and for their interaction effects with the predicted productivity of the laccase. On the Z-axis, the laccase titre was plotted against two process variables, while the other variables were held at zero levels, as illustrated in Fig. 3. It was observed from Fig. 3a that a relatively the highest yield of laccase was achieved at low and high levels of sugarcane bagasse (X1). While the laccase productivity was decreased at middle and high levels of NH4Cl (X2), via software program, the point prediction option was estimated to optimize the process parameters. At the optimum predicted levels (g/l) of sugarcane bagasse (13.14) and NH4Cl (0.2) at CuSO4·5H2O (0.112) and MnSO4·H2O (0.6), the maximum predicted laccase yield (2063.75 U L−1 min−1) has been achieved.
In Fig. 3b, the maximum laccase productivity is plainly supported at low and middle levels of sugarcane bagasse (X1) and CuSO4·5H2O (X3); an increase in their concentration tends to decrease in the productivity. The most extreme anticipated laccase activity of 2028.58 U L−1 min−1 has been achieved at the optimum predicted levels (g/l) of sugarcane bagasse (3), CuSO4·5H2O (0.037), NH4Cl (0.6), and MnSO4·H2O (0.6). Similar results are observed in Fig. 3d, at the highest levels of NH4Cl (X2) and CuSO4·5H2O (X3), the laccase productivity could be relatively low, the increase in laccase yield was noticed at middle and low levels of NH4Cl and CuSO4·5H2O. The maximum predicted productivity was 2207 U L−1 min−1 at the optimum predicted concentration (g/l) of NH4Cl (0.2), CuSO4·5H2O (0.037), MnSO4·H2O (0.6), and sugarcane bagasse (9). However, Fig. 3c illustrates that the laccase productivity was elevated sharply at high concentration of MnSO4·H2O (X4) and it went down cross high and low concentration of sugarcane bagasse (X1). The maximum predicted laccase yield (2559.5 U L−1 min−1) has been accomplished at an optimum predicted concentration (g/l) of sugarcane bagasse (3), MnSO4·H2O (1), NH4Cl (0.6), and CuSO4·5H2O (0.112). Similarly, in Fig. 3e, f, the high concentration of MnSO4·H2O (X4) causes the elevation of laccase productivity, while the high and low NH4Cl (X2), CuSO4·5H2O (X3) concentrations cause a decline in the productivity of laccase, respectively. In case of Fig. 3e, the maximum predicted laccase activity (2547.4 U L−1 min−1) has been achieved at an optimum predicted concentration (g/l) of NH4Cl (0.2), MnSO4·H2O (1), sugarcane bagasse (9), and CuSO4·5H2O (0.112), while the maximum predicted laccase titre (2314.4 U L−1 min−1) in case of Fig. 3f was obtained at an optimum predicted concentration (g/l) of CuSO4·5H2O (0.037), MnSO4·H2O (1), sugarcane bagasse (9), and NH4Cl (0.6). The highest interaction was found between sugarcane bagasse/CuSO4·5H2O > sugarcane bagasse/ NH4Cl > CuSO4·5H2O / NH4Cl at low levels (Fig. 3). On the other hand, higher levels of sugarcane bagasse/MnSO4·H2O, NH4Cl/MnSO4·H2O, and CuSO4·5H2O/-MnSO4·H2O increase the level of laccase activity (Fig. 3).
The ideal levels of the four variables under investigation were evaluated via polynomial model and found to be (g/l); sugarcane bagasse, 3; NH4Cl, 0.2; CuSO4·5H2O, 0.035; and MnSO4·H2O, 1.0, sucrose, 0.25; tryptone, 1; KNO3, 0.02; vanillic acid, 0.033; MgSO4·7H2O, 0.25; NaCl, 0.25; ZnSO4·7H2O, 0.001 with prediction calculated laccase productivity equal to 3111.7 U L−1 min−1. The cultivation condition was performed with agitation condition 200 rpm, at 30 °C and pH, 7.0 for 48 h. A verification experiment was conducted in the optimized medium under predicted optimal conditions to determine the accuracy of the quadratic polynomial. The bench-scale experiments show that experimental laccase activity was 3957 U L−1 min−1. The calculated model accuracy was 127.17%, which revealed the validity of the model. In this study, the mathematical approach, a combination of Plackett–Burman design and the RCCD, has been shown to be effective and reliable in identifying and finding optimum concentrations of the statistically significant variables. Many investigators (Sondhi and Saini 2019; Unuofin et al., 2019a, 2019b) exploited the CCD for the enhancement of the bacterial laccase productivity via using agro-industrial waste. Unuofin et al. (2019b) documented that the numerical optimization of significant factors for laccase production from Bordetella bronchiseptica HSO16 presented an overall maximum laccase output encountered throughout the study (45.22 U/ml). While Unuofin et al. (2019a) stated that about 20.93-fold increase in extracellularly secreted laccase titre from gammaproteobacteria was recorded after application of CCD that confirms RSM as a pivotal experimental tool in valorising maize as an ideal feedstock for laccase production. Moreover, Rajeswari and Bhuvaneswari (2017) recorded that the sequential statistical optimization strategy leads to enhancement of Bacillus sp. PK4 laccase production from 2.13 U/ml to 40.79 U/ml. Maximum laccase yield of 1645 IU g−1 was obtained from Bacillus sp. MSK-01 which is about a 470-fold more increase than those obtained under the unoptimized condition (Sondhi and Saini 2019).
