Abstract
In this study, sepiolite-nano zero valent iron composite was synthesized and applied for its potential adsorption to remove phosphates from aqueous solution. This composite was characterized by different techniques. For optimization of independent parameters (pH = 3–9; initial phosphate concentration = 5–100 mg/L; adsorbent dosage = 0.2–1 g/L; and contact time = 5–100 min), response surface methodology based on central composite design was used. Adsorption isotherms and kinetic models were done under optimum conditions. The results indicated that maximum adsorption efficiency of 99.43 and 92% for synthetic solution and real surface water sample, respectively, were achieved at optimum conditions of pH 4.5, initial phosphate concentration of 25 mg/L, adsorbent dosage of 0.8 g/L, and 46.26 min contact time. The interaction between adsorbent and adsorbate is better described with the Freundlich isotherm (R 2 = 0.9537), and the kinetic of adsorption process followed pseudo-second-order model. Electrostatic interaction was the major mechanisms of the removal of phosphates from aqueous solution. The findings of this study showed that there is an effective adsorbent for removal of phosphates from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of dissolved phosphates in water has been of serious concern due to eutrophication (Qiu et al. 2015). Discharge of phosphates from domestic, industrial, and agricultural wastewater into water bodies led to rapid growth of aquatic plants (algal bloom), depletion of dissolved oxygen, and resulting loss of aquatic life (Yan et al. 2014). According to the World Health Organization (WHO), the maximum discharge limit of phosphorous is 0.5–1 mg/L (Yu and Chen 2015; Su et al. 2013, 2015), which is being accepted in many countries and regions. Hence, in order to prevent eutrophication in the water bodies, removal of phosphates from water and wastewater is necessary.
Various techniques, including chemical precipitation, ion exchange, reverse osmosis, biological removal, membrane, and adsorption, have been investigated for removal of phosphates from water (Su et al. 2015; Choi et al. 2012; Qiu et al. 2014; Yoshino et al. 2014; Zhang et al. 2012). Among mentioned techniques, adsorption method has been widely used to remove phosphates from waters due to simplicity and flexibility of design, simple operation, and cost-effectiveness (Woumfo et al. 2015; Yan et al. 2014; Yu and Chen 2015; Xie et al. 2015). Various adsorbents, such as slag and fly ash (Ragheb 2013), chitosan (Liu and Zhang 2015), amorphous zirconium oxide nanoparticles (Su et al. 2013) and zero valent iron (Almeelbi and Bezbaruah 2012), have been used for phosphates removal.
Nano sized zero valent iron (NZVI) particles have high-capacity reactive sites capable of adsorbing various pollutants (Dai et al. 2015). Due to that property, they have been used in the remediation of different pollutants exist in soil and water (Sun et al. 2014). Despite the advantages of NZVI, there are some technical challenges in application of NZVI particles; for example, the agglomeration of NZVI particles in aqueous solution restricts their mobility, and surface dispersivity that in combination with major defect of rapid oxidization of NZVI particles led to low reactivity (Fu et al. 2015; Sun et al. 2014). In order to overcome these problems, different support media such as chitosan (Geng et al. 2009), kaolinite (Üzüm et al. 2009), clinoptilolite (Fateminia and Falamaki 2013), sineguelas waste (Arshadi et al. 2014), bentonite (Chen et al. 2011), resin (Shu et al. 2010), rectorite (Luo et al. 2013), and graphene (Liu et al. 2014a) have been used. Liu et al. (2014a, b) were using pumice-supported nanoscale zero valent iron for the adsorption of mercury (II) and chromium (VI); it was reported that the pumice as a support enhanced efficiency of NZVI to remove Hg and Cr from wastewater (Liu et al. 2014b). Also, the use of zeolite, due to chemical stability and low price, as a support had successfully reported (Kim et al. 2013). Regardless of previous studies, researches effort to explore efficient local support has been continued.
Sepiolite is a clay mineral with a wide range of applications (Suárez and García-Romero 2012; Lescano et al. 2014). The vast application of sepiolite is related to its sorptive, rheological, and catalytic characteristics (Suárez and García-Romero 2012). Sepiolite is a hydrous magnesium silicate with fibrous morphology and crystalline structure (Lescano et al. 2014). Sepiolite with needle-shape structure is made of blocks, which are characterized by two layers of tetrahedral silica and an octahedral magnesium layer in central point (Tunç et al. 2012). Because of high surface area per unit mass and porous surface, sepiolite is a suitable candidate as a support for immobilization of nanoparticles (Soylemez et al. 2013). But before synthesizing sepiolite-immobilized materials, it is better to enhance the sepiolite’s surface area and overcome its natural restriction through thin channels and low surface acidity modification (Lescano et al. 2014). Previously, Malakootian et al. conducted studies on the removal of organic and inorganic pollutants from aqueous solution by zeolite, alumina, bauxite, and clinoptilolite (Malakootian et al. 2011, 2015a, b, 2016a, b, c). Also, Daneshkhah et al. (2017) used supported NZVI as an oxidant to remove metoprolol from polluted water, and introduced SPT-NZVI as an efficient oxidant to remove micro-pollutant from water (Daneshkhah et al. 2017).
