Abstract
Phenol is one of the dangerous organic pollutants in industrial effluents, and it has high toxicity and numerous environmental problems. Thus, there is a growing need for more studies on its removal. The goal of this research is to provide a statistical modeling, analysis, and optimization of the simultaneous impact of temperature and phenol concentration on the efficiency of stabilization ponds in treatment of wastewater containing phenol. The experiments were performed using two stabilization ponds at laboratory scales. The experiments were designed by using response surface methodology and Design-Expert software. According to the presented model, optimum removal rate of phenol is achieve when temperature and phenol concentration are 14.20° C and 109.58 mg/l, respectively. The results showed that the removal rate dropped with decreased temperature and increased concentrations of phenol. At 20 °C, by increasing the concentration of phenol from 40 to 130 mg/l, the removal rate dropped from 73 to 45%. Based on this research results, it can be concluded that the stabilization pond had a proper performance in removing phenol, and stabilization ponds can be an alternative for complex and expensive systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, the issue of pollution is a major concern of many societies. A large quantity of industrial wastewater containing toxic and polycyclic aromatic hydrocarbon (PAHs) is produced every year (Qaderi et al. 2011; Nassef 2014). PAHs compounds are highly resistant to degradation and they may cause irreparable environmental damages. Therefore, addressing the pollution issue is critical from an environmental and economic perspective (Nassef 2014).
Phenol is an aromatic hydrocarbon pollutant with molecular formula of C6H5OH and molar mass of 94.11 g/mol. At room temperature, solid phenol is colorless, transparent and in the form of crystalline powder. It is also fragrant with high solubility in oil, glycerin, and alcohol (Almasi et al. 2014).
Due to their toxicity and persistence, phenols and phenolic compounds, even in low concentrations, are highly hazardous for humans and other living creatures. Phenol and its derivatives are extremely detrimental and can be absorbed by the body in various ways such as skin, respiratory system, eyes and the mouth (Pradeep et al. 2015). This hazardous chemical is rapidly absorbed by the body and cause to eye irritation, seizures and body tissue ablation (Wang et al. 2011). Excessive contacting to phenol may compromise the liver, lungs, kidneys, and the nervous and vascular system. According to the World Health Organization, the maximum allowable concentration of phenol in drinking water is 0.002 mg/l. Consuming 1 g of phenol is lethal in humans (Almasi et al. 2014). Phenol and its compounds are used in a variety of industries such as pharmaceuticals, paper, oil, paint, polycarbonate resins, pesticides, perfumes, coal tar, plastic and textile industries (Kiliç et al. 2012; Lee et al. 2014). Phenol could be removed using various physical–chemical and biological methods (Hussain et al. 2015), the most common methods for phenol wastewater treatment are using resin (Yamada et al. 2013), oxidation with ozone (Turhan and Uzman 2008), irradiation (Yao et al. 2017), adsorption though different materials (Hameed and Rahman 2008; Bizerea Spiridon et al. 2013), photocatalytic degradation (Lee et al. 2014; Jiang et al. 2017), and biological degradation (Kiliç et al. 2012).
Several studies have been conducted on using chemical and physical methods for treatment of phenol in wastewater. For example, in previous studies, phenol and COD removal by ozonation, adsorption on activated carbon and catalytic ozonation in a closed system have been studied in a fixed bed reactor (de Souza et al. 2012). Also, photocatalytic degradation of phenol using TiO2 particles was one of the methods which has been used for phenol removal (Faghih Nasiri et al. 2018). The removal of phenol through its adsorption on zeolite compounds including zeolitic tuff and cellulose has been examined in another study (Bizerea Spiridon et al. 2013). The removal of phenols from sour water with resin (Yamada et al. 2013) and with iron nanoparticles and iron microparticles has been studied in other studies (Singh et al. 2016). The physicochemical processes are less efficient due to their high energy consumption, high costs, production of secondary toxic pollutants, low productivity, and applicability in a limited concentration (Almasi et al. 2014).
