Abstract
Zooplankton assemblages in relation to water quality parameters at lentic and lotic habitats of Air Itam Reservoir, Penang, Malaysia, were analysed. Five sampling stations were designated, including three stations located in the reservoir (Stations A, B, and C) and two stations in the upstream inflow and downstream outflow (Stations D and E, respectively). The most common and dominant zooplankton found in the lake were Polyarthra vulgaris, Ceriodaphnia cornuta, Mesocyclops leuckarti, and Thermocyclops crassus. Water Quality Index, calculated as recommended by the Malaysian Department of Environment, ranged between 88.46 and 93.6, showed that the reservoir was clean. Low numbers of species and value of the Shannon–Wiener Diversity Index were recorded at stations located upstream and downstream of the reservoir. Cluster analysis based on the abundance of zooplankton species distinguished the sampling stations into two groups (lentic and lotic groups, comprised of Stations A, B, and C; and Stations D and E, respectively). This study showed that zooplankton occurrence and abundance were associated with the quality of their environment, and zooplankton community provides information about the reservoir ecosystem, thus reflecting the importance of biomonitoring in lake assessment and management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooplankton communities, including organisms such as rotifers, cladocerans, and copepods, were used as biological parameters because they play a central role in aquatic food webs between the primary producers (phytoplankton) and higher trophic levels and have an important role in energy flow and nutrient cycling in aquatic ecosystems (Sousa et al. 2008). Zooplankton studies may also be useful in the prediction of long-term changes in lake ecosystems, for example, zooplankton indices such as Wetland Zooplankton Index (WZI) that was used in water quality assessment (Khalifa et al. 2015). Monitoring of zooplankton community structure can also be useful in detecting patterns and changes in species composition that may be related to changes in water quality (Thorp and Rogers 2015; Overholt et al. 2016), for instance, Asplanchna brightwelli and cladoceran abundance reflected a eutrophic water body (Haberman et al. 2007), while Mesocyclops leuckarti prefers oligo-mesotrophic conditions (Ramdani et al. 2001).
The quality of water must be assessed in terms of its physical, chemical, and biological properties, as they are all interrelated. In Malaysia, lakes and reservoirs are important water resources, with at least 90 man-made lakes being used for various purposes. Fifty-five lakes are used for water supply and irrigation (61%), while the remaining 35 are used for hydropower, flood control, silt retention, and recreational activities (39%). In 2005, more than 60% of the 90 man-made lakes in Malaysia were assessed to be nutrient rich or eutrophic, while the remainder were believed to be mesotrophic (Zati and Zulkifli 2007). Since reservoirs are considered to be unstable and unpredictable environments, biomonitoring is necessary to help preserve and manage water resources by assessing their physical, chemical, and biological parameters (Wan Maznah and Mansor 2002; Wan Maznah and Makhlough 2015; DeBoer et al. 2016).
Air Itam Reservoir is the smallest and oldest reservoir in the Penang State, constructed for domestic water supply. The reservoir was one of the first major engineering projects undertaken by the City Council of George Town after gaining independence (PBA 2007). To date, little scientific studies have taken place and no data are available on its zooplankton communities. Therefore, this study was conducted to provide a comprehensive evaluation of the zooplankton community structure at various sampling stations, as part of the continuous biological monitoring in Air Itam Reservoir. Water Quality Index (WQI) (Department of Environment (DOE) 2010) was calculated to determine the status of the lake as an effort to develop better management and monitoring programmes.
Materials and methods
Sampling site
Air Itam Reservoir, constructed between 1958 and 1962, is the smallest reservoir in Penang, Malaysia, and is located in an area of tropical forested hills (PBA 2007). It was created by blocking the Air Itam River and can hold up to 500 million gallons of water. The reservoir receives an average annual rainfall of about 2300 mm/year, and the minimum and maximum water levels are 201.17 and 234.70 m above sea level, respectively. The catchment area of the reservoir includes recreational gardens, farms and fruit orchards, as well as forest reserves (PBA 2007).