Scaling-up fermentation strategies for Lysinibacillus macroides LSO laccase
Kinetics of cell growth and laccase production in shake flask under batch conditions
To better understand the kinetics of cell growth and laccase production, cell growth and production parameters have been monitored during cultivation periods. It has been clearly observed from Fig. 4a that the laccase production was inversely proportional with the bacterial growth. The laccase production occurred during adaptation (lag) phase as primary metabolites, which is essential for growth on sugarcane bagasse to build the bacterial cells. This finding is analogous to the result reported by Boulton and Large (1977) who found that the N-methylalanine dehydrogenase is secreted by Pseudomonas aminovorans at lag phase.
a Monitoring of Lysinibacillus macroides strain LSO growth and laccase productivity at shake flask scale, b1 and b2 scanning Electron Microscope (SEM) micrograph of Lysinibacillus macroides strain LSO during lag phase and log phase showing the morphology of the cell during the growth while b3 transmission Electron Microscope (TEM) micrograph of Lysinibacillus macroides strain LSO during stationary phase
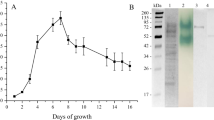
From the obtained results (Fig. 4a), it was noticed that the maximal laccase volumetric production of 4548.7 U L min−1 was achieved at 34 h with the production rate of 23 U L−1 h−1 and with yield coefficient (Yp/s) of 5326 U g−1 (Table 5), accompanied by increases in protein content to reach its maximum value of 1.66 g L−1. The cells are metabolically active during the lag phase and only increase in cell sizes and slowly grow rather than that of no growth; as shown in SEM micrographs (Fig. 4b1), the cell has undergone binary fission in which the cells were elongated and multiplied into several endospore-forming cells without complete cell division. Meanwhile, the total carbohydrate concentration was consumed slowly; however, the sharp elevation in the concentration of total carbohydrate has been observed at 48 h (1.044 g L−1) as a result of the action of laccase on lignin, which opened the route to other enzymes for acting on cellulose and hemicellulose and lead to an increase in total carbohydrate concentration. On the other hand, laccase volumetric production climbed down slowly till the time of 72 h it downfalls rapidly (2567 U L−1) concomitant to the accretion of bacterial growth exponentially over the time with the growth rate 0.0124 (g L−1 h−1), constant specific growth rate, µ of 0.00808 h−1, reaching the maximal cell growth (2.1453 g L−1) at 168 h. The protein content was diminished gradually throughout the time until the end of the incubation period. SEM micrographs (Fig. 4b2) demonstrated that through log phase the number of bacterial cells was increased via division of multiplied cells into a small size endospore-forming cell, resulting in a sharp falloff in the concentration of total carbohydrate, which reached to 0.045 g L−1. At the stationary phase, the proliferation of bacterial biomass was maintained constant, which is accompanied by depletion in yield of laccase, protein content, and total carbohydrate concentration. It was noticed that bacterial cells were converted to spore cells as shown in TEM micrographs (Fig. 4b3), which clarify the morphology of spore cells.
Kinetics of cell growth and laccase production in the bioreactor under batch conditions
Based on the above results, the kinetics of Lysinibacillus macroides strain LSO cell growth and laccase production were monitored in 10-L stirred tank bench-top bioreactor (Cleaver, Saratoga, USA) to further develop and investigate the effect of scaling up the cultivation process under batch cultivation conditions. From the obtained results (Fig. 5b), it can be observed that cell growth and enzyme production profiles run similarly to those obtained in shake flask cultivation. Similarly, to shake flask cultivation, the volumetric productivity of laccase was climbed up to its maximum value of 7653.2 U L−1 at 28 h with the production rate of 175.12 U L−1 h−1 and yield coefficient (Yp/s) of 8524.4 U g−1, accompanied by a notable decline in the concentration of total carbohydrate (0.897 g/l) with a consumption rate of − 0.01901 g L−1 h−1. Thereafter, the laccase yield, as well as protein content, became steady until 39 h.
