Abstract
The aim of this experiment was to investigate the exogenous application of salicylic acid (SA) on morpho-physiological and molecular characteristics of Impatiens walleriana plants grown under water deficit stress. Three levels of soil water contents (95, 85, and 75% of field capacity; FC) and three levels of SA (0, 1, and 2 mM) were applied on two impatient cultivars (‘Tempo’ and ‘Salmon’). The results showed that increasing water deficit stress negatively affected growth and flowering characteristics. On the contrary, the foliar application of SA reduced the adverse effect of water deficit stress and improved growth and ornamental plant attributes. Water deficit increased the amount of electrolyte leakage (EL), malondialdehyde (MDA), peroxidase (POD) and ascorbate peroxidase (APX) activities; and proline content. The expression of the gene encoding for Δ1-pyrroline-5-carboxylate synthetase (P5CS) was slightly increased under control treatment (95% FC + SA 0 mM) and then significantly increased at 75% FC and after the SA treatments. The expression pattern of P5CR (Δ1-pyrroline-5-carboxylate reductase gene) was similar to that of P5CS, with differences in terms of intensity. The application of SA reduced the amount of EL and MDA through increased antioxidant activities and water balance. Overall, the results of this study showed that ‘Salmon’ cultivar was able to tolerate drought stress conditions better than ‘Tempo.’ The application of 2 mM SA increased growth and physiological indices in drought-stressed impatient, mitigating the detrimental effects of water deficit in this important ornamental species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses are known as being the most substantial potential risks for agricultural efficiency and productivity all over the world (Anjum and Lopez-Lauri 2011; Khan et al. 2015). In particular, the harmful effect of drought stress on crops is ranked first among numerous abiotic stresses (Hayat et al. 2012; Khan et al. 2015). This type of stress, whose impact varies with plant species, growth stage, and its duration, can potentially affect almost all plant physiological and biochemical, from the early stage of seed germination to maturity (Hayat et al. 2012). To overcome water deficit, plant cells can decrease their osmotic potential and preserve cell turgor by the biosynthesis and accumulation of compatible solutes, such as proline, whose biosynthesis involves the glutamate (Glu) and ornithine (Orn) pathways (Farooq et al. 2009; Hayat et al. 2012). The Glu pathway generally occurs under stress conditions, while the Orn pathway takes place in seedling development (Armengaud et al. 2004). Glutamate can be reduced to glutamate-semialdehyde (GSA) via Δ1-pyrroline-5-carboxylate synthetase (P5CS) and spontaneously converted to Δ1-pyrroline-5-carboxylate (P5C) and then in turn reduced to proline by Δ1-pyrroline-5-carboxylate reductase (P5CR) (Hu et al. 1992; Roosens et al. 1998; Szabados and Savoure 2010).
Salicylic acid (SA) plays a crucial regulatory function, being involved in a number of plant physiological processes, including plant resistance both to environmental stresses (Singh and Usha 2003; La et al. 2019b), and for this reason SA is commonly used as exogenous phytohormone/plant growth regulator (Singh and Usha 2003; La et al. 2019b). Moreover, SA is also an important signaling molecule for the induction of systemic obtained resistance that protects plants against many microbial pathogens. Several evidences prove that SA, even at low concentrations (0.05 mM), resulted in a higher plant tolerance to many types of harsh environmental conditions, mainly because of the enhancement of plant antioxidant capacity (Horvath et al. 2007; Hayat et al. 2010; Bidabadi et al. 2012; La et al. 2019b). For instance, SA causes increase in hydrogen peroxide (H2O2) content in plants exposed to different abiotic stresses, that in turn induces the expression of gene-related antioxidant enzymes (Hayat et al. 2010; Bidabadi et al. 2012). The improvement of the activity of antioxidant enzymes including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) in plants can efficiently reduce the damage caused by reactive oxygen species (ROS) accumulation that ultimately induces the expression of several plant genes involved in stress reaction. Simultaneously, SA application also lowers the accumulation of malondialdehyde (MDA), a membrane lipid peroxidation product, and leaf membrane permeability (Maghsoudi et al. 2019). Additionally, it was demonstrated that exogenous SA causes an increase in ATP content, so providing sufficient energy for the metabolism of various substances able to improve plant resistance to high salt, low temperature, heavy metal, and other abiotic stresses (Hayat et al. 2010).
To date, several studies have reported the regulatory role of the exogenous application of SA, even if its effects also depend on the application methods (e.g., leaf spray, soaking the seeds, stem infusion, by irrigation), culture medium and timing, exogenous and endogenous SA levels, as well as on the stress environment, plant species, and its developmental stage (Horvath et al. 2007; Idrees et al. 2010; Miura and Tada 2014; La et al. 2019b). Several types of research indicated that exogenous SA can ameliorate drought-stressed plants in several species (Hayat et al. 2008; Bidabadi et al. 2012; Alam et al. 2013; Demiralay et al. 2013; Maghsoudi et al. 2019).