The synthesis and characterization of the NZVI-stabilized sepiolite material as well as the development of a mathematical relation between the removal parameters was the aim of this study. The selected parameters were initial phosphates concentration, contact time, adsorbent dosage, and pH to efficient removal of phosphates via response surface methodology (RSM).
Materials and methods
Materials
Sepiolite was provided from Dorkav Mining Co., Iran. Chemical materials such as ammonium meta vanadate (NH4VO3), sodium borohydride (NaBH4), iron (III) chloride hexahydrate (FeCl3·6H2O), sodium hydroxide (NaOH), hydrochloric acid (HCl), sulfuric acid (H2SO4), absolute ethanol (C2H5OH), potassium dihydrogen orthophosphates (KH2PO4), and ammonium molybdate (NH4)Mo7O24·4H2O were of analytical grade and purchased from Merck Co., Germany.
Acid treatment of natural sepiolite
To prepare the acid modified sepiolite, 20 g/L of sepiolite (60-mesh sieved) suspension was treated by adding hydrochloric acid (0.5 mol/L) until the bubble was formed. It was stirred at 200 rpm for 24 h at room temperature. Then, the suspension was filtered and washed repeatedly with deionized water to neutralize. The neutralized sepiolite was dried in an oven at 50 °C for 24 h, and after sieved through a sieve # 60, it was used as a support in nano zero valent iron synthesis.
Preparation of Sep-NZVI
Sepiolite-nano zero valent iron particles (Sep-NZVI) used in this study was synthesized by using liquid phase in sodium borohydride reduction method (O’Carroll et al. 2013; Esfahani et al. 2014). Briefly, 1.8 g of iron (III) chloride was dissolved in a 4:1 ethanol: water mixture (48 mL absolute ethanol + 12 mL deionized water) and placed into a three-necked flask; then, 0.5 g of acid modified sepiolite was added to it (solution A). The prepared solution was stirred using a mechanical stirrer at room temperature for 10 min under N2 atmosphere. Then, 200 mL of 0.1 mol/L NaBH4 (solution B) was added drop-wise (2 drops in per second) into solution A under agitation and N2 atmosphere to avoid oxidation. After observing black precipitate particles in solution, in order to minimizing the size of Sep-NZVI, it was stirred for another 10 min. The black precipitate was separated from the liquid solution by vacuum filtration and washed three times with 30 mL absolute ethanol. Finally, Sep-NZVI was dried at 50 °C for 24 h under vacuum.
Sep-NZVI characterization
The shape and surface morphology of Sep-NZVI was obtained using Ziess (Germany) scanning electron microscopy (SEM). The elemental content of Sep-NZVI was recorded by energy-dispersive X-ray (EDAX) (SDD detector, Oxford instruments) coupled with SEM. FTIR spectra were recorded in the range of 4000–400 cm−1 by a Tensor 27, Brouker, Germany model fourier transform infrared spectrometer for analyzing of functional groups of natural sepiolite and Sep-NZVI. The crystalline phases present in Sep-NZVI were determined by x-ray diffractometer (XRD) (XPERT model) at 40 mA and 40 kV using Cokα radiation (λ = 1.78897Å) at 2θ between 10° and 100°.
Adsorption experiment
The batch experiments were conducted in Erlenmeyer flask. Phosphates stock solution was prepared by dissolving 0.2195 g of KH2PO4 into 1000 mL deionized water. For each adsorption experiment, 50 mL of known initial phosphates concentration was transferred to the flask, the solution pH was adjusted using a pH meter (HANNA, Romania) with dilute NaOH or H2SO4 solutions, and the proper amount of Sep-NZVI was added to the phosphates solution and was placed on the mechanical stirrer at 200 rpm during certain contact time. At the end of contact time, suspension was centrifuged at 3800 rpm for 10 min and filtered through whatman filter paper in order to separate the adsorbent. The phosphates concentration of the filtrate was determined by using vanadomolybdophosphoric acid colorimetric method (4500-P C) according to standard methods for the examination of water and wastewater (Clesceri et al. 1998), with UV–Vis spectrophotometer (Shimadzu, UV-1800, Japan). In order to enhance precision of the results, all experiments were done in duplicate and mean of values was used for data analysis. The removal efficiency (%) and adsorption capacity (mg/g) were calculated as follows:
where, C 0 , C t, and C e are the phosphates concentration at time 0, at time t, and at equilibrium (mg/L), respectively. V is the volume of solution (L), and m is the weight of adsorbent (g).
Experimental design
In order to design adsorption experiments, the response surface methodology (RSM) was used. RSM is a collection of mathematical and statistical method for improving, developing, and optimizing chemical and biochemical processes (Ye et al. 2016). In this study, the adsorption of phosphates into Sep-NZVI was optimized using RSM based on central composite design (CCD) to predict the influence of the parameters on the adsorption process. In the CCD, total of experimental runs was calculated as follows:
where N, c, and n are the total of experimental runs, the number of central points, and the number of parameters, respectively (Dasgupta et al. 2015). According to Eq. (3) and α of 1.96, the total runs of experiments was equal to 30. The independent variables and their levels in this study are shown in Table 1.
