Abstract
In this work, nanoparticles zerovalent iron (NFe°) and silty clay supported nano zerovalent iron (SC–NFe°) were utilized as a granular third electrode (3D) in the electrochemical technique. The two electrodes (2D) with aluminum plates as anode and cathode) and 3D electrochemical cells were utilized to remove aqueous phenol. For 2D electrochemical system, the optimal operating conditions were as follows: time = 0.5 h, pH = 4, current density = 50 mA/cm2, distance between electrodes plates = 4 cm, and phenol concentration = 500 mg/L, and the highest removal rate of phenol was 82%. The results demonstrated the considerable efficiency of the 3D electrochemical process (with NFe° and SC–NFe° as a granular third electrode) in treating phenolic wastewater. For 3D electrochemical process, the optimal operating conditions were as follows: pH = 2, operation time = 0.5 h, current density = 50 mA/cm2, electrode distance = 4 cm, and phenol concentration = 500 mg/L. The highest removal rates of phenol in 3D electrochemical technique with NFe° or SC–NFe° were 96.1 and 97.8%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol and its compounds exist in the wastewater of various industries, including petroleum refineries, petrochemical industries, coking operations, pharmaceutical production, and phenolic resins industries (Laura et al. 2016). According to the US Environmental Protection Agency (EPA) and the National Pollutant Release Inventory (NPRI) of Canada, phenol is classified as a priority pollutant (Albayati and Doyle 2014; Albayati et al. 2019). People exposed to phenol acutely or chronically may suffer adverse health consequences, which may be quite serious depending on the degree of exposure (Mohammadi et al. (2014)). Polluted phenolic wastewater were treated using various processes such as chemical oxidation (Yavuz and Koparal 2006; Wang and Gu 2009), biological degradation (Shourian et al. 2009; EL-Naas et al. 2010), membranes (Albayati 2019), photocatalysts (Busca et al. 2008), solvent extraction (Agrios et al. 2003), ultrasonic technique (Yang et al. 2006), enzymatic polymerization (Pandit et al. 2001), Fenton-like reactions (Buchanan and Micell 1997), adsorption (Kumar et al. 2011; Valente Nabais et al. 2009), and electrochemical oxidation (Britto-Costa and Ruotolo 2012; Pillai and Gupta 2015).
In general, an electro-oxidation system includes an oxidation process at the anode and a reduction process at the cathode (redox reaction). Additionally, the electro-oxidation processes used for removing organic materials from wastewater depend upon the potential of gaining partial degradation or full mineralization by using anodic oxidation (Garcia-Segura et al. 2018). Electrooxidation processes can be divided into direct (anodic oxidation) and indirect (using intermediary redox reagents) pathways (Brillas and Martínez-Huitle 2015). In recent years, the green synthesis of nano zerovalent iron (NFe°) and its potential as an adsorbent have gained considerable attention. This interest is due to their natural, eco-friendly, low cost, and efficiency as an adsorbent. Also, there is a great interest in nanomaterial synthesis from biowaste and use it in wastewater remediation (Faisal et al. 2020). The high conductivity, high surface energy, and large specific surface area of nano zerovalent iron make it a useful alternative as a granulated electrode in wastewater treatment. Regrettably, iron nanoparticles subject to magnetic attraction and van der Waals forces which cause aggregation and oxidation (which decrease their efficiency) unless it modified (Yang et al. 2015). Also, employing bare NFe° in the wastewater treatment process will likely cause a rapid loss of nano zerovalent iron as well as a high concentration of iron ions (Zhang et al. 2014). So, supporting NFe° by applying it on a material that can decrease iron leaching and elongate the lifespan of the NFe° became an important process (Bang et al. 2005).
Recently, the electrochemical technique has gained considerable attention in wastewater remediation as a result of its quality, simplicity, and eco-friendly character in the removal of persistent organic contaminants (Yang and Tang 2018). However, the conventional electrochemical technique is considered unworkable because of its low current density. Therefore, the addition of a granular electrode to the traditional electrochemical unit is deemed an excellent improvement to increase the activity and raise the current density of the electrochemical technique (Yan et al. 2011). The space between the granular electrodes in the electrochemical process that employed granular electrodes (3-D) systems was shorter, which allowed for more rapid electron transfer and treatment (Yang and Tang 2018). For these reasons, many researchers have been reporting excellent results using a 3-D electrochemical technique as a tertiary process in wastewater treatment (Canizares et al. 2004; Singh et al. 2019; Meng et al. 2020).