Due to their beauty and flowering period of time, many Impatiens species are cultivated worldwide as flowering or bedding plants. Among them, Impatiens walleriana is one of the most favorite species due to its fleshy, floriferous leaves, and wide variegation of flower colors. The main problems of this ornamental species occur during the production, transportation, and sale periods, due to its high vulnerability to drought and/or physical damage. The biochemical and physiological responses of Impatiens spp. to drought stress have not been deeply studied and are poorly known. On this basis, the morpho-physiological, biochemical, and molecular responses of Impatiens walleriana plants subjected to drought stress and treated with exogenous SA are here reported and discussed. The aim is to elucidate whether exogenous usage of SA could alleviate the negative effects of water shortage in this economically important ornamental species.
Materials and methods
Plant material and experimental conditions
The experiment was done at research greenhouse of the Faculty of Agriculture, Lorestan University, Khoramabad, Iran, during 2016–2017. Day and night temperatures ranged between 22–30 °C and 16–20 °C, respectively. The range of relative humidity was 55–65% and that of average daily PAR was 400–600 μmol photons m−2 s−1. Seeds were sown manually in pots (17 cm for both height and maximum diameter) containing equal proportions of soil, sand, and cow manure. After plants establishment, SA was sprayed weekly until the end of the experiment. Soil water content at field capacity (FC) was measured using pressure plate (Soil moisture equipment Corp., Santa Barbara, Ca., USA). Then, irrigation interval at 95, 85 and 75% of FC was determined using the time domain reflectometry (TDR) (Dobriyal et al. 2012).
Morpho-physiological characteristics
Growth characteristics were recorded in all the plants, including plant height (cm), number of leaves per plant, stem diameter (mm), number of auxiliary shoots, bud appearance (days from sowing), time of flower opening (days), number of flowers, flower diameter (mm), and flower longevity (days). Then, plants were harvested and separated into roots, leaves, and stems and their fresh weight (g plant−1), root length (cm), and root volume (cm3) were measured. Dry weight was measured after oven-drying at 80 °C for 48 h. Leaf area (cm2) was measured using a leaf area meter (Delta T-scan, Version 2.03; Delta-T Devices Ltd., Burwell, and Cambridge, UK). The water use efficiency measurements were done in order to estimate the water productivity over time using the following equation (Nazarli et al. 2010).
\({\text{WUE }}\left( \% \right) \, = \, \left( {{\text{DW}}/{\text{WU}}} \right) \, \times 100\) Where WUE is water use efficiency, DW is dry weight, and WU is water use (amount of irrigation (g) during the experiment).
Photosynthetic pigments assays
Chlorophyll a (Chl a), chlorphyll b (Chl b), and carotenoids were measured in fresh leaf samples at the end of the experiment. According to Lichtenthaler (1987), leaf samples (0.1 g) were powdered in liquid nitrogen and homogenized with 10 mL pure acetone and filtered, in order to obtain a final volume of 10 mL. Pigment concentrations were calculated from the absorbance of extract at A662, A645 and A470, using the following equations (Lichtenthaler 1987):
Determination of relative water content
To determine plant water status, relative water content (RWC) was measured according to the method of Yamasaki and Dillenburg (1999):
where FW is fresh weight, \(\mathrm{DW}\) is dry weight, and TW is turgid weight.
Electrolyte leakage (%) assay
Electrolyte leakage (IL) was measured and calculated according to the method described by Huo et al. (2016). In order to determine the degree of electrolyte leakage (%), leaves were collected and washed quickly three times with deionized water. Ten fresh leaves were cut in 1 × 1 cm sections, placed in 10 mL deionized water, and left into the dark at 25 °C for 2 h. Subsequently, total electrical conductivity (EC1) was measured. Following these steps, samples were autoclaved, cooled at 25 °C, and the total electrical conductivity (EC2) was measured again. Electrolyte leakage (EL) was measured as follows:
Malondialdehyde (MDA)
Malondialdehyde content, an index of lipid peroxidation, was measured using the method of Buege and Aust (1978). In brief, 1 mL of the supernatant derived from 100–200 mg of powdered fresh leaf tissue was mixed to 2 mL of (1:1:1, v/v) TCA-TBA-HCL reagent [0.37% (w/w) solution of thiobarbituric acid (TBA), 15% tricarboxylic acid (TCA), and 0.24 N hydrochloric acid (HCl)]. The TCA-TBA-HCl reagent was boiled at 100 °C for 15 min and allowed the cooling of the solution in an ice bath. Flocculent materials were eliminated via centrifuging at 3.000 × g for 10 min. The supernatant was separated, and the absorbance reading was done at 532 nm against a blank. MDA concentration was calculated using the molar extinction coefficient for MDA-TBA-complex of 1.56 × 105 M−1 cm−1.