Biodegradation can transform pollutants into simpler substances (Pradeep et al. 2011). Biological degradation of different pollutants has been widely investigated (Qaderi et al. 2018; Babanezhad et al. 2017). Among various methods of phenol removal, biodegradation is more environment-friendly as it produces harmless products. For this reason, growing attention has been paid to this technique (Pradeep et al. 2015).
Biological methods can be proper alternative to the usual methods of pollutants removal from water. These methods have been extensively used in the previous researches (Hussain et al. 2015). In one study, phenol biodegradation has been analyzed using triacontanol hormone in the presence and absence of light (Kiliç et al. 2012). Another study has been focused on the simultaneous removal of nitrate and phenol in anaerobic bioreactors (Zhu et al. 2007). The simultaneous removal of nitrate and phenol in anaerobic fluidized bed reactor (AFBR) has been studied in another research (Omena et al. 2013). In the same vein, the use of microbial fuel cells (MFCs) as an effective method for biodegradation of phenol has been assessed in another study and a removal rate of 86.44% after 41 h at 30 °C for 500 mg/lit of phenol was obtained (Wei et al. 2017). The ability of Rhodococcus UKMP-5 M bacteria in biodegradation of phenol has been studied in different batch and continuous bioreactors, with the results indicating that the removal rate of phenol in continuous mode was 3.28 times greater than the batch mode (Yaacob et al. 2016). Phenol degradation and Cr(VI) reduction by sulfate-reducing bacteria (SRB) have been assessed (Han et al. 2017).
In all of above studies, the operation of studied reactors is complex and consumes a great deal of energy. Therefore, in this paper, stabilization pond is used for treating wastewater containing phenol, as one of the most convenient and economic biological systems (Filho et al. 2013; Pham et al. 2014). The stabilization ponds have been extensively used to remove pollutants from municipal wastewater (Sabah et al. 2016).
According to the literature review, modeling and optimization of effective parameters on stabilization ponds efficiency have not been studied. Also, analyzing of parameters effects on degradation of phenol in stabilization ponds has not been simultaneously studied.
The main novelty of this research is designing serial ponds for treatment of phenol wastewater. Also, for the first time, modeling, optimization, and statistical analyses were performed on the phenol removal results of this reactor. Furthermore, the mutual and simultaneous impact of temperature and concentration were analyzed using RSM.
Materials and methods
Research biological reactor
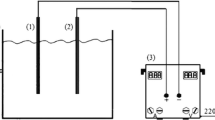
In this study, two optional stabilization ponds were developed at a laboratory scale with a capacity of 100 L using metal sheets with a thickness of 6 mm. The retention time of each pond was 3 days. The required light was provided by fluorescent lamps (690 lx) and an electric blower supplied the air flow. The sewage of the feed tank, including the thermostat and the heater system, was directed by the flow control valve to the first stabilization pond and after 3 days, it was transferred to the second stabilization pond. Fluorescent lamps were installed on top of each stabilization pool.
Materials
Phenol and other chemical compounds used in this study were purchased from Merck Company, Germany, with laboratory purity.
Inoculation of stabilization ponds and seeding
To pace up the treatment of phenol wastewater, before launching the systems first the sludge recycled from the activated sludge unit of Tehran Oil Refinery wastewater treatment plant was used as the biomass after integration.
Response surface methodology and variables
Today, modeling and prediction were used widely in environmental studies (Qaderi and Babanezhad 2017; Babanezhad et al. 2018). In this study, pond series were used in biological processes for the purpose of modeling, statistical analysis and the determination of optimal conditions of treating wastewater containing phenol in the reactor. To achieve these objectives, an experimental design was developed using response surface methodology (RSM).
RSM is a set of statistical and mathematical methods used to optimize a variety of processes. RSM helps describe the behavior of the set. RSM applies a multivariate equation to the data sets for statistical prediction (Bezerra et al. 2008). RSM is useful for the analysis of experiments in which one or more independent variables (as a response) are influenced by several other variables to optimize the mentioned response. One of the advantages of RSM, besides reducing the number of experiments, is that it forges a mathematical relationship between independent and dependent variables. Moreover, in addition to the analysis of numerical variables, it allows studying the impact of qualitative variables (Bashi et al. 2012).