In this study, the synthesis and characterization of the bare nanoparticles zerovalent iron (NFe°) and the supported with silty clay (SC–NFe°) are reported. Due to their large specific surface area, both silty clays supported and unsupported NFe° have proved to be highly reactive and efficient adsorbents, which makes them both excellent candidates for use as a third electrode. Furthermore, the efficiency of NFe° and SC–NFe° as granular third electrodes for the removal of aqueous phenol was examined. Various significant operating conditions were optimized, such as pH, electrolysis time, current density, electrode plate distance, granular electrode dose, and phenol concentration.
Materials and Methods
Materials
The green tea (biomaterial waste) that used in synthesis of NFe° and SC–NFe° was the commercial type Ahmed brand. The chemicals that have been used consist of phenol crystal (C6H5OH) with 99.5% purity, ferric chloride anhydrous (FeCl3), sodium sulfate (Na2SO4), 0.01 M NaOH, 0.01 M H2SO4, and deionized H2O. These chemicals were bought from India (Thomas Baker Chemicals). The origin of the natural clay utilized in this work was Mosul, (Smehlla 36º 31′ 25 N, 43º 53′ 52E), in the north of Iraq.
Synthesis of NFe° and SC–NFe°
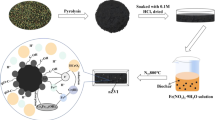
In this study, NFe° and SC–NFe° were synthesized according to the method used in our previous study (Kadhum et al. 2020). The synthesis methods are demonstrated in Fig. 1.
Characterization of NFe° and SC–NFe°
The characterization of the prepared adsorbents NFe° and SC–NFe° was achieved using the following: X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), zeta potential (ζ), and Brunauer–Emmett–Teller (BET). A detailed characterization of NFe° and SC–NFe° is given in our previous study (Kadhum et al. 2020).
Electrolytic cell
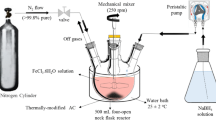
The electrolytic cell used in this work is illustrated in Fig. 2. A reaction tank was made of a glass box, with dimensions 10 × 6 × 5 cm. The two parallel aluminum plates (2.5 × 3 × 0.1 cm) were used as anode and cathode electrodes. This study also used a 60 V 5A DC power supply (Maisheng MS-605D) and magnetic stirrer.
Batch experiments
The efficiency of the NFe° and SC–NFe° as granulated electrodes in an electrochemical reactor to remove an aqueous phenol was evaluated by two groups of batch experiments. The first group researched the electrochemical processes without any modification. The second group studied the electrochemical processes in the presence of a granulated electrode (i.e., NFe° or SC–NFe°). All experiments were performed with 250 ml of deionized water and 0.25 g/l of the electrolyte (Na2SO4). Experimental solutions were made by dissolving a specific weight of phenol in deionized water. The pH values were regulated utilizing 0.1 N NaOH and 0.1 N H2SO4 solutions. For the second set of experiments, certain weights of NFe° or SC–NFe° were added to the synthesized solution of the certain concentration of phenol (C6H5OH) along with (Na2SO4) and deionized water. A magnetic stirrer with 250 rpm was used for solution agitation. Samples were filtered using a 0.45-µm syringe filter. A calibration curve of the phenol was made by UV 9200 Biotech Engineering as shown in Fig. S1, with a λmax. of 268 nm and Fig. S2. Depending on the absorbance (measured by UV spectroscopy) and calibration curve, the concentration of phenol was determined. The removal rate of phenol was computed using Eq. (1).
where C0 and Ct are the concentration of phenol (mg/L) at times zero and t, respectively.