Antioxidant enzymes activity
Peroxidase (POD)
For extraction according to MacAdam et al. (1992) method, 0.3 g leaf samples were weighed; liquid nitrogen was added and ground into fine powder with mortar and pestle. 1.5 mL of enzyme extraction buffer (including 50 mM potassium phosphate + 2% PVP + 1 mM EDTA) was added to completely crushed leaf tissue. The suspension was poured into 2 mL microtubes and centrifuged in a refrigerated centrifuge for 20 min at 4 °C at 14.000 rpm. Then, 20 μl of the supernatant was taken to measure the activity of the enzyme.
Peroxidase activity was assayed according to MacAdam et al. (1992) with some modifications regarding the concentrations used. The assay mixture comprised of 0.1 M potassium phosphate buffer (pH 6.0), 0.03 M H2O2, and 0.02 M guaiacol. The reaction was initiated by adding 50 µL of extract to the assay solution. The POD activity was recorded at A475 over a 3 min period.
Catalase (CAT)
The activity of catalase enzyme was evaluated via the method reported by Abassi et al. (1998). Two different buffer solutions were used, one containing 12.5 mM solution of H2O2 in 50 mM KPO4 buffer (pH 7.0) and another one with 50 mM KPO4 buffer (pH 7.0). The reaction was triggered by adding 100 \(\mu\)L of enzyme extract to each buffer solution in 3 mL cuvettes, and then, absorbance value was recorded at A240 with the intervals of 10 s for 3 min.
Ascorbate peroxidase (APX)
The APX was extracted from 100 to 200 mg of powdered fresh leaf tissue in 1.5 mL of the following buffer: 50 mM K2PO4 (pH 7.8), containing 5 mM reduced ascorbate (ASA), 5 mM EDTA, 5 mM DTT, 100 mM NaCl, and 2% PVPP. The extracts were then centrifuged at the rate of 15,000 × g for 15 min, and the supernatants were used for the next analyses. APX enzyme activity was determined based on the decrease at A290 over a period of 20 s due to the oxidation of ASA (Nakano and Asada 1981).
Proline
Proline content was measured following Bates et al. (1973). Briefly, 0.5 g of the fresh leaf were ground in 10 mL of sulfosalicylic acid, and the mixture was centrifuged at 14.000 rpm for 10 min at 4 °C. Two mL of filtrate was mixed with 2 mL of acid-ninhydrin and 2 mL of glacial acetic acid in a test tube. The mixture was incubated in a water bath for 60 min at 100 °C and then immediately cooled with ice. To each tube, 4 mL of toluene was added into the reaction mixture. Then the solution was vortexed for 20 s, and the absorbance of the organic phase was recorded at 520 nm. The results were compared with a standard curve of proline, and the concentration was expressed in µmol g−1 leaf fresh weight.
RNA extraction, cDNA synthesis, and qRT-PCR analysis
Total RNA was extracted according to the manufacturer’s instructions of BioBasic kit (BioBasic, Canada; BS8231450). The first-strand cDNA was synthesized with Primerscript RT reagent kit (RR037Q; Takara Bio Inc., Tokio, Japan) according to the manufacturer’s instructions. Quantitative real-time (qRT) PCR was performed using SYBR Premix ExTaq II (TliRNaseH Plus; Takara Bio Inc., Japan) on Master cycler system (ABI, Biosystem, USA) in triplicate. The qRT-PCR analysis was carried out with actin gene as the internal standard. The gene-specific primers were designed by Primer3 (http://primer3.ut.ee/) and evaluated by OligAnalyzer (Supplementary Table S1). Details on the amplification can be found in Tavakoli et al. (2016). Relative gene expressions were analyzed using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
The trial was implemented as a factorial experiment in a completely randomized block design with three replications (n = 3). Three factors, i.e., variety (‘Salmon ‘and ‘Tempo’), SA concentration (0, 1 and 2 mM), and soil water content (95, 85, and 75% FC) were considered. All the data regarding morpho-physiological, biochemical, and molecular attributes were analyzed by analysis of variance (ANOVA). Means were separated by LSD (P = 0.05) (Sokal and Rohlf, 1997) using Statistica software (version 8.1; Stat Soft, Paris, France).
Results and discussion
Effect of drought stress and SA application on morphological and physiological characteristics
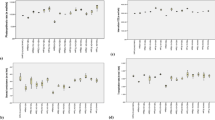
The main problem with plants belonging to Balsaminaceae family, like I. walleriana here studied, is the rapid loss of water and their wilting, so drought stress strongly reduces the plant height, plant dry weight, and the number of flowers, with consequent negative economic repercussions (Blanusa et al. 2009). The ANOVA showed that the effects of water deficit, SA, and their interactions were significant (p < 0.05) on morpho-physiological characteristics of the two studied cultivars (Table 1). In the present study, drought stress negatively affected plant height, leaf area, number of auxiliary shoots, root volume, stem diameter, number of leaves, while the application of SA (both at 1 and 2 mM) significantly increased their values (Figs. 1 and 2). Drought stress and SA had no significant effect on root length (Fig. 2). The highest and lowest values of the studied characteristics were observed in 95% FC + SA 1 or 2 mM, and 75% FC + SA 0 mM, respectively (Fig. 2). Like the above-mentioned traits, the characteristics of leaf fresh weight, leaf dry weight, stem fresh weight, stem dry weight, root fresh weight, root dry weight, total fresh weight, and total dry weight had the same trends observed for the above-cited parameters (Fig. 3).