For designing the experiments and analyzing the results, Design-Expert software (version: 10.0.6) was used. In this research, a central composite design was used for the statistical analysis in RSM. The design requirements of the variables are presented in Table 2. In this table, names, types, and units of variables as well as the rate of variables (between − 1 and 1) are displayed.
According to the conditions set forth in Table 1, since there are two independent variables (the temperature of influent wastewater and the concentration of phenol in influent wastewater) and one dependent variable (phenol removal), 13 tests were conducted by the software in the original trial period, as shown in Table 2.
Feeding and operation of reactors and the test reference
pH levels at all stages were periodically measured and maintained in the range of 6.8–7.2. The C/N/P ratio was 100:5:1 which was injected into the bioreactor at the beginning of each period. The carbon was provided by the phenol.
In this study, a new concentration of phenol feeding was used at each stage and this feeding concentration was retained until the reactor outflow concentration reached the steady state. In the course of the research, urea, as a major source of nitrogen, and phosphate buffer salts compounds (K2HPO4, KH2PO4), as a source of phosphorus, were injected into the system to provide C/N/P ratio (100:5:1). Other compounds of the synthetic wastewater formula were used as trace elements in order to increase the efficiency. The specifications of these compounds are shown in Table 3.
All the experiments of this research were performed based on the instructions provided in “Standard Methods for the Examination of Water and Wastewater” (Greenberg et al. 2000). Phenol was determined based on 5530-D of Standard Methods for the Examination of Water and Wastewater. The Unico spectrophotometer was used. In every step of the experiment, pH was kept constant (Neutral Range); so that microorganisms could continue living and serving as purifier of the pollutants. All of the experiments were repeated at least three times. Relative Standard Deviation (RSD) of the reported results being less than 5%.
Results and discussion
In this study, two independent variables under study were influent phenol concentration (mg/l) and temperature (°C) in the stabilization pond. Table 4 shows the test results. In this table, two major factors are considered: (A) is the temperature and (B) is the concentration of the influent phenol. A comparison of the results reported in Table 4 suggests that temperature has a positive effect on the removal rate while the concentration of influent phenol has a negative impact on the removal efficiency. These findings were observed in all samples.
Simultaneous effect of parameters
Figure 1 shows the effects of two independent variables of temperature and concentration on the phenol removal rate. As can be seen, the phenol removal rate at 20 °C and a concentration of 40 mg/l was approximately 73%. According to Fig. 1, phenol removal percentage was directly related to the temperature, so that increased temperature was caused by improving the removal rate. Microorganisms constitute a main factor in the pond treatment. As temperature rises, the efficiency of microorganisms’ activity increases as well. Thus, it can be stated that temperature rise has a positive effect on the phenol removal efficiency.
On the other hand, as shown in Fig. 1, the removal efficiency dropped with an increase in the concentrations of phenol. This could be due to the toxic effects of phenol on the microorganisms. A study on the removal of phenol from oil refinery wastewater in anaerobic stabilization pond reported similar results, according to which the removal efficiency dropped with the increased concentration of phenols, and soared with rising temperature (Dargahi et al. 2016). In a study on the simultaneous removal of phenol and nitrate in anaerobic fluidized bed reactor (AFBR), it was observed that the removal efficiency dropped with excessive increase in phenol levels (Omena et al. 2013).
Individual effect of parameters
At this stage, the relationship between each parameter and the removal rate was evaluated separately by drawing one-dimensional graphs.