Results and discussion
Characterization
The SEM images demonstrated that the manufactured NFe° particles seemed as cubic, and a lot of them were about 100 nm in diameter Fig. S3. Also, SC–NFe° appeared with a high degree of dispersion. XRD spectra of NFe° and SC–NFe° proved the existence of the organics Fe2O3, Fe3O4, and Fe° (Fig. S4). The source of these organic materials, which act as capping/stabilizing agent, is the green tea waste extract. For NFe° and SC–NFe°, FTIR data revealed the existence of functional polyphenols Fig. S5. Zeta potential results proved that the stability of SC–NFe° is higher than that for NFe° Fig. S6. Also, the high specific surface area of SC–NFe° was detected by BET analysis Table S1.
Electrochemical experiments
Effect of pH
The initial pH value has a considerable influence on the removal rate of phenol (Abdelwahab et al. 2009). The removal rates of phenol were determined in the pH range from 2 to 6 and at an initial phenol concentration of 500 mg/L and 250 mg/L (Na2SO4), temperature of 45 °C, current density (CD) of 40 mA/cm2, and plate distance of 4 cm. Figure 3 demonstrates the influence of the phenolic solution pH on the efficiency of phenol removal. The removal rates of phenol had their lower value at both low and high pH values, while the highest removal rate was at a pH of 4. These results occurred because at the lower pH value, Al (OH)3 does not precipitate due to its amphoteric property (Adhoum and Monser 2004). Additionally, at the higher pH value, Al (OH)4 forms, and this compound is ineffective for phenol adsorption (Zhu et al. 2007).
Effect of electrolysis time
The impact of the electrolysis time on the removal rate of phenol was investigated over 1.5 h and at an initial phenol concentration of 500 mg/L and 250 mg/L (Na2SO4), temperature of 45 °C, pH of 4, CD of 40 mA/cm2, and plate distance of 4 cm as initial conditions, as illustrated in Fig. 4. The removal rates of phenol increased with the elongation of the electrolysis time until 0.5 h. However, there was no significant rise in the removal efficiency of phenol after this time. After a specific time within the electrochemical process, the reduction in the concentration of pollutants leads to a decrease in the chance of contact with the electrode, which reduces the removal rate. Another potential cause is the formation of intermediate recalcitrant compounds due to the insufficient oxidation of organics (Chi et al. 2018; Sharma et al. 2019).
Effect of current density
The experiments were conducted with a current density (CD) from 20 to 60 mA/cm2 and with an initial phenol concentration of 500 mg/L and 250 mg/L Na2SO4, temperature of 45 °C, pH of 4, electrolysis time of 0.5 h, and plate distance of 4 cm. As displayed in Fig. 5, the removal rate of phenol increased with the increase in CD. The growing in CD value meant an increase in the transmission of electrons, which promoted the redox reactions of the pollutants on the electrodes (Faisal et al. 2020). However, when the CD surpassed 40 mA/cm2, the increase in the removal rate of phenol was slight and insignificant. These results occurred because the increase in CD generated additional oxygen and hydrogen bubbles, which limited the contact of the pollutants with the electrodes (Wang et al. 2014). For maximal energy savings and an appropriate removal rate of phenol, the optimum current density was 50 mA/cm2. The consumption of electrical energy is represented in the following:
where EC is the energy consumption (kWh/m3), V is the voltage (V), I is the applied current (A), t is the electrolysis time (min), and v is the volume of wastewater (m3). For a CD of 50 mA/cm2 and an electrolysis time of 30 min, the energy consumption was 6.34 KW.h/m3.
Influence of electrode plate distance
The effect of the electrode plate distance was investigated using a 3–6-cm plate distance, phenol concentration = 500 mg/L, Na2SO4 = 250 mg/L, temperature = 45 °C, pH = 4, CD = 50 mA/cm2, and electrolysis time = 0.5 h, as illustrated in Fig. 6. The phenol removal rate increased when the distance between the plates was less than 4 cm. When the electrode distance exceeded 4 cm, the removal rate of phenol decreased slightly. This decrease was perhaps due to the decline in the formation of aluminum cations, which resulted from the increment in the ohmic potential. Hence, there was a decline in the effectiveness of the electrochemical technique (Sahu 2019). The maximum phenol removal rate was 82% at the optimum electrode distance of 4 cm.