The simplest model in RSM based on a linear function was tested, and responses were adjusted with the following equation:
In above equation, β 0, β i, and X i are constant, linear regression coefficients, and independent parameters, respectively (Olya et al. 2015). Y shows the predicted response of parameters. In order to validate the model, analysis of variance (ANOVA) was used (Chakraborty et al. 2014).
Results and discussion
Characterization of natural sepiolite and Sep-NZVI
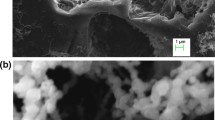
The morphology and shape of the Sep-NZVI analyzed by SEM imaging is shown in Fig. 1. The good dispersibility of NZVI on the needle-structure sepiolite was observed, and the spherical and linear chain of NZVI was successfully formed on the sepiolite structure with a size range from 20 to 70 nm without aggregation.
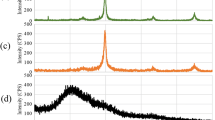
The EDAX spectrum (Fig. 2) provides the evidence for the presence of Fe0 on the surface of sepiolite elemental structure. The signal of Fe0 nanoparticle was at about 6–8 keV. Presence of Mg, O, and Si in the spectrum related to sepiolite elemental structure.
The analysis of FTIR spectra of natural sepiolite and Sep-NZVI was indicated in Fig. 3. The index bands of natural sepiolite are 3687, 3420, 1628, 1025, and 472 cm−1. The bands of 3420 and 1628 related to –OH bending vibration of adsorbed water. The height of bands decreased after synthesis of NZVI. Synthesize of NZVI on the surface of sepiolite led to displacement of band at 1025–1021 cm−1 and decrease in intensity. The addition of NZVI on the surface of sepiolite led to the creation of new band at 624 cm−1. The band at 473 relates to Si–O–Si bending vibration.
The observed narrow band at ~3687 cm−1 is attributed to OH vibration of hydroxyl group (belong to Mg3OH) in the blocks of natural sepiolite and Sep-NZVI (Perraki and Orfanoudaki 2008). The broad band at around 3420 cm−1 with band at ~1628 cm−1 is due to H–O–H vibration of powerful hydrogen bonded in the structure of the samples (Özcan and Gök 2012; Bakhtiary et al. 2013). The band at around 1020 cm−1 shows the stretching of Si–O in the Si–O–Si group of the tetrahedral sheet (Bakhtiary et al. 2013). The additional band at around 624 cm−1 in spectra of Sep-NZVI was observed corresponding to the Fe–O stretching vibrations, which is in line with the results of Arshadi et al. (2014), Singh et al. (2014) and Xiao et al. (2015a, b) (Arshadi et al. 2014; Singh et al. 2014; Xiao et al. 2015a). The band at 473 cm−1 is attributed to the Si–O–Si bending vibration of both samples (Gök et al. 2008).
The XRD pattern of Sep-NZVI is shown in Fig. 4. XRD pattern of Sep-NZVI showed strong peak (JCPDS card no. 06-0696) at about 52.37°, which is a characteristic reflection for nano zero valent iron (Sun et al. 2013). This represented that the iron exists in sepiolite surface is mainly in its Fe0 state.
Results of RSM based on CCD
Following linear equation in terms of coded factors for phosphates removal as well as the effects of each parameter on the response was obtained:
The synergistic and antagonistic effects on the response are shown by positive and negative sign, respectively. The results of experimental runs showed that at the selected range of four parameters, time, anion concentration, pH, and amount of Sep-NZVI, phosphate anions removal were 24–100%. It is obvious that lower amount of Sep-NZVI and highest amount of phosphates were led to the lowest removal efficiency, but to better understand the effect of parameters and their significance, the statistical test of ANOVA was done.
The results of analysis of variance (ANOVA) for the linear model were presented in Table 2. ANOVA is needed to determine the significance and adequacy of the model by large F value and low P value (Berkani et al. 2015). Based on Table 2, F value of 13.51 and P value less than 0.05 (0.0001) indicated that the model is significant.
According to the analysis of variation (ANOVA), the linear model is significant and can describe the relation between the response and variable adequately. Based on the Eq. (5), the influence of quota of each parameter on the percentage of removal was shown in Fig. 5; in this manner, among all parameters, the initial phosphates concentration and adsorbent dosage were found to be most effective parameters.
The optimization of parameters, including the solution pH, initial phosphates concentration, contact time, and adsorbent dosage, was the main target of this study. The optimal conditions were obtained at solution pH of 4.5, initial phosphates concentration of 25 mg/L, contact time of 46.26 min, and adsorbent dosage of 0.8 g/L with a predicted phosphates removal efficiency of 99.43%. The confirming experiment was carried out at optimal conditions and verified the predicted result (value equal to 99.31%). In order to test the applicability of Sep-NZVI in removal of phosphates from polluted water, under optimal condition, the removal efficiency of Sep-NZVI was examined toward phosphates removal from real river water with spiked amount of phosphates (25 mg/L). Some physicochemical characteristics of river water are presented in Table 3. Under these conditions, removal percentage of 92% was obtained and confirmed the applicability of adsorbent for real water.