Effect of temperature
Figure 2 shows the phenol removal rate in different temperatures (for 85 mg/l of influent phenol concentration). As temperature rises, the phenol removal rate also increases, rapidly. According to Fig. 2, there is a nonlinear relationship between the phenol removal rate and the temperature. The removal rate was approximately 65% at 20 °C. As discussed earlier, with increasing in temperature, the removal efficiency enhanced because of increased activity of the microorganisms in the pond. The growth and metabolism of microorganisms decreases at low temperatures. The tests were conducted at temperatures near to ambient temperature; therefore, maintenance did not require a lot of energy and expense. Given their proximity to the ambient temperature, the tests were economically justifiable. Similar results have been reported in other biological studies (Alemzadeh et al. 2002; Lakshmi et al. 2009).
Effect of influent phenol concentration
Figure 3 shows the effect of initial phenol concentration on the phenol removal rate at a temperature of 15.5 °C. According to Fig. 3, phenol removal rate has a reverse relationship with the influent phenol concentrations. So that with an increase in the influent phenol concentration, the nonlinear decreasing was observed in phenol removal rate. The removal rate of the phenol shown in Fig. 3 is approximately 57.75% for a concentration of 40 mg/l. Furthermore, the removal rate of the phenol for a phenol concentration of 130 mg/l is about 38%. Phenol has toxicity and it has negative effect on the growth of algae and bacteria in the stabilization pond. It seems that phenol toxicity is the main factor for the lower efficiency of stabilization ponds in removing phenol during high influent phenol concentration. This finding is consistent with other biological studies on this subject (Moussavi and Heidarizad 2010; Omena et al. 2013; Almasi et al. 2014). The results have shown that in other biological systems such as MBBR and activated sludge processes, removal efficiency reduces by increased phenol (Kukadiya 2016). According to the results of biodegradation of phenol in the RBC system, there seems to be a reverse relationship between loading and removal of phenols (Alemzadeh et al. 2002).
According to the World Health Organization, the permissible concentration of phenol in drinking water is 0.002 mg/l (WHO 1984), and in accordance with the provisions of the Environmental Protection Agency, the maximum permissible concentration of phenol content in wastewater is 1 mg/l (Senturk et al. 2009). According to the Iranian national standard, the maximum permissible concentration of phenol outlet in surface water, agriculture and irrigation is 1 mg/l. Thus, it can be stated that the stabilization pond is effective in removing phenol and its compounds, and it can be used as a pre-treatment for industrial effluents and domestic wastewater.
According to above points, the stabilization pond provides a simple and flexible system with optimum productivity and efficiency for the removal of phenol and phenolic compounds in wastewater. The system is cost-effective and can be used as an alternative to complex and expensive systems.
Interaction and perturbation graphs
Figures 4 and 5 show interaction effects of parameters and perturbation, respectively. As mentioned, the removal efficiency rises with reduced phenol concentration and increased temperature. According to Fig. 4, at 11 °C, with an increase in phenol concentrations from 40 to 130 mg/l, the removal rate drops from 34 to 20%. At 20 °C, the removal rate declines with increased concentration of phenol, but this time, the change is doubled and the removal rate is reduced by about 28%, from 73% in a phenol concentration of 40 mg/l to about 45% in a phenol concentration of 130 mg/l. Therefore, these two variables seem to have a synergistic effect. In other words, as noted earlier, at constant temperature, an increase in the concentration of influent phenol leads to a decrease in the phenol removal rate; nevertheless, the intensity of this reduction depends on the ambient temperature.
Variance analysis and mathematical model
After a complete analysis of the results, the model is presented in Eq. (1). According to the presented model, the temperature had the greatest impact on the removal efficiency. In Table 5, the values predicted by Eq. (1) for 13 experiments (shown in Table 2) are depicted. Equation (1) was validated based on laboratorial data; root-mean-square error was less than 5%.
where T is temperature (C) and C (mg/l) is the influent phenol concentration.
According to Table 6, the results of the analysis of the variance demonstrated the validity of the proposed model with p value of 0.0001. An acceptable lack of fit is shown in this table. Furthermore, the p value for temperature and influent phenol concentration was less than 0.0001. Therefore, the hypothesis that these two factors have no impact was rejected, as these two parameters had a significant effect on phenol removal efficiency.