Growth characteristics of I. walleriana plants under drought stress and treated with foliar applications of salicylic acid (SA): a plant height, b number of leaves per plant, c leaf area, d stem diameter, e number of auxiliary shoots, f root length, and g root volume. Data represent means ± standard deviation (SD). Comparison of the means was done using the LSD test at p < 0.05. Means with the same letter are not significantly different
Leaf, root, stem and total weights of I. walleriana plants under drought stress and treated with foliar applications of salicylic acid (SA): a leaf FW, b leaf DW, c stem FW, d stem DW, e root FW, f root DW, g total FW, and h total DW. Data represent means ± standard deviation (SD). Comparison of the means was done using the LSD test at p < 0.05. Means with the same letter are not significantly different
The results of the analysis of variance showed that all of the main factors studied had a significant effect (p < 0.05) on RWC, WUE, Chl a, Chl b, total Chl, and carotenoids (Table 1). Chl a, Chl b, total and carotenoids had the same trends in both Salmon and Tempo. With increasing drought stress, the levels of RWC (Fig. 4b), WUE (Fig. 4a), Chl a (Fig. 4c), Chl b (Fig. 4d), total Chl (Fig. 4e), and carotenoids (Fig. 4f) decreased. The highest and lowest values of Chl a, Chl b, total Chl, and carotenoids were observed in 95% FC + SA 2 mM and in 75% FC + SA 0 mM, respectively (Fig. 4). The results showed that Tempo had higher amounts of Chl a, Chl b, total chlorophyll, and carotenoids compared to Salmon (Fig. 4).Our results showed SA application enhanced chlorophyll content both under well water and water deficit stress conditions (Fig. 4c–e). There is some evidence that SA application increased N assimilation via activity of nitrate reductase and N content. The increased N assimilation in plants receiving SA provides N backbone for chlorophyll and proline synthesis (Khan et al. 2013).
WUE, RWC, and pigments in I. walleriana plants under drought stress and treated with foliar applications of salicylic acid (SA): a WUE, b RWC, c Chl a, d Chl b, e total Chl, and f total carotenoids. Data represent means ± standard deviation (SD). Comparison of the means was done using the LSD test at p < 0.05. Means with the same letter are not significantly different. Chl: chlorophyll
Among the environmental stresses, drought stress is the most important limiting factor in production and decreasing the growth and yield of many crops, gardens, and medicinal plants, especially in arid and semiarid regions of the world. Drought stress in plants is associated with loss of growth and photosynthesis, production of free radicals, decreasing of water potential turgor pressure (Lipiec et al. 2013; Damalas 2019). In our experiment, drought stress had a significant effect on the traits of I. walleriana (Figs. 1 and 2) so that, with increasing drought stress, all growth characteristics decreased and plants flowered earlier. Several studies showed that drought stress reduced the desired traits and applied SA treatment also reduced the effect of drought stress on the studied traits of I. walleriana. (Lipiec et al. 2013; Blanusa et al. 2009; Farooq et al. 2009; Damalas 2019).
Effect of drought stress and SA application on flower characteristics
All the main factors and their interactions had a significant effect (p < 0.05) on flower characteristics (Table 1). The results revealed that 75% FC of drought stress reduced flower diameter, flower longevity, and the number of flowers, while SA application (1 mM) increased these parameters (Fig. 5). Drought stress and SA had no significant effect on the time of flower bud appearance and time of flower opening (Fig. 5e and a). The highest and lowest values of flower characteristics were observed in 95% FC + SA 1 or 2 mM and 75% FC + SA 0 mM, respectively (Fig. 5). Flower longevity in 75% FC + SA 2 mM was 5 and 6 days in ‘Salmon’ and ‘Tempo,’ respectively (Fig. 5c). ‘Salmon’ cultivar had a significantly higher number of flowers than ‘Tempo’ (Fig. 5d).
Flower characteristics of I. walleriana plants under drought stress and treated with foliar applications of salicylic acid (SA): a time of flower opening, b flower diameter, c flower longevity, d number of flowers, and e time of flower bud appearance. Data represent means ± standard deviation (SD). Comparison of the means was done using the LSD test at p < 0.05. Means with the same letter are not significantly different
Loss of water is the main after effect of water stress (Farooq et al. 2009), reflecting the water status of plants and the most significant indicator for dehydration (Alam et al. 2013). Relative water content was observed to be remarkably reduced in various species subjected to a water deficit, such as in mustard (Alam et al. 2013), wheat (Bajji et al. 2000), tomato (Hayat et al. 2008), and I. walleriana (Antonić et al. 2016). Drought stress can cause membrane detriment, thus enhancing electrolyte leakage due to negative water balance (Hayat et al. 2008; Antonić et al. 2016), that can explain the observed significant loss of water (Table 1).