Isotherm studies
Adsorption isotherms are important in characterizing the overall surface phenomena. The most common isotherms, Freundlich and Langmuir, were used to explain the interaction between phosphate anions (adsorbate) and Sep-NZVI (adsorbent). The Freundlich isotherm is a usual pattern for heterogeneous surfaces, while the Langmuir isotherm is common for homogeneous surfaces (Moussavi et al. 2013). The linear equation of Freundlich (Xiao et al. 2015b) and Langmuir (Moussavi et al. 2013) models were shown as Eqs. (6) and (7), respectively.
In above-mentioned equations, q e and q m are the phosphate anions adsorption capacity at equilibrium and maximum quantity of adsorbed phosphate anions per unit mass in Langmuir model (mg/g), respectively. C e is the amount of phosphate anions at equilibrium in solution (mg/L), and K f (mg/g (L/mg)1/n) and K L (L/mg) are the constants of Freundlich and Langmuir, respectively.
The adsorption isotherm studies were done in various concentration of adsorbent (0.5–2 g/L), pH of solution equal to 4.5, initial phosphates concentration of 25 mg/L, and 6 h contact time. The results obtained from the isotherm study are presented in Table 4. Freundlich isotherm was better fitted with experimental data than the Langmuir one (R 2 = 0.9537). The results suggested a multilayer adsorption onto a heterogeneous surface. Additionally, value of 1/n between 0 and 1 shows a favorable process for phosphates adsorption onto Sep-NZVI. Other studies have reported different results as Freuendlich isotherm (Ragheb 2013; Wang et al. 2015; Yin et al. 2011), Langmuir isotherm (Arshadi et al. 2015; Dai et al. 2014; Su et al. 2013), and both Fruendlich and Langmuir isotherm (Lu et al. 2015). This difference in studies results can be attributed to adsorbent type and characteristics of adsorbent surface.
Kinetic studies
In order to describe the rate controlling step in the phosphates adsorption onto Sep-NZVI, two kinetic models of pseudo-first order [Eq. (8)] and pseudo-second order [Eq. (9)] (Dai et al. 2014; Yao et al. 2013) were studied:
In these equations, q e and q t are the amounts of adsorbed phosphates at equilibrium and any time (mg/g), respectively. k 1 (1/min) and k 2 (g/mg min) are the adsorption rate constant of pseudo-first and pseudo-second-order model, respectively.
The kinetics models were investigated at optimum condition such as pH of 4.5, various phosphate concentrations of 5–100 mg/L, adsorbent dosage of 0.8 g/L, and 46.26 min contact time. The results of kinetic models were summarized in Table 5.
Adsorption of phosphates was better described with the pseudo-second-order model (R 2 = 0.9601). Based on the pseudo-second order, adsorption process is related to the amount of phosphates and the number of binding sites on the Sep-NZVI surface (adsorbent). In fact, limiting step for the adsorption process is the chemical reaction (Li et al. 2014). Wang et al. (2015) were investigated adsorption of phosphates onto magnetic core–shell composite MFC, they were reported that the pseudo-second-order model was better fitted with experimental data (Wang et al. 2015). The results of other research (Li et al. 2014; Arshadi et al. 2015; Lu et al. 2015; Zong et al. 2013; Yan et al. 2014) were in agreement with the finding of this study. The rate constant of pseudo-second-order model (K2) of other adsorbents was indicated in the Table 6 in comparison with the adsorbent in this study.
Interaction of studied parameters on adsorption of phosphates
The effect of parameters including adsorbent dosage, pH solution, contact time, and phosphates concentration on phosphates removal efficiency using Sep-NZVI are shown in Fig. 6.
Figure 6a shows the related influence of phosphates concentration and pH solution on removal efficiency of phosphates (%). It is observed that removal efficiency at higher value of pH and phosphates concentration was decreased. The effect of pH in adsorption of phosphates could be explained by attraction between adsorbate (phosphates concentration) and adsorbent (Sep-NZVI). At low solution pH, H+ species are present, which are useful for the protonation of adsorbent surface; as a result, the electrostatic attraction between positively charged Sep-NZVI particles and the phosphate anions enhanced and facilitated phosphates adsorption. But, at higher pH, present of OH− species charged the surface of adsorbent negatively; thereby, the electrostatic repulsion between negatively charged surface of adsorbent and the phosphate anions increased, which resulted in phosphates adsorption efficiency reduction (Wang et al. 2015). Arshadi et al. (2015) also obtained the similar result in the case of the adsorption of phosphates onto S-NaOH-NZVI (Arshadi et al. 2015).
In Fig. 6b, the influence of phosphates concentration and contact time on removal efficiency of phosphates (%) was shown. It can be understood that the removal efficiency of phosphates increased with increasing time and decreased with increasing phosphates concentration. For a fixed amount of adsorbent, at higher phosphates concentration, removal percentage decrease due to high ratio of phosphates concentration to active adsorption sites on the adsorbent surface, which leads to saturation of adsorbent surface and decrease in interaction between adsorbent and phosphate anions (Hassani et al. 2015; Asfaram et al. 2014). Liu et al. (2013) used NZVI derived from natural goethite as adsorbent for removal of phosphorus and found that percentage removal decreased with increasing phosphorus concentration (Liu et al. 2013).