In this period, the consumption of total carbohydrate is maintained constant with some fluctuation; these fluctuations result from the acting of laccase on lignin portion, which enables other enzymes like cellulase and amylase to work on the hemicellulose and cellulose portions to produce reducing and non-reducing sugar, which is consumed by the organism for cell proliferation. Thus, it can be concluded that bioreactor cultivation resulted in a significant increase in volumetric productivity of laccase in comparison with shake flask cultivation. It is worth mentioning that the concentration of total carbohydrate was gone down pointedly reached to 0.17 g L−1; at the beginning of exponential (log) phase (48 h), concomitant with ascending of bacterial cell growth, it has been seen that cell clearly grew exponentially with a growth rate of 0.0334 (g L−1 h−1) and constant specific growth rate, µ of 0.024 h−1. Under bioreactor cultivation conditions, the maximal cell growth of 2.13 g L−1 was recorded at 88 h of cultivation, which was similar to obtain the shake flask cultivation (2.15 g L−1 at 168 h).
From the data of dissolved oxygen, it can be shown that the percentage of DO was decreased owing to high oxygen consumption during the growth phase as well as cellular activities, reaching a minimal DO% of 0% at 48 h. Afterwards, the percentage of dissolved oxygen began in increment gradually rate from 52 h to reach 100% at 87.5 h. Figure 5a shows the profile of pH throughout the fermentation process. It has been clearly observed that the pH value of the medium at zero time was 7.0; pH value was raised gradually to reach 8.35 after 24 h of cultivation time, which accompanied with a gradual elevation of the laccase production. Subsequently, the pH went down slowly to pH 6 at 48 h, which associated with the descent of the concentration total carbohydrate, the altitude of bacterial cell weight, and fall off laccase productivity. Afterwards, through log and stationary phase the pH profile increased gradually until the end of the fermentation process. The maximal volumetric productivity increased by 168.24% from that obtained in shake flask cultivation (4548.745 U L−1 at 34 h).
Such productivity improvements are attributable to better cultivation of oxygenation and mixing conditions as well as the vessel’s bioreactor capacity. Comparing the obtained results with those cited by the other investigators, through an early study carried out by Hussein et al. (2017), the biomass of Pseudomonas aeruginosa was conjugated with a volumetric productivity of laccase, whereas the maximum value (6214 U/mg protein) of Pseudomonas aeruginosa laccase productivity was achieved when the biomass was reached to its optimal value 4.5 g L−1 after 24 h on the laboratory-scale bioreactor, that finds contrary to the present study. Chauhan et al. (2018) have recently shown that the dependency of pilot-scale production of laccase from Staphylococcus arlettae uses tea waste on bacterial growth pattern, which is contradictory to the results obtained from the current study, while the pH profile during the fermentation showed consistent with that obtained results. Chen et al. (2013) reported that after 36 h of fermentation in a 5-L fermentor, the culture broth of Thermobifida fusca accumulated 4.96 U/ml laccase activity.
Lysinibacillus macroides strain LSO laccase productivity has been systematically improved through Plackett–Burman (1829.99 U L−1 min−1), Rotatable Central Composite Designs (3957 U L−1 min−1), and batch cultivation strategy designs (7653.2 U L−1 min−1) compared with basal medium (108 U L−1 min−1).
Lignin residual content
According to the method described by Templeton and Ehrman (1995) for the determination of residual of lignin (Klason lignin) from biodegradation of sugarcane bagasse, the obtained result referred that the laccase enzyme from Lysinibacillus macroides strain LSO had the capacity to degrade the sugarcane bagasse to about 7% under batch fermentation conditions. The lignin content for raw sugarcane bagasse was approximately 25.5%, whilst the estimation lignin content after bio-ligninolysis process via Lysinibacillus macroides strain LSO was 18.5%. This study is the first report to show that the production of extracellular Lysinibacillus macroides laccase in lag phase was up-scaled using bio-waste raw material.