Optimum conditions
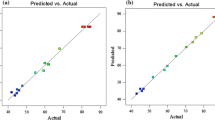
According to the results of RSM, the optimal phenol removal rate conditions were achieved in a phenol concentration of 109.58 mg/l and at 14.20° C. Using the presented model, a removal rate of 41.2% was achieved under these conditions, which was less than 5% different from the lab results. A difference of less than 5% is considered acceptable in biological research (Rastegar et al. 2011; Langroudi et al. 2014; Arshadi et al. 2016). According to Fig. 5, the accuracy of the proposed model can be assessed. The proximity of the points to the trend line suggests that the results were identical to the tests results, and the model was able to predict the behavior of the response variable.
Conclusion
The results of this study showed that pond systems due to featured such as high efficiency, simplicity, and low costs could be an appropriate alternative to complex and expensive systems. In the present study, the stabilization pond system has low efficiency in low concentrations of phenol (between 40 and 130 mg/l). Based on the result, the interaction effect between influent phenol concentration and temperature can be concluded. The model designed by the software showed that optimum conditions for removal were at a phenol concentration of 109.58 mg/l and temperature of 14.20 °C. Therefore, given their low costs, stabilization ponds can be used for treating wastewater containing phenol, provided that the variables affecting their performance are set in the proper range.
References
Alemzadeh I, Vossoughi F, Houshmandi M (2002) Phenol biodegradation by rotating biological contactor. Biochem Eng J 11:19–23. https://doi.org/10.1016/S1369-703X(02)00011-6
Almasi A, Dargahi A, Amrane A et al (2014) Effect of the retention time and the phenol concentration on the stabilization pond efficiency in the treatment of oil refinery wastewater. Fresenius Environ Bull 23:2541–2548
Arshadi M, Mousavi SM, Rasoulnia P (2016) Enhancement of simultaneous gold and copper recovery from discarded mobile phone PCBs using Bacillus megaterium: RSM based optimization of effective factors and evaluation of their interactions. Waste Manag 57:158–167. https://doi.org/10.1016/j.wasman.2016.05.012
Babanezhad E, Amini Rad H, Hosseini Karimi SS, Qaderi F (2017) Investigating nitrogen removal using simultaneous nitrification-denitrification in transferring wastewater through collection networks with small-diameter pipes. Water Pract Technol 12:396–405. https://doi.org/10.2166/wpt.2017.044
Babanezhad E, Qaderi F, Salehi Ziri M (2018) Spatial modeling of groundwater quality based on using Schoeller diagram in GIS base: a case study of Khorramabad, Iran. Environ Earth Sci 77:339. https://doi.org/10.1007/s12665-018-7541-0
Bashi DS, Mortazavi SA, Rezaei K et al (2012) Optimization of ultrasound-assisted extraction of phenolic compounds from yarrow (Achillea beibrestinii) by response surface methodology. Food Sci Biotechnol 21:1005–1011. https://doi.org/10.1007/s10068-012-0131-0
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Bizerea Spiridon O, Preda E, Botez A, Pitulice L (2013) Phenol removal from wastewater by adsorption on zeolitic composite. Environ Sci Pollut Res 20:6367–6381. https://doi.org/10.1007/s11356-013-1625-x
Dargahi A, Atafar Z, Mohammadi M et al (2016) The survey of phenol removal from oil refinery wastewater by anaerobic stabilization in high temperature. Res J Appl Sci 11(7):567–572
de Souza SMDGU, de Souza FB, de Souza AAU (2012) Application of individual and simultaneous ozonation and adsorption processes in batch and fixed-bed reactors for phenol removal. Ozone Sci Eng 34:259–268. https://doi.org/10.1080/01919512.2012.688711