Figure 6c shows interactive influence of contact time and adsorbent dosage in removal efficiency of phosphates. It can be deduced from Fig. 6c that at low amount of adsorbent dosage, increasing contact time led to elevate removal efficiency. Increasing contact time can be attributed to enhance accessibility of active adsorption sites for phosphate anions in the solution which is leading to increasing removal percentage (Al-Ghouti et al. 2009). Finding of Ragheb (2013) also is consistent with this study (Ragheb 2013).
Adsorption mechanism
In addition to major surface mechanisms including electrostatic interaction (outer sphere surface complex) and ion exchange (inner sphere surface complex) which have been proposed to describe the removal of phosphates from aqueous solution (Lu et al. 2009) on mineral adsorbents, a combination of reduction/precipitation and adsorption of phosphorus newly formed iron oxides caused further removal of phosphates (Fiedor et al. 1998).
The solution pH plays an important role in the above-mentioned mechanisms of the adsorption process. Under acidic condition, the surface of sepiolite will be positively charged; thus, interaction between phosphate anions and adsorbent will be electrostatic. While under alkaline condition, interaction between phosphate anions and FeOOH lead to incorporation of phosphates in the Fe3(PO4)3 percipitate structure. It should be mentioned that, the adsorptive nature of ferric oxyhydrate (FeOOH) is well documented (Sun et al. 2011).
In this study, the phosphates adsorption capacity at optimal conditions obtained 17.18 and 31 mg/g of natural sepiolite and Sep-NZVI, respectively, which this discrepancy of adsorption capacity can be related to influence of nano zero valent iron in removal efficiency. Thus, it can be stated that the electrostatic interaction was the main mechanism of phosphates removal, and co-precipitation also enhanced the adsorption removal efficiency.
Conclusion
The study successfully synthesized Sep-NZVI by using sodium borohydride reduction method and characterized it using various techniques such as SEM, EDAX, FTIR, and XRD. The maximum removal efficiency observed from synthetic solutions (phosphate model solutions) and surface water sample under optimum condition (Sep-NZVI dose: 0.8 g/L, pH: 4.5 and phosphates concentration: 25 mg/L) was 99.43 and 92%, respectively at a set contact time of 46.26 min. The experimental data obtained from this study indicated that the initial phosphates concentration and adsorbent dosage were most effective parameters. The sorption followed a pseudo-second-order kinetics and prescribed to the Freundlich adsorption model. This implies that the sorption was a multi-site interaction. Thus, electrostatic interaction could have been the major mechanism of the adsorption of phosphate. The findings of this study indicated that the Sep-NZVI has potential application in the removal of phosphates from aqueous media.
References
Al-Ghouti MA, Khraisheh MA, Ahmad MN, Allen S (2009) Adsorption behaviour of methylene blue onto Jordanian diatomite: a kinetic study. J Hazard Mater 165:589–598. doi:10.1016/j.hazmat.2008.0.018
Almeelbi T, Bezbaruah A (2012) Aqueous phosphate removal using nanoscale zero-valent iron. J Nanopart Res 14:1–14. doi:10.1007/s11051-012-0900-y
Arshadi M, Soleymanzadeh M, Salvacion J, Salimivahid F (2014) Nanoscale zero-valent iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: kinetics, thermodynamic and mechanism. J Colloid Interf Sci 426:241–251. doi:10.1016/j.jcis.2014.04.014
Arshadi M, Foroughifard S, Gholtash JE, Abbaspourrad A (2015) Preparation of iron nanoparticles-loaded Spondias purpurea seed waste as an excellent adsorbent for removal of phosphate from synthetic and natural waters. J Colloid Interf Sci 452:69–77. doi:10.1016/j.jcis.2015.04.019
Asfaram A, Fathi M, Khodadoust S, Naraki M (2014) Removal of Direct Red 12B by garlic peel as a cheap adsorbent: kinetics, thermodynamic and equilibrium isotherms study of removal. Spectrochim Acta A 127:415–421. doi:10.1016/j.saa.2014.02.092
Bakhtiary S, Shirvani M, Shariatmadari H (2013) Characterization and 2, 4-D adsorption of sepiolite nanofibers modified by N-cetylpyridinium cations. Microporous Mesoporous Mat 168:30–36. doi:10.1016/j.micromeso.2012.09.022
Berkani M, Bouhelassa M, Bouchareb MK (2015) Implementation of a venturi photocatalytic reactor: optimization of photodecolorization of an industrial azo dye. Arab J Chem. doi:10.1016/j.arabjc.2015.07.004