Moreover, improving plant growth and flowering characteristics in water deficit stressed plants under SA application in the present study could be due to a better water balance and higher antioxidant activities (Figs. 4 and 6). It has been shown that SA induce stomatal closure under drought stress (Okuma et al. 2014), resulting in higher relative water content and water use efficiency. Further, decreased electrolyte leakage and increased antioxidant activities under SA application have been reported (Shi et al. 2006; Wang and Li 2006).
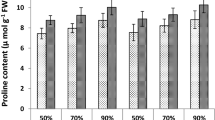
Effects of drought stress and treated with foliar applications of salicylic acid (SA) on a electrolyte leakage, b MDA content, c CAT activity, d POD activity, e APX activity, and f proline content in leaves of I. walleriana plants. Data represent means ± standard deviation (SD). Comparison of the means was done using the LSD test at p < 0.05. Means with the same letter are not significantly different. APX: ascorbate peroxidase; CAT: catalase; MDA: malondialdehyde; POD: peroxidase
Effect of drought stress and SA application on oxidative stress indicator malondialdehyde (MDA)
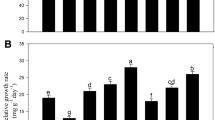
Except for the interaction of SA × cultivar and drought stress × SA × cultivar, all of the studied factors had a significant effect (p < 0.05) on the amount of MDA (Table 1). The levels of MDA in Tempo were higher than those found in Salmon. Generally, MDA was affected by drought stress and SA. Increased drought stress caused parallel increases of MDA, while the application of SA decreases the amount of MDA at all the levels of drought stress. The highest and lowest levels of MDA were found in Tempo at 75% FC + SA 2 mM (0.35 (µmol g−1 FW) and 95% FC + SA 2 mM (0.24 µmol g−1 FW), respectively (Fig. 6b).
ROS aggregates can cause different damage in compartment of plant cells to oxidative stress (Farooq et al. 2009). The oxidative stress caused by drought stress in I. walleriana with the accumulation of H2O2 has been confirmed, according to Antonić et al. (2016) and, with increasing MDA content, indicates peroxidation lipid damage (Fig. 6b). These findings are consistent with literary data about increased H2O2 content in drought-stressed mustard (Alam et al. 2013) and banana (Bidabadi et al. 2012). The same increases were observed for MDA in many plant species subjected to drought (Hayat et al. 2008; Liu et al. 2011; Bidabadi et al. 2012; Odjegba and Adeniyi 2012; Alam et al. 2013). One of the outstanding effects of SA on I. walleriana was the significant protection against membrane lipid peroxidation found in plants under all the drought levels (Fig. 6b). The reduction of MDA in drought-stressed under SA application is reported in different plants such as mustard, banana, tomato (Hayat et al. 2008; Bidabadi et al. 2012; Alam et al. 2013). Reducing the amount of oxidative stress and increasing the amount of proline, SA also protect the membranes and cellular organs, preserving enzymes structure and reducing their oxidation or decomposition (Costa et al. 2005; Alam et al. 2013; Antonić et al. 2016). The foliar application of SA is able to increase antioxidant capacity, reduce the amount of lipids peroxidation and oxidative damage, and protect photosynthetic membranes and pigments, so preventing chlorophyll degradation (Costa et al. 2005).
Effect of SA and drought stress on antioxidant enzymes
Peroxidase (POD) activity
Except for the cultivar factor, all of the studied factors had a significant (p < 0.05) effect on POD activity (Fig. 6d). Mean comparison of treatment showed that drought stress and the application of SA increased the amount of POD enzyme. The application of 2 mM SA increased the activity of POD up to 25.19 and 19.64 units g−1 FW in Salmon and Tempo, respectively, while the level of 1 mM SA decreased the POD activity from 23.27 to 18.40 units g−1 FW in Salmon and from 19.12 to 17.33 units g−1 FW in Tempo, compared to the control plants maintained at 95% FC (Fig. 6d). POD activity of Tempo cultivar in 85% and 95% FC under 0 mM SA were not significantly different compared to 85% and 95% FC under 1 or 2 mM SA, respectively (Fig. 6d).
Increased POD activity in plants under water deficit stress and SA application suggests the high demands of H2O2 quenching. In line with the present study, it has also been shown that SA generally increases the total POD activity in mustard, tomato, and I. walleriana (Hayat et al. 2008; Alam et al. 2013; Antonić et al. 2016).
Catalase (CAT) and ascorbate peroxidase (APX) activities
Drought stress caused increases in CAT and APX activities at both the SA concentrations used, while the application of SA reduced both CAT and APX activities at all levels of drought stress (Fig. 6). Mean comparison of the main effects showed that CAT activity in ‘Salmon’ cultivar is generally higher than in ‘Tempo.’ The highest and lowest values of CAT activity were observed in ‘Tempo ‘cultivar in 75% FC + SA 0 mM (1.57 units g−1 FW), and 95% FC + SA 2 mM (1.29 units g−1 FW), respectively (Table1 and Fig. 6c).