Faghih Nasiri E, Yousefi Kebria D, Qaderi F (2018) An experimental study on the simultaneous phenol and chromium removal from water using titanium dioxide photocatalyst. Civ Eng J 4:585–593. https://doi.org/10.28991/cej-0309117
Filho SSF, Piveli RP, Cutolo SA, De Oliveira AA (2013) Water treatment plant sludge disposal into stabilization ponds. Water Sci Technol 67:1017–1025. https://doi.org/10.2166/wst.2013.652
Greenberg AE, Clesceri LS, Eaton AD (2000) Standard methods for the examination of water and wastewater, 20th edn. American Public Health, Washington
Hameed BH, Rahman AA (2008) Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. 160:576–581. https://doi.org/10.1016/j.jhazmat.2008.03.028
Han X, Zhou TX, Xu SW et al (2017) Removal of Cr(VI) and phenol coupled with the reduction of sulfate by sulfate-reducing bacteria sludge. Int J Environ Sci Technol 14:2173–2180. https://doi.org/10.1007/s13762-017-1302-6
Hussain A, Dubey SK, Kumar V (2015) Kinetic study for aerobic treatment of phenolic wastewater. Water Resour Ind 11:81–90. https://doi.org/10.1016/j.wri.2015.05.002
Jiang J, Wang H, Chen X et al (2017) Journal of colloid and interface science enhanced photocatalytic degradation of phenol and photogenerated charges transfer property over BiOI-loaded ZnO composites. J Colloid Interface Sci 494:130–138. https://doi.org/10.1016/j.jcis.2017.01.064
Kiliç NK, Karacakaya P, Duygu E, Dönmez G (2012) Biodegradation of phenol by Synechocystis sp. in media including triacontanol hormone. Water Environ J 26:94–99. https://doi.org/10.1111/j.1747-6593.2011.00267.x
Kukadiya MA (2016) Study of removal of phenol by biological treatment methods-with reference to moving bed biofilm reactor & activated sludge process. Int J Eng Res Technol 5:400–404
Lakshmi MVVC, Sridevi V, Neharika E et al (2009) Effect of temperature and carbon source on phenol degradation by pseudomonas aeruginosa (NCIM 2074) and pseudomonas desmolyticum (NCIM 2028) and their comparison. Int J Chem Sci 7:2591–2601
Langroudi LO, Pahlavanzadeh H, Mousavi SM (2014) Statistical evaluation of a liquid desiccant dehumidification system using RSM and theoretical study based on the effectiveness NTU model. J Ind Eng Chem 20:2975–2983. https://doi.org/10.1016/j.jiec.2013.11.031
Lee SC, Lintang HO, Yuliati L (2014) Photocatalytic removal of phenol under visible light irradiation on zinc phthalocyanine/mesoporous carbon nitride nanocomposites. J Exp Nanosci 9:78–86. https://doi.org/10.1080/17458080.2013.814172
Moussavi G, Heidarizad M (2010) Biodegradation of mixture of phenol and formaldehyde in wastewater using a single-basin MSCR process. J Biotechnol 150:240–245. https://doi.org/10.1016/j.jbiotec.2010.08.012
Nassef EMR (2014) Removal of polyaromatic hydrocarbons from waste water by electrocoagulation. J Pet Gas Eng 5:32–42. https://doi.org/10.5897/JPGE
Omena SPF, Sader LT, Silva EL (2013) Simultaneous removal of phenol and nitrate in an anoxic fluidized bed reactor. Journal of environmental science and health. Part A Toxic Hazard Subs Environ Eng 48:581–591. https://doi.org/10.1080/10934529.2013.730459
Pham DT, Everaert G, Janssens N et al (2014) Algal community analysis in a waste stabilisation pond. Ecol Eng 73:302–306. https://doi.org/10.1016/j.ecoleng.2014.09.046
Pradeep NV, Anupama S, Hampannavar US (2011) Biodegradation of phenol using rotating biological contactor. Int J Environ Sci 2:105–114. https://doi.org/10.6088/ijes.00202010011
Pradeep NV, Anupama S, Navya K et al (2015) Biological removal of phenol from wastewaters: a mini review. Appl Water Sci 5:105–112. https://doi.org/10.1007/s13201-014-0176-8