Third granulated electrode experiments
pH effect
The phenolic solution pH effect on the removal efficiency of phenol in the electrochemical cell in the presence of NFe° and SC–NFe° as granulated electrodes was investigated at a plate distance = 4 cm, CD = 50 mA/cm2, phenol concentration = 500 mg/L, Na2SO4 = 250 mg/L, NZVI or SC–NZVI = 1 g/L, temperature = 45 °C, and operating time = 0.5 h. The pH was adjusted from 2 to 5, as shown in Fig. 7. The maximum removal rates of phenol decreased at a lower pH value (i.e., 2.5) and with the increment of pH for both NFe° and SC–NFe°. During the electrolysis process, H2O2 was produced by the reduction of oxygen at a low pH. An indirect Fenton reaction will occur between NFe°and hydrogen peroxide, producing OH radicals (Thi et al. 2015). In addition, a reduction of Fe3+ to Fe2+ on the cathode electrode will occur. The OH will combine with the organic pollutants, thereby degrading them. At a very low pH (i.e., < 2), the removal rate of phenol will decline because the saturated hydrogen ions will supply a proton for hydrogen peroxide to form hydroxonium ions (Eq. 3), thus decreasing the activity of the hydrogen peroxide (Faisal et al. 2020). Furthermore, the strong scavenging action of H + to OH (Eq. 4) will be evident (Shemer and Linden 2006). Thus, the optimum pH value is 2 for NFe° and SC–NFe°.
Impact of granular electrode dose
The impact of the granular electrode dose was investigated using 0.75–2 g/L of NFe° or SC–NFe°, plate distance = 4 cm, CD = 50 mA/cm2, phenol concentration = 500 mg/L, Na2SO4 = 250 mg/L, pH = 2, temperature = 45 °C, operating time = 0.5 h, along with a magnetic stirrer. As illustrated in Fig. 8, the removal efficiency increased when the dose of NFe° was less than 1 g/L and the dose of SC–NFe° was below 1.25 g/L. However, a declined line took place when the doses were greater. The growing in NFe° and SC–NFe° doses led to an increase in the contact chance between the granular electrodes and the phenol molecule, which enhanced the adsorption and reduction of the latter. In addition, this increase in granular electrode doses can support a Fenton reaction. The possible cause of this phenomena is the aggregation of the granular electrodes due to the increase in their doses and that minimize the specific surface area and elongate diffusion pathway of phenols molecules. The second probable cause is the high doses of granulated electrodes which lead to unsaturated adsorption sites and oxidation of the excessive doses of NFe° or SC–NFe° to Fe2O3 and Fe3O4. So, when the granulated electrodes (NFe° and SC–NFe°) reached specific values, the equilibrium adsorption and reduction capacity is decreased (Basha Allabaksh et al. 2010). The maximum phenol removal rates using NFe° and SC–NFe° were 96.1% and 97.8%, respectively.
Phenol concentration effect
The initial phenol concentration effect was studied utilizing phenol concentrations ranging from 500 to 1500 mg/L. The electrolysis process was conducted with a magnetic stirrer under the following conditions: plate distance = 4 cm, NFe° = 1 g/L, SC–NFe° = 1.25 g/L, Na2SO4 = 250 mg/L, pH = 2, temperature = 45 °C, and operating time = 0.5 h. As illustrated in Fig. 9, the removal rate of phenol decreased gradually as the phenol concentration increased. A possible explanation for this result is that the aluminum oxides that formed during the electrolysis process were insufficient to oxidize all of the additional amounts of the phenol molecules (Abdelwahab et al. 2009).
Perfect experimental conditions
The perfect experimental conditions for different studied variables were gained for highest removal rate of phenol in two cases, as shown in Fig. 10. First, when using only the electrolysis process, where pH = 4, electrolysis time = 0.5 h, CD = 50 mA/cm2, and distance between electrodes plates = 4 cm, the removal rate of phenol was 82%. Second, when using the electrolysis process in the presence of granular electrodes (i.e., NFe° or SC–NFe°), the perfect operating conditions were pH = (2), operating time = 0.5 h, CD = 50 mA/cm2, distance between electrodes plates = 4 cm, granular electrode doses (NFe° = 1 g/L and SC–NFe° = 1.25 g/L), and phenol = 500 mg/L. The second case provided removal rates of 96.1 and 97.8% using NFe° and SC–NFe°, respectively.