Exogenous SA was found to inhibit the CAT enzyme activity in I. walleriana under different dose-level (Antonić et al. 2016). This effect is more effective in the plants exposed to abiotic stress, where CAT activity is very higher than in control plants at 95% FC (Fig. 6). In the similar studies, SA was found to enhance the CAT activity (Ananieva et al. 2004; Hayat et al. 2008; Kadioglu et al. 2011; Alam et al. 2013; Demiralay et al. 2013). Moreover, Chen et al. (1997) reported that different CAT isoform may have a different degree of sensitivity to SA. The results on the activity of CAT showed that drought stress increased the activity of this enzyme, but the use of SA reduced its activity (Fig. 6c). The increased activities of antioxidant enzymes under drought conditions can be considered an indicator for the activity of the enzyme under abiotic stress and plant protection against abiotic stress (Kukreja et al. 2005).
Effect of SA and drought stress on proline accumulation and its synthesis-related genes
Except for the interaction of SA × drought and drought stress × SA × cultivar, all of the studied factors had a significant effect (p < 0.01) on the amount of proline (Table 1). The levels of proline in Tempo were higher than those found in Salmon. Generally, proline was affected by drought stress and SA. Increasing drought stress and foliar application of SA increased amount of proline in both cultivar. The highest and lowest level of proline were found in Tempo at 75% FC + SA 1 mM (3.90 µmol g−1 FW) and in Salmon at 95% FC + SA 0 mM (1.29 µmol g−1 FW), respectively (Fig. 6f).
Proline accumulation is one of the most commonly solutes that is often associated with drought tolerance, since it contributes to all the important characteristics of drought tolerance, such as osmotic regulation, osmotic protection, antioxidation, and ROS scavenging (Verbruggen and Hermans 2008; Farooq et al. 2009; Antonić et al. 2016). Proline is also involved in the stabilization of membranes and proteins, buffering cellular redox potential under stress, metal chelation, and signaling, also acting as a sink for carbon and nitrogen for their use after stress relief (Hayat et al. 2012). Exogenous application of SA stimulates proline accumulation in stressed plants (Misra and Saxena 2009; La et al 2019b), particularly when drought-induced proline accumulation is low to moderate. This has been observed in different plant species (Hayat et al. 2008; Bidabadi et al. 2012; Marcińska et al. 2013) and confirmed also by our results on in I. walleriana (Fig. 6f). Besides upregulation of genes involving proline synthesis that enhanced proline accumulation discussed below, Nazar et al. (2015) showed increasing of proline could be observed in mustard under SA treatment through the increase in γ-glutamyl kinase (GK) and decrease in proline oxidase (PROX) activity.
Here, the expression of P5CS gene was slightly increased in 95% FC + SA 0 mM and then significantly increased in 75% FC and SA treatment (Fig. 7a). The expression pattern of P5CS showed a similar trend in both the studied cultivars, but in Salmon it reached higher levels than in control plants at 95% FC (Fig. 7a). Gene expression of P5CR in both cultivars increased in 75% FC and SA treatment, and its pattern was similar to that of P5CR with differences in terms of intensity (Fig. 7b). From these data, it appears that the observed increases in proline accumulation (Fig. 6f) were likely due to the due to up-regulation of P5CS and P5CR genes (Fig. 7). This is in agreement with the model proposed by Verslues and Sharma (2010), who found proline accumulation linked to P5CS up-regulation in Arabidopsis under saline stress. Moreover, the expression of P5CS1 and P5CS2 was induced under different abiotic stresses in Brassica napus before the accumulation of proline (Xue et al. 2009), and Tavakoli et al. (2016) reported the expression of P5CS and P5CR in salinized wheat.
Relative expression of a P5CS and b P5CR genes in I. walleriana plants under drought stress and treated with foliar applications of salicylic acid (SA). For drought stress, seedlings were subjected to water deficit at 75%, 85%, and 95% field capacity (FC). Data represent means ± standard deviation (SD). Comparison of the means was done using the LSD test at p < 0.05. Means with the same letter are not significantly different. P5CS: encoding Δ1-pyrroline-5-carboxylate synthetase gene; P5CR: Δ1-pyrroline-5-carboxylate reductase (P5CR) gene
There are two pathways in proline biosynthesis including the glutamate (Glu) and ornithine (Orn) pathways (Hu et al. 1992; Roosens et al. 1998). Glu pathway generally occurs under abiotic stress, while pathway of Orn is involved in development of seedling (Armengaud et al. 2004). SA causes the dynamic transport process of proline and also vital for the protective role of proline in plants. Thus, under SA treatment glutamic-γ -semi-aldehyde (GSA) converted to pyrroline-5- carboxylate (P5C) in cytosol and chloroplasts and so increased the proline transport (La et al. 2019a).