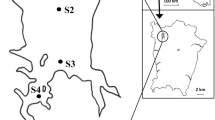
In this study, five sampling stations in the Air Itam Reservoir were investigated. Three stations were in the reservoir itself (A, B, and C), one upstream of the lake (D), and one downstream of the lake (E) (Fig. 1). Station A (05°.23′.41.80″N, 100°.15′.42.23″E) was located in the limnetic zone, with a maximum depth of 30 m. Station B (05°.23′.37.69″N, 100°.15′.37.01″E) was also located in the limnetic zone, and its mean depth was about 25 m. Station C (05°.23′.31.90″N, 100°.15′.32.14″E) was located in the littoral zone near the river mouth. The mean depth of Station C ranged between 8 and 10 m. Station D (05°.23′.26.76″N, 100°.15′.24.40″E) was located in the small stream that flows into the reservoir and was surrounded by tropical forest. Station E (05°.24′.01.02″N, 100°.16′20.82″E) was located about 1.14 km downstream of the reservoir. Station E received water seepage from the reservoir and was located near human settlements. The depth was determined by using high-frequency digital depth sounder (Hondex, Japan).
Sampling design
Samples were collected monthly from May 2006 to February 2007 in the reservoir and streams to assess the water quality and community structure of zooplankton. Temperature, pH, dissolved oxygen (DO), and conductivity were recorded in situ approximately at 2 m below the surface (in the lentic stations) using a Hydrolab SRV3-DL (Surveyor 3 Data Logger). Total dissolved solids (TDS) were measured using a TDS meter (Model sesnION5) according to APHA (1998). Biological oxygen demand (BOD) was measured through differential oxygen content using the Winkler method (APHA 1998). The chemical oxygen demand (COD) in the sample was determined using the ampule method (APHA 1998). The absorbance of the solution was measured using a spectrophotometer (Hitachi U-1100) for low-level COD concentration. Total suspended solids (TSS) were analysed using the drying method (APHA 1998), and water transparency was measured using a Secchi disk.
Concentration of ammonium (NH4–N) in water samples was determined using the ammonia low-level indophenol method (APHA 1998). Nitrite (NO2–N) concentration was measured using the calorimetric method, and nitrate (NO3–N) was determined by calculating the column reduction efficiency (cadmium reduction method) and the NO2 concentration (APHA 1998). Phosphate (PO4–P) concentration was measured using the ascorbic acid method (APHA 1998).
To obtain zooplankton samples, 40 L of water was collected at each sampling station. Collected water samples were filtered through a Wisconsin plankton net with a mesh size of 80 µm. A quantity of 100 mL filtrates containing the zooplankton were preserved with 4% buffered formaldehyde in pre-cleaned polyethylene bottles, capped and labelled. Triplicate samples were collected from each station.
Zooplankton were enumerated in a Bogorov tray under an inverted microscope (Olympus CK2, Japan). Copepod specimens were sorted and counted under a dissecting microscope (Olympus SZ61, Japan) magnified at 20× and 40×. Individual copepods were transferred onto glass slides coated with lactophenol and dissected for identification, using a compound microscope (Olympus CX41, Japan). All zooplankton were identified with the help of taxonomic keys, drawings, and descriptions (Pennak 1978; Idris and Fernando 1981; Idris 1983; Smirnov 1996; Dumont 2003).
The Species Richness Index was determined by the number of species present, while species diversity was determined using the Shannon–Wiener Index (H′) (Ludwig and Reynolds 1998). The Evenness Index (E) was calculated to measure how evenly the individuals in the community were distributed across the different species present following Ludwig and Reynolds (1998). The dominant zooplankton species at all sampling stations was determined based on the Importance Species Indices (ISI) (Rushforth and Brock 1991) by multiplying the percentage frequency of the taxon by its average relative density. This index is preferable because it reflects both the distribution and abundance of a taxon in the ecosystem (Wan Maznah and Mansor 2002).
Water Quality Index (WQI)
The assessment of water quality status was carried out based on the Water Quality Index recommended by the Department of Environment Malaysia (Department of Environment (DOE) 2010). Deepthi et al. (2014) defined WQI as a rating reflecting the composite influence of different water quality parameters, with the purpose of expressing complex water quality data as information that is accessible and usable by the public. Six water quality parameters are included in this index: dissolved oxygen (DO), biological oxygen demand (BOD), chemical oxygen demand (COD), ammonium-nitrogen (AN), total suspended solids (TSS), and pH. The use of WQI was based on the pollution load of the six parameters, which were converted to subindices (SI) using the best fit equations (Department of Environment (DOE) 2010). The index ranged from 0 to 100, where 100 represented excellent water quality condition. Based on the range of WQI obtained, the water condition was categorised either as clean (81–100), slightly polluted (60–80) or polluted (0–59). Water Quality Index was computed according to the following equation:
\({\text{WQI}} = (0.22 \times {\text{SI}}\;{\text{DO}}) + (0.19 \times {\text{SI}}\;{\text{BOD}}) + (0.16 \times {\text{SI}}\;{\text{COD}}) + (0.15 \times {\text{SI}}\;{\text{AN}}) + (0.16 \times {\text{SI}}\;{\text{TSS}}) + (0.12 \times {\text{SI}}\;{\text{pH}}),\) where SI is the subindex of each parameter (DOE 2010).