Qaderi F, Babanezhad E (2017) Prediction of the groundwater remediation costs for drinking use based on quality of water resource, using artificial neural network. J Clean Prod 161:840–849. https://doi.org/10.1016/j.jclepro.2017.05.187
Qaderi F, Ayati B, Ganjidoust H (2011) Role of moving bed biofilm reactor and sequencing batch reactor in biological degradation of formaldehyde wastewater. Iran J Environ Health Sci Eng 8:295–306
Qaderi F, Sayahzadeh AH, Azizi M (2018) Efficiency optimization of petroleum wastewater treatment by using of serial moving bed biofilm reactors. J Clean Prod 192:665–677. https://doi.org/10.1016/j.jclepro.2018.04.257
Rastegar SO, Mousavi SM, Shojaosadati SA, Sheibani S (2011) Optimization of petroleum refinery effluent treatment in a UASB reactor using response surface methodology. J Hazard Mater 197:26–32. https://doi.org/10.1016/j.jhazmat.2011.09.052
Sabah A, Bancon-Montigny C, Rodier C et al (2016) Occurrence and removal of butyltin compounds in a waste stabilisation pond of a domestic waste water treatment plant of a rural French town. Chemosphere 144:2497–2506. https://doi.org/10.1016/j.chemosphere.2015.11.006
Senturk HB, Ozdes D, Gundogdu A et al (2009) Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: equilibrium, kinetic and thermodynamic study. J Hazard Mater 172:353–362. https://doi.org/10.1016/j.jhazmat.2009.07.019
Singh J, Prasanna L, Yang J et al (2016) Effect of pH values on recovery of nano particles (NPs) from the fine fraction of automobile shredder residue (ASR): an application of NPs for phenol. Process Saf Environ Prot. https://doi.org/10.1016/j.psep.2016.10.011
Turhan K, Uzman S (2008) Removal of phenol from water using ozone. Desalination 229:257–263. https://doi.org/10.1016/j.desal.2007.09.012
Wang Y, Song J, Zhao W et al (2011) In situ degradation of phenol and promotion of plant growth in contaminated environments by a single Pseudomonas aeruginosa strain. J Hazard Mater 192:354–360. https://doi.org/10.1016/j.jhazmat.2011.05.031
Wei G, Xia D, Li-Li W, Hong Y (2017) Isolation, selection, and biological characterization research of highly effective electricigens from MFCs for phenol degradation. Folia Microbiol 63:73–83. https://doi.org/10.1007/s12223-017-0536-5
WHO (1984) Guidelines for drinking water quality (vol. II): health criteria and supporting information. World Health Organization, Geneva
Yaacob NS, Mohamad R, Ahmad SA et al (2016) The influence of different modes of bioreactor operation on the efficiency of phenol degradation by Rhodococcus UKMP-5M. Rend Lincei 27:749–760. https://doi.org/10.1007/s12210-016-0567-x
Yamada A, Matsui A, Tsuji H (2013) Removal of phenol from saline water by polyamine chelating resin. Water Sci Technol 68:1819–1824. https://doi.org/10.2166/wst.2013.411
Yao J, Chen H, Jiang F et al (2017) Titanium dioxide and cadmium sulfide co-sensitized graphitic carbon nitride nanosheets composite photocatalysts with superior performance in phenol degradation under visible-light irradiation. J Colloid Interface Sci 490:154–162. https://doi.org/10.1016/j.jcis.2016.11.051
Zhu J, Lin J, Zhang B et al (2007) Simultaneous removal of phenol and nitrate in an anaerobic bioreactor. J Environ Eng 132:1073–1077
Acknowledgements
The authors would like to thank Dr. Hamid Amiri for his contribution to the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Qaderi, F., Sayahzadeh, A.H., Azizpour, F. et al. Efficiency modeling of serial stabilization ponds in treatment of phenolic wastewater by response surface methodology. Int. J. Environ. Sci. Technol. 16, 4193–4202 (2019). https://doi.org/10.1007/s13762-018-1816-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1816-6