Chakraborty S, Dasgupta J, Farooq U, Sikder J, Drioli E, Curcio S (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Membr Sci 456:139–154. doi:10.1016/j.memsci.2014.01.016
Chen ZX, Jin XY, Chen Z, Megharaj M, Naidu R (2011) Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J Colloid Interf Sci 363:601–607. doi:10.1016/j.jcis.2011.07.057
Choi JW, Choi YS, Hong SW, Kim DJ, Lee SH (2012) Effect of pH and coexisting anions on removal of phosphate from aqueous solutions by inorganic-based mesostructures. Water Environ Res 84:596–604. doi:10.2175/106143012X13373575830755
Clesceri L, Greenberg A, Eaton A (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC (variously paged)
Dai L, Wu B, Tan F, He M, Wang W, Qin H, Tang X, Zhu Q, Pan K, Hu Q (2014) Engineered hydrochar composites for phosphorus removal/recovery: lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw. Biores Technol 161:327–332. doi:10.1016/j.biortech.2014.03.086
Dai Y, Hu Y, Jiang B, Zou J, Tian G, Fu H (2015) Carbothermal synthesis of ordered mesoporous carbon-supported nano zero-valent iron with enhanced stability and activity for hexavalent chromium reduction. J Hazard Mater 309:249–258. doi:10.1016/j.jhazmat.2015.04.013
Daneshkhah M, Hossaini H, Malakootian M (2017) Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. J Environ Chem Eng 5:3490–3499. doi:10.1016/j.jece.2017.06.040
Dasgupta J, Singh M, Sikder J, Padarthi V, Chakraborty S, Curcio S (2015) Response surface-optimized removal of Reactive Red 120 dye from its aqueous solutions using polyethyleneimine enhanced ultrafiltration. Ecotox Environ Safe 121:271–278. doi:10.1016/j.ecoenv.2014.12.041
Esfahani AR, Hojati S, Azimi A, Farzadian M, Khataee A (2014) Enhanced hexavalent chromium removal from aqueous solution using a sepiolite-stabilized zero-valent iron nanocomposite: impact of operational parameters and artificial neural network modeling. J Taiwan Inst Chem Eng 49:172–182. doi:10.1016/j.jtice.2014.11.011
Fateminia FS, Falamaki C (2013) Zero valent nano-sized iron/clinoptilolite modified with zero valent copper for reductive nitrate removal. Process Saf Environ 91:304–310. doi:10.1016/j.psep.2012.07.005
Fiedor JN, Bostick WD, Jarabek RJ, Farrell J (1998) Understanding the mechanism of uranium removal from groundwater by zero-valent iron using X-ray photoelectron spectroscopy. Environ Sci Technol 32:1466–1473
Fu R, Yang Y, Xu Z, Zhang X, Guo X, Bi D (2015) The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 138:726–734. doi:10.1016/j.chemosphere.2015.07.051
Geng B, Jin Z, Li T, Qi X (2009) Kinetics of hexavalent chromium removal from water by chitosan-Fe 0 nanoparticles. Chemosphere 75:825–830. doi:10.1016/j.chemosphere.2009.01.009
Gök Ö, Özcan AS, Özcan A (2008) Adsorption kinetics of naphthalene onto organo-sepiolite from aqueous solutions. Desalination 220:96–107. doi:10.1016/j.desal.2007.01.025
Hassani A, Soltani RDC, Karaca S, Khataee A (2015) Preparation of montmorillonite–alginate nanobiocomposite for adsorption of a textile dye in aqueous phase: isotherm, kinetic and experimental design approaches. J Ind Eng Chem 21:1197–1207. doi:10.1016/j.jiec.2014.05.034
Kim SA, Kamala-Kannan S, Lee KJ, Park YJ, Shea PJ, Lee WH, Kim HM, Oh BT (2013) Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem Eng J 217:54–60. doi:10.1016/j.cej.2012.11.097
Lescano L, Castillo L, Marfil S, Barbosa S, Maiza P (2014) Alternative methodologies for sepiolite defibering. Appl Clay Sci 95:378–382. doi:10.1016/j.clay.2014.05.001
Li G, Gao S, Zhang G, Zhang X (2014) Enhanced adsorption of phosphate from aqueous solution by nanostructured iron (III)–copper (II) binary oxides. Chem Eng J 235:124–131. doi:10.1016/j.cej.203.09.021
Liu X, Zhang L (2015) Removal of phosphate anions using the modified chitosan beads: adsorption kinetic, isotherm and mechanism studies. Powder Technol 277:112–119. doi:10.1016/j.powtec.2015.02.055
Liu H, Chen T, Zou X, Xie Q, Qing C, Chen D, Frost RL (2013) Removal of phosphorus using NZVI derived from reducing natural goethite. Chem Eng J 234:80–87. doi:10.1016/j.cej.2013.08.061
Liu F, Yang J, Zuo J, Ma D, Gan L, Xie B, Wang P, Yang B (2014a) Graphene-supported nanoscale zero-valent iron: removal of phosphorus from aqueous solution and mechanistic study. J Environ Sci 26:1751–1762. doi:10.1016/j.jes.2014.06.016
Liu T, Wang ZL, Yan X, Zhang B (2014b) Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: pumice-supported nanoscale zero-valent iron. Chem Eng J 245:34–40. doi:10.1016/j.cej.2014.02.011
Lu S, Bai S, Zhu L, Shan H (2009) Removal mechanism of phosphate from aqueous solution by fly ash. J Hazard Mater 161:95–101. doi:10.1016/j.jhazmat.2008.02.123