Statistical analysis
Analysis of variance (one-way ANOVA) and the Tukey HSD tests were used to determine statistically significant differences (P < 0.01) of water quality parameters between sampling stations. The data were transformed to natural logarithms (log10(x)) to normalise them. The tests were performed using MINITAB® Release 14.12.0. Correlations between the 10 most dominant zooplankton species with the water quality parameters were identified using Pearson’s correlation (SPSS version 11.0, Chicago, IL, USA). Canonical correspondence analysis (CCA) was conducted using multi-variate statistical package (MVSP) version 3.13d (Kovach Computing Services, UK). CCA was performed in order to identify any relationships between the abundance of zooplankton species and the environmental parameters analysed. Cluster analysis (CA), whose primary purpose is to categorise objects of the system into clusters or categories based on their similarities or dissimilarities, was used in this study. Hierarchical agglomerative CA was performed by the means of Ward’s method, using Euclidean distances as a measure of similarity (Fatema et al. 2015). Cluster analysis was carried out using the MVSP software.
Results and discussion
Water quality
The average water quality parameters at each sampling station in the Air Itam Reservoir are presented in Table 1, with significant differences being measured for temperature (P < 0.01), DO (P < 0.01), conductivity (P < 0.01), and PO4–P (P < 0.01). Temperature recorded at Stations A, B, and C (reservoir) was significantly higher than Stations D (upstream) and E (downstream) with the lowest value shown at Station D. Dissolved oxygen showed a significant increase from Stations A to C, but Station C was not significantly different from Stations D and E. Conductivity and TDS were significantly lower at Station A compared to Station B, while no significant differences were observed between Stations C, D, and E. Phosphate content increased from Stations A to C, but decreased to the lowest value at Station E. No significant differences were observed among stations for BOD, COD, pH, TSS, NH4–N, NO2–N, and NO3–N (all P > 0.01).
Average values of the WQI at each station are shown in Table 2. The highest WQI was observed at Station C (93.64), followed by Station B (92.61), Station A (92.02), Station D (91.55), and Station E (88.46). Water conditions at all stations were categorised as clean, with Station C in Class I and other stations in Class II.
The Water Quality Index (WQI) categorised the water quality of Air Itam Reservoir as clean for all stations (Table 2). Among the five sampling stations evaluated, the lowest WQI was observed at Station E (88.46), indicating relatively polluted water. This is most likely due to its location (downstream of the lake), indirectly receiving discharges from untreated domestic waste water and farming through runoff during the rainy season, which occurred between May and September 2006 (Malaysia Meteorological Centre, unpublished data). Based on the WQI obtained at all stations (Table 2), Station C was classified as Class I, where no treatment is necessary, while the remaining stations were in Class II, which requires conventional treatment, as recommended by DOE (2010). However, all the stations are interconnected; hence, there is a need for a detailed continuous monitoring program to be conducted in order to ensure good health status of the reservoir, especially when various developments are expected to take place around the reservoir in the near future.
Zooplankton
Zooplankton collected in this study included Rotifera (15 species), Cladocera (6 species), and Copepoda (5 species) (Table 3), with Copepoda having the highest abundance (74.77%), followed by Rotifera (16.77%) and Cladocera (7.72%) (Fig. 2). Stations B, C, D, and E showed higher relative abundance of Copepoda (74.3, 68.7, 54.2, and 56.0%, respectively), followed by Rotifera and Cladocera, while Station A had higher relative abundance of Rotifera (50.5%) compared to Copepoda (27.2%). Cladocera was not found at Station D (Fig. 3). All species were abundant (61–100.00%) at Stations A, B, and C. However, Thermocyclops crassus and Tropocyclops prasinus were absent at Station A. Average relative abundance (31–60.00%) was recorded for Lecane luna at Station B and Brachionus falcatus, Pompholyx complanata, and Trichocerna similis at Station C. At Stations D and E, most species were absent, except for Asplanchna brightweli, Lecane luna, Pompholyx complanata, Testudinella patina, and L. (Monostilla) bulla, Harpaticoida, Microcyclops varicans, and Tropocyclops prasinus. In addition to Station E, Brachionus patulus and Diaphanosoma excism were recorded at average relative abundance, while a rare species was identified as Ceriodaphnia cornuta (Table 3). Based on ISI values (Table 4), the reservoir was dominated by Polyarthra vulgaris, Tropocyclops prasinus, and Microcyclops varicans, especially at Stations A, B, and C.