Conclusion
It is of primary importance to explore bacterial species with novel ligninolytic abilities. Therefore, the current study was aimed to isolate the most potent extracellular laccase-producing bacteria from different discharged sites of Alexandria paper and pulp industries, increase efficiency laccase productivity by a local isolate Lysinibacillus macroides strain LSO using low-cost carbon sources, such as the sugarcane bagasse on large-scale cultivation through a sequential optimization strategy. The data obtained revealed that the selected isolate is designated as Lysinibacillus macroides LSO (MN216323) used as a laccase producer model. The sugarcane bagasse, NH4Cl, CuSO4·5H2O, and MnSO4·H2O were chosen among the examined variables dependent on their high positive impact on the laccase efficiency through 2-level Plackett–Burman design. A near-optimum medium formulation was obtained that the laccase yield increased by more than 16.94 times comparing with the initial basal medium. RCCD was used to develop a quadratic polynomial model that correlates the relationship between all four variables and laccase productivity. The predicted optimum laccase activity was 3957 U L−1 min−1, which was 36.6 times the activity with the unoptimized medium. Subsequently, the monitoring of experimental bacterium growth and laccase productivity was carried out under shake flask and submerged batch fermentation using 10-L bench-top bioreactor. The independency of enzyme production on bacterial growth was observed, where the laccase productivity reached maximum titre (7653 U L−1 min−1) at lag phase, which is 70.8 times more than that obtained by the unoptimized basal medium.
References
Abdel-Fattah R, Hesham S, Gohar Y, El-Baz A (2005) Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem 40:1707–1714. https://doi.org/10.1016/j.procbio.20-04.06.048
Abdel-Fattah YR, El-Enshasy HA, Soliman NA, El-Gendi H (2009) Bioprocess development for production of alkaline protease by Bacillus pseudofirmus Mn6 through statistical experimental designs. J Microbiol Biotechnol 19(4):378–386
Abdelgalil AS, Morsi A, Reyed M, Soliman A (2018) Application of experimental designs for optimization the production of Alcaligenes faecalis nyso laccase. J Sci Ind Res 77:713–722
Al Fattah Amara A (2011) Experimental design for simultaneous production of PHB, mesophilic proteases and lipases. IIUM Eng J 12:155–184. https://doi.org/10.31436/iium-ej.v1-2i2.137
Arora S, Gill P (2000) Laccase production by some white-rot fungi under different nutritional conditions. Bioresour Technol 73:283–285. https://doi.org/10.1016/S0960-8524(99)00141-8
Boulton A, Large J (1977) Synthesis of certain assimilatory and dissimilatory enzymes during bacterial adaptation to growth on trimethylamine. Microbiology 101:151–156. https://doi.org/10.1099/00221287-101-1-151
Box E, Behnken W (1960) Some new three-level designs for the study of quantitative variables. Technometrics 2:455–475. https://doi.org/10.1080/00401706.1960.10489912
Chauhan S, Goradia B, Saxena A (2017) Bacterial laccase: recent update on production, properties and industrial applications. 3 Biotech 7:323–343. https://doi.org/10.1007/-s13205-017-0955-7
Chauhan S, Goradia B, Jha B (2018) Optimization and up-scaling of ionic liquid tolerant and thermo-alkali stable laccase from a marine Staphylococcus arlettae S1–20 using tea waste. J Taiwan Inst Chem E 86:1–8. https://doi.org/10.1016/j.jtice.2018.02.032
Chen Y, Huang C, Wei M, Meng M, Liu H, Yang H (2013) Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxidation. AMB Express 3:49–58. https://doi.org/10.1186/2191-0855-3-49
Chhaya R, Modi A (2013) Statistical optimization of laccase producing Streptomyces chartreusis by solid-state fermentation. CIBTech J Microbiol 3:8–17
El-Sersy A, Ebrahim A, Abou-Elela M (2010) Response surface methodology as a tool for optimizing the production of antimicrobial agents from Bacillus licheniformis SN 2. Curr Res Bacteriol 3:1–14. https://doi.org/10.3923/crb.2010.1.14
Ferreira F, Dall’Antonia B, Shiga A, Alvim J, Pessoni B (2018) Sugarcane bagasse as a source of carbon for enzyme production by filamentous fungi. Hoehnea 45:134–142. https://doi.org/10.1590/2236-8906-40/2017
Hussein I, Haider H, Aziz M, Hussein A (2017) Determination the optimum conditions of laccase production by a local isolate of Pseudomonas aeruginosa SR3 using lab-scale fermenter. Int J Sci Nat 8:1–10
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev-/msw054