Conclusions
The results indicated that drought stress, especially at 75% FC, applied to Impatiens walleriana reduced flower diameter, number of flowers, and flower longevity, while salicylic acid treatment was effective in increasing these characteristics. Generally, SA had beneficial effects on plant growth through facilitating water uptake, higher antioxidant enzyme activities, and better membrane stability. On the other side, the exogenous application of SA, particularly at a concentration of 2 mM, mitigated the deleterious effects of drought stress, causing increasing growth indices, better morpho-physiological traits, and enhanced water use efficiency. The results also showed that Salmon cultivar is more tolerant to drought compared to Tempo cultivar, being so recommended in areas with low available water.
References
Abassi NA, Kushad MM, Endress AG (1998) Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci Hort 74(3):183–194. https://doi.org/10.1016/S0304-4238(98)00077-6
Alam MM, Hasanuzzaman M, Nahar K, Fujita M (2013) Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 7(7):1053
Ananieva EA, Christova KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to Paraquat. J Plant Physiol 161:319–328. https://doi.org/10.1078/0176-1617-01022
Anjum NA, Lopez-Lauri F (2011) Plant Nutrition and Abiotic Stress Tolerance III. Global Science Books, Ikenobe
Antonić S, Milošević A, Cingel M, Lojić M, Trifunović-Momčilov M, Petrić A, Subotić A, Simonović A (2016) Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. South Afr J Bot 105:226–233. https://doi.org/10.1016/j.sajb.2016.04.002
Armengaud P, Thiery L, Buhot N, Grenier-DeMarch G, Savouré A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120(3):442–450. https://doi.org/10.1111/j.0031-9317.2004.00251.x
Bajji M, Lutts S, Kinet JM (2000) Physiological changes after exposure to and recovery from polyethylene glycol-induced water deficit in roots and leaves of durum wheat (Triticum durum Desf.) cultivars differing in drought resistance. J Plant Physiol 157:100–108. https://doi.org/10.1016/S0176-1617(00)80142-X
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bidabadi SS, Mahmood M, Baninasab B, Ghobadi C (2012) Influence of salicylic acid on morphological and physiological responses of banana (Musa acuminate cv. Berangan, AAA) shot tips to in vitro water stress induced by polyethylene glycol. Plant Omics J 5:33–39
Blanusa T, Vysini E, Cameron RW (2009) Growth and flowering of Petunia and Impatiens: effects of competition and reduced water content within a container. HortScience 44(5):1302–1307. https://doi.org/10.21273/HORTSCI.44.5.1302
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Meth Enzymol 52:302–310. https://doi.org/10.1016/s0076-6879(78)52032-6
Chen Z, Iyer S, Caplan A, Klessig DF, Fan B (1997) Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol 114:193–201. https://doi.org/10.1104/pp.114.1.193
Costa M, Civell PM, Chaves AR, Martinez GA (2005) Effects of ethephon and 6- benzylaminopurine on chlorophyll degrading enzymes and peroxidase-linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20°C. Postharvest Biol Technol 35:191–199. https://doi.org/10.1016/j.postharvbio.2004.07.007
Damalas CA (2019) Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci Hort 246:360–365. https://doi.org/10.1016/j.scienta.2018.11.005
Demiralay M, Sağlam A, Kadioğlu A (2013) Salicylic acid delays leaf rolling by inducing antioxidant enzymes and modulating osmoprotectant content in Ctenanthe setosa under osmotic stress. Turk J Bio 37:49–59
Dobriyal P, Qureshi A, Badola R, Hussain SA (2012) A review of the methods available for estimating soil moisture and its implications for water resource management. J Hydrol 458:110–117. https://doi.org/10.1016/j.jhydrol.2012.06.021
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C (eds) Sustainable agriculture. Springer, Dordrecht, pp 153–188
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
Hayat S, Hasan SA, Fariduddin Q, Ahmad A (2008) Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Interact 3:297–304. https://doi.org/10.1080/17429140802320797
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
Horvath E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300. https://doi.org/10.1007/s00344-007-9017-4
Hu CA, Delauney A, Verma DS (1992) A bifunctional enzyme (delta1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA 89:9354–9358. https://doi.org/10.1073/pnas.89.19.9354
Huo Y, Wang M, Wei Y, Xia Z (2016) Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front Plant Sci 6:1223. https://doi.org/10.3389/fpls.2015.01223
Idrees M, Masroor M, Khan A, Aftab T, Naeem M, Hashmi N (2010) Salicylic acid-induced physiological and biochemical changes in lemongrass varieties under water stress. J Plant Interact 5:293–303. https://doi.org/10.1080/17429145.2010.508566
Kadioglu A, Saruhan N, Sağlam A, Terz R, Acet T (2011) Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul 64:27–37. https://doi.org/10.1007/s10725-010-9532-3