Three-dimensional electrochemical mechanism
The three-dimensional electrochemical process for organic materials removal is dependent on several parameters, such as the electrode material, current density, properties and concentrations of the pollutant, granular electrode type, and pH value. Figure 11 demonstrates a generalized mechanism of the processes (i.e., electrolysis, adsorption, and reduction) that occur in a hybrid system and that assist NFe° and SC–NFe° as granular electrodes in an electrochemical reactor for phenol removal.
Due to the low pH value, the possible reason for phenol degradation was a Fenton-like reaction. The addition of a granular third electrode (i.e., NFe° or SC–NFe°) of a high specific surface area provides an excellent adsorbent for the organic pollutant (i.e., phenol). In addition to adsorption, NFe° or SC–NFe° will reduce the organic pollutant, and mineralization of organics to CO2 and H2O will occur.
Oxidation of NFe° or SC–NFe° by O2 and the formation of Fe2+ will take place (Eq. 5) (Shimizu et al. 2012). At strong acidic conditions, a Fenton reaction between either NFe° or SC–NFe° and hydrogen peroxide will occur. Generation of H2O2 due to the reduction of O2 by H+ will take place near the cathode (Eq. 6). Therefore, as a result of the Fenton reaction of H2O2 with Fe2+, an abundance of •OH with a high oxidation capability is formed (Eq. 7) (Brillas et al. 2009). Also, the immediate reduction of the generated Fe3+ to Fe2+ creates a cycle of iron ion transformations that decrease NFe° or SC–NFe° exhaustion and oxidize organic pollutants effectively (Chi et al. 2018).
Comparative study
A comparative study between this research and the literature can be done by assessing the derived results of the removal rate of phenol using different granular electrodes in hybrid 3-D electrochemical systems, as shown in Table 1. This table illustrates that using nanoparticle zerovalent iron manufactured from adjusted green tea biowaste and supported on silty clay provides the highest removal rate of aqueous phenol as well as providing the benefit of using eco-friendly materials as a granular third electrode.
Conclusion
In this study, synthesized NFe° and SC–NFe° were successfully used as granular electrodes in an electrochemical reactor. This work revealed that the electrochemical treatment of aqueous phenol using aluminum as the anode and cathode electrodes is an effective method, which produces a maximum phenol removal rate of 82%. The following perfect conditions were determined: pH = 2, operating time = 0.5 h, current density = 50 mA/cm2, distance between electrodes plates = 4 cm, and phenol concentration = 500 mg/L. It was proved that either NFe° or SC–NFe° improve the treatment process when they are employed as efficient and effective granular electrodes in hybrid systems. In the hybrid system, the removal rate of phenol increased to 96.1 and 97.8% using NFe° and SC–NFe°, respectively. This study demonstrated that a 3-D electrode process was an effective technique for the remediation of phenolic wastewater in an electrochemical reactor.