Lu J, Liu D, Hao J, Zhang G, Lu B (2015) Phosphate removal from aqueous solutions by a nano-structured Fe–Ti bimetal oxide sorbent. Chem Eng Res Des 93:652–661. doi:10.1016/j.cherd.2014.05.001
Luo S, Qin P, Shao J, Peng L, Zeng Q, Gu JD (2013) Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem Eng J 223:1–7. doi:10.1016/j.cej.2012.10.088
Malakootian M, Yousefi N, Jaafarzadeh HN (2011) Kinetics modeling and isotherms for adsorption of phosphate from aqueous solution by modified clinoptilolite. Water Wastewater 22:21–22
Malakootian M, Javdan M, Iranmanesh F (2015a) Fluoride removal study from aqueous solutions using Jajarm bauxite: case study on Koohbanan water. Fluoride 48:113–122
Malakootian M, Mansoorian HJ, Hosseini A, Khanjani N (2015b) Evaluating the efficacy of alumina/carbon nanotube hybrid adsorbents in removing azo Reactive Red 198 and Blue 19 dyes from aqueous solutions. Process saf Environ 96:125–137. doi:10.1016/j.psep.2015.05.002
Malakootian M, Nori Sepehr M, BahrainiS Zarrabi M (2016a) Capacity of natural and modified zeolite with cationic surfactant in removal of antibiotic tetracycline from aqueous solutions. Koomesh 17:779–788
Malakootian M, Pourshaban-Mazandarani M, Hossaini H, Ehrampoush MH (2016b) Preparation and characterization of TiO2 incorporated 13X molecular sieves for photocatalytic removal of acetaminophen from aqueous solutions. Process saf Environ 104:334–345. doi:10.1016/j.psep.2016.09.018
Malakootian M, Ehrampoush MH, Hossaini H, Pourshaban-Mazandarani M (2016c) Acetaminophen removal from Aqueous Solutions by TiO2-X photo catalyst. Tolooebehdasht 14:200–213
Moussavi G, Hosseini H, Alahabadi A (2013) The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4 Cl-induced activated carbon. Chem Eng J 214:172–179. doi:10.1016/j.cej.2012.10.034
O’carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122. doi:10.1016/j.advwatres.2012.02.005
Olya M, Vafaee M, Jahangiri M (2015) Modeling of acid dye decolorization by TiO2–Ag2O nano-photocatalytic process using response surface methodology. J Saudi Chem Soc. doi:10.1016/j.jscs.2015.07.006
Özcan AS, Gök Ö (2012) Structural characterization of dodecyltrimethylammonium (DTMA) bromide modified sepiolite and its adsorption isotherm studies. J Mol Struct 1007:36–44. doi:10.1016/j.molstruc.2011.09.044
Perraki T, Orfanoudaki A (2008) Study of raw and thermally treated sepiolite from the Mantoudi area, Euboea, Greece. J Therm Anal Calorim 91:589–593. doi:10.1007/s10973-007-8329-8
Qiu L, Wang G, Zhang S, Huang K (2014) Phosphate removal through crystallization using hydrothermal modified steel slag-based material as seed crystal. Desalin Water Treat 52:384–389. doi:10.1080/19443994.2013.795725
Qiu L, Zheng P, Zhang M, Yu X, Abbas G (2015) Phosphorus removal using ferric–calcium complex as precipitant: parameters optimization and phosphorus-recycling potential. Chem Eng J 268:230–235. doi:10.1016/j.cej.2014.12.107
Ragheb SM (2013) Phosphate removal from aqueous solution using slag and fly ash. HBRC J 9:270–275. doi:10.1016/j.hbrcj.2013.08.005
Shu HY, Chang MC, Chen CC, Chen PE (2010) Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution. J Hazard Mater 184:499–505. doi:10.1016/j.jhazmat.2010.08.064
Singh P, Raizada P, Kumari S, Kumar A, Pathania D, Thakur P (2014) Solar-Fenton removal of malachite green with novel Fe0-activated carbon nanocomposite. Appl Catal A-Gen 476:9–18. doi:10.1016/j.apcata.2014.02.009
Soylemez S, Kanik FE, Tarkuc S, Udum YA, Toppare L (2013) A sepiolite modified conducting polymer based biosensor. Colloid Surf B 111:549–555. doi:10.1016/j.colsurfb.2013.07.013
Su Y, Cui H, Li Q, Gao S, Shang JK (2013) Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res 47:5018–5026. doi:10.1016/j.watres.2013.05.044
Su Y, Yang W, Sun W, Li Q, Shang JK (2015) Synthesis of mesoporous cerium–zirconium binary oxide nanoadsorbents by a solvothermal process and their effective adsorption of phosphate from water. Chem Eng J 268:270–279. doi:10.1016/j.cej.2015.01.070
Suárez M, García-romero E (2012) Variability of the surface properties of sepiolite. Appl Clay Sci 67:72–82. doi:10.1016/j.clay.2012.06.003
Sun Y, Wang Q, Yang S, Sheng G, Guo Z (2011) Characterization of nano-iron oxyhydroxides and their application in UO2 2+ removal from aqueous solutions. J Radioanal Nucl Chem 290:643–648. doi:10.1007/s10967-011-1325-2
Sun Z, Zheng S, Ayoko GA, Frost RL, Xi Y (2013) Degradation of simazine from aqueous solutions by diatomite-supported nanosized zero-valent iron composite materials. J Hazard Mater 263:768–777. doi:10.1016/j.jhazmat.2013.10.045