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:1–17. https://doi.org/10.3389/fpls.2015.00462
Khan MIR, Iqbal N, Masood A, Per TS, Khan NA (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8(11):e26374. https://doi.org/10.4161/psb.26374
Kukreja S, Nandval AS, Kumar N, Sharma SK, Sharma SK, Unvi V, Sharma PK (2005) Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant 49:305–308. https://doi.org/10.1007/s10535-005-5308-4
La VH, Lee BR, Islam MT, Park SH, Jung HI, Bae DW, Kim TH (2019a) Characterization of salicylic acid-mediated modulation of the drought stress responses: reactive oxygen species, proline, and redox state in Brassica napus. Environ Exp Bot 157:1–10. https://doi.org/10.1016/j.envexpbot.2018.09.013
La VH, Lee BR, Zhang Q, Park SH, Islam MT, Kim TH (2019b) Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic Environ Biotechnol 60(1):31–40. https://doi.org/10.1007/s13580-018-0099-7
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lipiec J, Doussan C, Nosalewicz A, Kondracka K (2013) Effect of drought and heat stresses on plant growth and yield: a review. Int Agrophys 27(4):463–547. https://doi.org/10.2478/intag-2013-0017
Liu CC, Liu YG, Guo K, Fan DY, Li GG, Zheng YR, Yu LF, Yang R (2011) Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ Exp Bot 71:174–183. https://doi.org/10.1016/j.envexpbot.2010.11.012
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
MacAdam JW, Nelson CJ, Sharp RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99(3):872–878. https://doi.org/10.1104/pp.99.3.872
Maghsoudi K, Emam Y, Ashraf M, Arvin MJ (2019) Alleviation of field water stress in wheat cultivars by using silicon and salicylic acid applied separately or in combination. Crop Pasture Sci 70(1):36–43. https://doi.org/10.1071/CP18213
Marcińska I, Czyczyło-Mysza I, Skrzypek E, Grzesiak MT, Janowiak F, Filek M, Dziurka M, Dziurka K, Waligórski P, Juzoń K, Cyganek K, Grzesiak S (2013) Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int J Mol Sci 14:13171–13193. https://doi.org/10.3390/ijms140713171
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci 177:181–189. https://doi.org/10.1016/j.plantsci.2009.05.007
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:4. https://doi.org/10.3389/fpls.2014.00004
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nazar R, Umar S, Khan NA, Sareer O (2015) Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S Afr J Bot 98:84–94. https://doi.org/10.1016/j.sajb.2015.02.005
Nazarli H, Zardashti MR, Darvishzadeh R, Najafi S (2010) The effect of water stress and polymer on water use efficiency, yield and several morphological traits of sunflower under greenhouse condition. Not Sci Biol 2(4):53–58
Odjegba VJ, Adeniyi AM (2012) Responses of Celosia argentea L. to simulated drought and exogenous salicylic acid. Nat Sci 10:252–258
Okuma E, Nozawa R, Murata Y, Miura K (2014) Accumulation of endogenous salicylic acid confers drought tolerance to Arabidopsis. Plant Signal Behav. https://doi.org/10.4161/psb.28085
Roosens NH, Thu TT, Iskandar HM, Jacobs M (1998) Isolation of ornithine-d-amino transferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol 117:263–271. https://doi.org/10.1104/pp.117.1.263
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135. https://doi.org/10.1007/s10725-005-5482-6
Singh B, Usha K (2003) Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul 39:137–141. https://doi.org/10.1023/A:1022556103536
Sokal RR, Rohlf FJ (1997) Biometry: the principles and practice of statistic in biological research. WH Freeman, New York
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Tavakoli M, Poustini K, Alizadeh H (2016) Proline accumulation and related genes in wheat leaves under salinity stress. J Agr Sci Tech 18:707–716
Verbruggen N, Hermans C (2008) Pro accumulation in plants: a review. Amino Acids 35:753–759. https://doi.org/10.1007/s00726-008-0061-6
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant- environment interaction. Arabidopsis Book 8:344–342. https://doi.org/10.1199/tab.0140
Wang LJ, Li SH (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170(4):685–694.
Xue X, Liu A, Hua X (2009) Proline accumulation and transcriptional regulation of proline biosynthesis and degradation in Brassica napus. BMB Rep 42:28–34. https://doi.org/10.5483/bmbrep.2009.42.1.028
Yamasaki S, Dillenburg LR (1999) Measurements of leaf relative water content in Araucaria angustifolia. R Bras Fisiol Veg 11(2):69–75
Acknowledgements
This study was supported by two research grants at Lorestan University (FPN: B.5107-1397-02-30), Khoramabad, Iran, and Shahid Chamran University, Ahvaz (AG1397 Grant Faculty of Agriculture), Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Mohamed F. Yassin.
Supplementary information
Rights and permissions
About this article
Cite this article
Safari, M., Mousavi-Fard, S., Rezaei Nejad, A. et al. Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 19, 969–984 (2022). https://doi.org/10.1007/s13762-020-03092-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-03092-2