References
Abdelwahab O, Amin NK, El-Ashtoukhy E-SZ (2009) Electrochemical removal of phenol from oil refinery wastewater. J Hazard Mater 163:711–716
Adhoum N, Monser L (2004) Decolorization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem Eng Process 43:1281–1287
Agrios A, Gray K, Weitz E (2003) Photocatalytic transformation of 2,4,5- trichlorophenol on TiO2 under sub-band-gap illumination. Langmuir 19:1402–1409
Albayati TM (2019) Application of nanoporous material MCM-41 in a membrane adsorption reactor (MAR) as a hybrid process for removal of methyl orange. Desalin Water Treat 151:138–144
Albayati TM, Doyle AM (2014) Purification of aniline and nitrossubistituted aniline contaminants from aqueous solution using beta zeolite. Chem Bulgar J Sci Educ 23(1):105–114
Albayati TM, Sabri AA, Abed DB (2019) Adsorption of binary and multi heavy metals ions from aqueous solution by amine functionalized SBA-15 mesoporous adsorbent in a batch system. Desalin Water Treat 151:315–321
Bang S, Korfiatis GP, Meng X (2005) Removal of arsenic from water by zero-valent iron. J Hazard Mater 121:61–67
Basha Allabaksh M, Kumar Mandal B, Kumar Kesarla M, Siva Kumar K, Sreedhara Reddy P (2010) Preparation of stable zero valent iron nanoparticles using different chelating agents. J Chem Pharm Res 2(5):67–74
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ 166–167:603–643
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631
Britto-Costa PH, Ruotolo LAM (2012) Phenol removal from wastewater by electrochemical oxidation using boron doped diamond (BDD) and Ti/Ti07Ru03O2 DSA electrodes. Braz J Chem Eng 29(4):763–773
Buchanan ID, Micell JA (1997) Peroxidase catalyzed removal of aqueous phenol. Biotechnol Bioeng 54:251–261
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams, a short review of recent developments. J Hazard Mater 160:265–288
Canizares P, Lobato J, Garcia-Gomez J, Rodrigo MA (2004) Combined adsorption and electrochemical processes for the treatment of acidic aqueous phenol wastes. J Appl Electrochem 34:111–117
Chi Z, Wang Z, Liu Y, Yang G (2018) Preparation of organosolv lignin-stabilized nano zero-valent iron and its application as granular electrode in the tertiary treatment of pulp and paper wastewater. Chem Eng J 331:317–325
El-Naas MH, Al-Zuhair S, Makhlouf S (2010) Batch degradation of phenol in a spouted bed bioreactor system. J Ind Eng Chem 16:267–272
Faisal A, Al-Wakel S, Naji L, Naushad M (2020) Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J Water Process Eng 33:101112
Garcia-Segura S, Ocon D, Meng Nan C (2018) Electrochemical oxidation remediation of real wastewater effluents - a review. Process Saf nd Environ Protect 113:48–67
Kadhum ST, Alkindi GY, Albayati TM (2020) Eco friendly adsorbents for removal of phenol from aqueous solution employing nanoparticle zero-valent iron synthesized from modified green tea bio-waste and supported on silty clay. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2020.07.031
Kumar PS, Ramakrishnan K, Kirupha SD, Sivanesan S (2011) Thermodynamic, kinetic, and equilibrium studies on phenol removal by use of cashew nut shell. Can J Chem Eng 89(2):284–291
Laura G, Villegas C, Mashhadi N, Chen M, Mukherjee D, Taylor KE, Biswas N (2016) A short review of techniques for phenol removal from wastewater. Curr Pollution Rep 2:157–167
Meng Y, Fangrong Y, Yige C, Jujie L (2020) and Li Ziyan, A three-dimensional electrochemical oxidation system with a-Fe2O3/PAC as the particle electrode for ammonium nitrogen wastewater treatment. R Soc Chem 10:8773–8779
Mohammadi SH, Kargari A, Sanaeepur H, Abbassian K, Najafi A, Mofarrah E (2014) Phenol removal from industrial wastewaters: a short review. Desalin Water Treat 1:20
Pandit AB, Gogate PR, Mujumdar S (2001) Ultrasonic degradation of 2:4:6 trichlorophenol in presence of TiO2 catalyst. Ultrason Sonochem 8:227–231
Pillai IM, Gupta AK (2015) Batch and continuous flow anodic oxidation of 2,4-dinitrophenol: modeling, degradation pathway and toxicity. J Electroanal Chem 75:108–117
Ping L, Wangfeng C, Yue X, Wang Y, Jiangyang F (2017) Electrochemical degradation of phenol wastewater by Sn-Sb-Ce modified granular activated carbon. Int J Electrochem Sci 12:2777–2790