Lowry H, Rosebrough J, Farr L, Randall J (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Marahatta B, Punyarit P, Bhudisawasdi V, Paupairoj A, Wongkham S, Petmitr S (2006) Polymorphism of glutathione S-transferase omega gene and risk of cancer. Cancer Lett 236:276–281. https://doi.org/10.1016/j.canlet.2005.05.020
Mongkolthanaruk W, Tongbopit S, Bhoonobtong A (2012) Independent behaviour of bacterial laccases to inducers and metal ions during production and activity. Afri J Biotechnol 11:9391–9398. https://doi.org/10.5897/AJB11.3042
Morales-Álvarez ED et al (2017) Plackett-Burman Design for rGILCC1 laccase activity enhancement in Pichia pastoris: concentrated enzyme kinetic characterization. Enz Res. https://doi.org/10.1155/2017/5947581
Morris L (1948) Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science (Washington) 107:254–255. https://doi.org/10.1126/science.107.2775-.254
Nakhla D, Haggar S (2014) A proposal to environmentally balanced sugarcane industry in Egypt. Int J Agric Pol Res 2:321–328. https://doi.org/10.15739/IJAPR.003
Neelkant S, Shankar K, Jayalakshmi K, Sreeramulu K (2019) Optimization of conditions for the production of lignocellulolytic enzymes by Sphingobacterium sp. ksn-11 utilizing agro-wastes under submerged condition. Prep Biochem Biotech 49:927–934. https://doi.org/10.1080/10826068.2019.1643735
Pandey A, Soccol C, Nigam P, Soccol V, Vandenberghe L, Mohan R (2000) Biotechnological potential of agro-industrial residues. II: cassava bagasse. Bioresour Technol 74:81–87. https://doi.org/10.1016/S0960-8524(99)00143-1
Piccinno F, Hischier R, Seeger S, Som C (2016) From laboratory to industrial scale: a scale-up framework for chemical processes in life cycle assessment studies. J Clean Prod 135:1085–1097. https://doi.org/10.1016/j.jclepro.2016.06.164
Plackett L, Burman P (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Rajeswari M, Bhuvaneswari V (2016) Production of extracellular laccase from the newly isolated Bacillus sp. PK4. Afr J Biotechnol 15:1813–1826. https://doi.org/10.5897/AJB-2016-.15509
Rajeswari M, Bhuvaneswari V (2017) Optimization of laccase production from Bacillus sp. PK4 through statistical design of experiments. Microbiol Biotechnol Lett 45:330–342. https://doi.org/10.4014/mbl.1709.09003
Reksohadiwinoto BS (2019) Novelties of laccase enzyme from bacteria of sago waste. IOP Conference Series: Earth and Environmental Science 406:012013
Sondhi S, Saini K (2019) Response surface-based optimization of laccase production from Bacillus sp. MSK-01 using fruit juice waste as an effective substrate. Heliyon 5:1–8. https://doi.org/10.1016/j.heliyon.2019.e01718
Sondhi S, Sharma P, George N, Chauhan S, Puri N, Gupta N (2015) An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to bio-bleach softwood pulp. 3 Biotech 5:175–185. https://doi.org/10.1007/s13205-014-0207-z
Templeton D, Ehrman T (1995) Determination of acid-insoluble lignin in biomass. In: Laboratory Analytical Procedures No. 003, National Renewable Energy Laboratory, Golden, Co.
Unuofin O, Okoh I, Nwodo U (2019a) Maize Stover as a feedstock for enhanced laccase production by two gammaproteobacteria: a solution to agro-industrial waste stockpiling. Ind Crop Prod 129:611–623. https://doi.org/10.1016/j.indcrop.2018.12.043
Unuofin O, Okoh I, Nwodo U (2019b) Utilization of agro-industrial wastes for the production of laccase by Achromobacter xylosoxidans HWN16 and Bordetella bronchiseptica HSO16. J Environ Manage 231:222–231. https://doi.org/10.1016/j.jenvm-an.2018.10.016
Zhu D, Nian L, Rongxian Z, Fiaz A, Weimin Z, Bin Y, Jian W, Alei G, Murillo G, Jianzhong S (2020) Insight into depolymerization mechanism of bacterial Laccase for lignin. ACS Sustain Chem Eng 8:12920–12933. https://doi.org/10.1021/acssuschemeng-.0c03457
Acknowledgements
The authors gratefully acknowledge the A City of Scientific Research and Technological Applications (SRTA-City), Alexandria, 21934, Egypt, for providing financial support for laboratory measurements and analyses of this paper within the framework of SRTA-City Central Laboratories Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
About this article
Cite this article
Abdelgalil, S.A., Soliman, N.A., Abo-Zaid, G.A. et al. Bioprocessing strategies for cost-effective large-scale production of bacterial laccase from Lysinibacillus macroides LSO using bio-waste. Int. J. Environ. Sci. Technol. 19, 1633–1652 (2022). https://doi.org/10.1007/s13762-021-03231-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03231-3