Sun X, Yan Y, Li J, Han W, Wang L (2014) SBA-15-incorporated nanoscale zero-valent iron particles for chromium (VI) removal from groundwater: mechanism, effect of pH, humic acid and sustained reactivity. J Hazard Mater 266:26–33. doi:10.1016/j.jhazmat.2013.12.001
Tunc S, Duman O, Kancı B (2012) Rheological measurements of Na-bentonite and sepiolite particles in the presence of tetradecyltrimethylammonium bromide, sodium tetradecyl sulfonate and Brij 30 surfactants. Colloid Surf A 398:37–47. doi:10.1016/j.colsurfa.2012.02.006
Üzum Ç, Shahwan T, Eroğlu AE, Hallam KR, Scott TB, Lieberwirth I (2009) Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl Clay Sci 43:172–181. doi:10.1016/j.clay.2008.07.030
Wang W, Zhang H, Zhang L, Wan H, Zheng S, Xu Z (2015) Adsorptive removal of phosphate by magnetic Fe3 O4@ C@ ZrO2. Colloid Surf A 469:100–106. doi:10.1016/j.colsurfa.2015.01.002
Woumfo ED, Siewe JM, Njopwouo D (2015) A fixed-bed column for phosphate removal from aqueous solutions using an andosol-bagasse mixture. J Environ Manag 151:450–460. doi:10.1016/j.jenvman.2014.11.029
Xiao J, Gao B, Yue Q, Gao Y, Li Q (2015a) Removal of trihalomethanes from reclaimed-water by original and modified nanoscale zero-valent iron: characterization, kinetics and mechanism. Chem Eng J 262:1226–1236. doi:10.1016/j.cej.2014.10.080
Xiao L, Xiong Y, Tian S, He C, Su Q, Wen Z (2015b) One-dimensional coordination supramolecular polymer [Cu (bipy)(SO4)] n as an adsorbent for adsorption and kinetic separation of anionic dyes. Chem Eng J 265:157–163. doi:10.1016/j.cej.2014.11.134
Xie J, Lin Y, Li C, Wu D, Kong H (2015) Removal and recovery of phosphate from water by activated aluminum oxide and lanthanum oxide. Powder Technol 269:351–357. doi:10.1016/j.powtec.2014.09.024
Yan Y, Sun X, Ma F, Li J, Shen J, Han W, Liu X, Wang L (2014) Removal of phosphate from wastewater using alkaline residue. J Environ Sci 26:970–980. doi:10.1016/S1001-0742(13)60537-9
Yao Y, Gao B, Chen J, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47:8700–8708. doi:10.1021/es4012977
Ye J, Cong X, Zhang P, Zeng G, Hoffmann E, Liu Y, Wu Y, Zhang H, Fang W, Hahn HH (2016) Application of acid-activated Bauxsol for wastewater treatment with high phosphate concentration: characterization, adsorption optimization, and desorption behaviors. J Environ Manag 167:1–7. doi:10.1016/j.jenvman.2015.ll.023
Yin H, Yun Y, Zhang Y, Fan C (2011) Phosphate removal from wastewaters by a naturally occurring, calcium-rich sepiolite. J Hazard Mater 198:362–369. doi:10.1016/j.jhazmat.2011.10.072
Yoshino H, Tokumura M, Kawase Y (2014) Simultaneous removal of nitrate, hydrogen peroxide and phosphate in semiconductor acidic wastewater by zero-valent iron. J Environ Sci Heal A 49:998–1006. doi:10.1080/10934529.2014.894841
Yu Y, Chen JP (2015) Key factors for optimum performance in phosphate removal from contaminated water by a Fe–Mg–La tri-metal composite sorbent. J Colloid Interf Sci 445:303–311. doi:10.1016/j.jcis.2014.12.056
Zhang G, Liu H, Liu R, Qu J (2009) Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J Colloid Interf Sci 335:168–174. doi:10.1016/j.jcis.2009.03.019
Zhang Y, Wang L, Lu D, Shi X, Wang C, Duan X (2012) Sensitive determination of bisphenol A base on arginine functionalized nanocomposite graphene film. Electrochim Acta 80:77–83. doi:10.1016/j.electacta.2012.06.117
Zhu Y, Zhu Z, Chen Y, Yang F, Qin H, Xie L (2013) Kinetics and thermodynamics of sorption for as (V) on the porous biomorph-genetic composite of α-Fe2O3/Fe3O4/C with eucalyptus wood hierarchical microstructure. Water Air Soil Pollut 224:1–19. doi:10.1016/j.seppur.2013.05.048
Zong E, Wei D, Wan H, Zheng S, Xu Z, Zhu D (2013) Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem Eng J 221: 193-203. DOI: http://dx.doi.org/!0.1016/j.cej.2013.01.088
Acknowledgements
This research was conducted at the Environmental Health Engineering Research Center and was sponsored by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences. The authors take this opportunity to express their gratitude for the support and assistance extended by the facilitators during the conduct of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Hari Pant.
Rights and permissions
About this article
Cite this article
Malakootian, M., Daneshkhah, M. & Hossaini, H. Removal of phosphates from aqueous solution by sepiolite-nano zero valent iron composite optimization with response surface methodology. Int. J. Environ. Sci. Technol. 15, 2129–2140 (2018). https://doi.org/10.1007/s13762-017-1520-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1520-y