Qiao N, Ma H, Hu M (2015) Design of a neutral three-dimensional electro-Fenton system with various bentonite-based Fe particle electrodes: a comparative study. Mater Res Innovations 19:137–141
Sahu O (2019) Electro-oxidation and chemical oxidation treatment of sugar industry wastewater with ferrous material: an investigation of physicochemical characteristic of sludge. S Afr J Chem Eng 28:26–38
Sharma G, Kumar A, Naushad M, Sharma S, Ghfar A, Ahamad T, Si C, Stadler F (2019) Graphene oxide supported La/Co/Ni trimetallic nano-scale systems for photocatalytic remediation of 2-chlorophenol. J Mol Liq 249:111605
Shemer H, Linden KG (2006) Degradation and by-product formation of diazinon in water during UV and UV/H2O2 treatment. J Hazard Mater B 136:553–559
Shimizu A, Tokumura M, Nakajima K, Kawase Y (2012) Phenol removal using zerovalent iron powder in the presence of dissolved oxygen: roles of decomposition by the Fenton reaction and adsorption/precipitation. J Hazard Mater 201–202:60–67
Shourian M, Noghabi K, Zahiri H, Bagheri T, Karaballaei G, Mollaei M, Rad I, Ahadi S, Raheb J, Abbasi H (2009) Efficient phenol degradation by a newly characterized Pseudomonas sp. SA01isolated from pharmaceutical wastewaters. Desalination 246:577–594
Singh S, Mahesh S, Sahana M (2019) Three-dimensional batch electrochemical coagulation (ECC) of health care facility wastewater—clean water reclamation. Environ Sci Pollut Res 26:12813–12827
Song N, Zhaoxi Z (2011) Experimental study on the oxidation degradation of phenolic compound from refinery wastewater by three-dimensional electrode. Adv Fine Petrochem 12(5):28–31
Thi XHL, Bechelany M, Champavert J, Cretin M (2015) A highly active based graphene cathode for the electro-Fenton reaction. RSC Adv 5:42536–42539
Valente Nabais JM, Gomes Suhas JA, Carrott PJM, Laginhas C, Roman S (2009) Phenol removal onto novel activated carbons made from lignocellulosic precursors: influence of surface properties. J Hazard Mater 167:904–910
Wang Y, Gu B (2009) Electro-catalytic degradation of phenol on several metal-oxide anodes. J Hazard Mater 162:1159–1164
Wang C, Huang YK, Zhao Q, Ji M (2014) Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment and disinfection effects. Chem Eng J 243:1–6
Xia Y, Yafeng L, Lin C (2010) Experimental study on the oxidation degradation of phenol wastewater by three-dimensional electrode. Ind Water Treat 30(5):27–29
Yan L, Ma H, Wang B, Wang Y, Chen Y (2011) Electrochemical treatment of petroleum refinery wastewater with three-dimensional multi-phase electrode. Desalination 276:397–400
Yang B, Tang J (2018) Electrochemical oxidation treatment of wastewater using activated carbon electrode. Int J Electrochem Sci 13:1096–1104
Yang C, Qian Y, Zhang L, Feng J (2006) Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem Eng J 117:179–185
Yang Z, Qiu X, Fang Z, Pokeung T (2015) Transport of nano zero-valent iron supported by mesoporous silica microspheres in porous media. Water Sci Technol 17(12):1800–1805
Yavuz Y, Koparal S (2006) Electrochemical oxidation of phenol in a parallel plate reaction using ruthenium mixed metal oxide electrode. J Hazard Mater B 136:292–302
Zhang C, Zhou L, Yang J, Yu X, Jiang Y, Zhou M (2014) Nanoscale zero-valent iron/AC as heterogeneous Fenton catalysts in three-dimensional electrode system. Environ Sci Pollut Res 21:8398–8405
Zhigang L, Wei S, Yansheng L, Shaomin Z (2011) Continuous electrochemical oxidation of phenol using a three-dimensional electrode reactor. Appl Mech Mater 71–78:2169–2172
Zhu J, Zhao H, Ni J (2007) Fluoride distribution in electrocoagulation defluoridation process. Sep Purif Technol 56:184–191
Acknowledgments
The authors gratefully acknowledge the scientific support and help of the Civil Engineering Department and Chemical Engineering Department, University of Technology, Baghdad, Iraq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
Authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Gaurav Sharma.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kadhum, S.T., Alkindi, G.Y. & Albayati, T.M. Remediation of phenolic wastewater implementing nano zerovalent iron as a granular third electrode in an electrochemical reactor. Int. J. Environ. Sci. Technol. 19, 1383–1392 (2022). https://doi.org/10.1007/s13762-021-03205-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03205-5