The distribution of zooplankton varied among stations, corresponding with changes in water quality parameters. In this study, Rotifera dominated the Air Itam Reservoir in terms of species richness (15 species), but occurred at low density, representing only 16.77%. The fact that Rotifera can be found at all sampling stations may be related to their species-specific characteristics. Rotifera were less abundant at Stations D and E compared to Stations A, B, and C, probably due to higher water velocity in the upstream and downstream sampling stations. These weak swimmers, in which their swimming ability ranges from 0.17–2.5 mm/s (Thorp and Rogers 2015), may not be able to resist the fast flowing water and were thus drifted by the water. The most dominant species of Rotifera in this study was P. vulgaris, which was reported to be particularly abundant in tropical and subtropical areas (Nogueira 2001).
Cluster analysis distinguished the sampling stations into two groups, based on the abundance of zooplankton species (Fig. 4). High percentage similarities were recorded among Stations A, B, and C, while Stations D and E, located on the streams, were clustered together. Figure 4 shows the cluster analysis based on the abundance of zooplankton species at all sampling stations. The dendrogram showed that Stations D and E were separated from Stations A, B, and C. Streams are characterised by a continual downstream movement of water, dissolved substances, and suspended particles, therefore affecting the zooplankton species composition compared to the stations located in the reservoir (Stations A, B, and C). The stations situated within streams (Stations D and E) showed the lowest percentage of similar occurrence of zooplankton species.
Diversity, evenness indices, and species richness for all stations are presented in Table 5. The highest and lowest diversity indices were observed at Stations C and D, respectively. The highest species richness was observed at Stations B and C, both with 26 species, while the lowest species richness was recorded at Station D with 8 species.
The low numbers in diversity of zooplankton in these stations (Stations D and E) may be due to the current velocity/lotic conditions reducing the composition and abundance of the zooplankton communities compared to stations located in the reservoir (lentic system). Based on the Water Quality Index (WQI), both stream stations were classified as clean. These stations were clustered together to form Group 2, and the zooplankton species that formed this group were Lecane (Monostilla) bulla and L. luna. Group 1 (Stations A, B, and C; classified as clean and very clean stations) was dominated by Thermocyclops crassus (Stations B and C) and Mesocyclops leuckarti (Stations A, B, and C). Among the cyclopoid copepods, Mesocyclops leuckarti prefers oligo-mesotrophic conditions of lake and is a cosmopolitan and fairly stenothermic species (Ramdani et al. 2001).
Pearson’s correlation coefficients between dominant zooplankton species and water quality parameters are presented in Table 6. C. cornuta and Moina micrura showed significant positive correlations with temperature (r = 0.236 and 0.213, respectively), while Diaphanosoma sarsi had significant negative correlation with NH4–N (r = −0.175). Harpaticoida was positively correlated with temperature (r = 0.379), pH (r = 0.195), and PO4–P (r = 0.224). M. leuckarti revealed significant positive correlations with temperature (r = 0.2330), but was negatively correlated with DO (r = −0.502) in this study. In contrast, M. varicans had significant negative correlation with NH4–N (r = −0.194), but showed positive correlation with NO2–N (r = 0.206). Both Polyarthra vulgaris and T. similis revealed significant positive correlations with temperature (r = 0.431 and 0.340, respectively), DO (r = 0.180 and 0.291, respectively), and pH (r = 0.266 and 0.199, respectively). T. crassus showed positive correlation with temperature (r = 0.321), but negative correlation with DO (r = −0.350).
In the present study, P. vulgaris and T. similis had significant positive correlations with DO and pH (Table 6). Dissolved oxygen has been reported as one of the most important factors limiting the occurrence of Rotifera (Arora and Mehra 2003), while P. vulgaris thrive at higher pH (Dorak et al. 2013). Although temperature and NO2 did not vary in this study, significant correlations with P. vulgaris and T. similis (Table 6) indicated the importance of these factors in providing favourable conditions for the rotifers. In addition, decreasing abundance of Rotifera could be linked with the increasing abundance of Copepoda through predation or competition (Diéguez and Gilbert 2002), as Copepoda are larger and better swimmers than Rotifera (Armengol and Miracle 2000). Rotifera are comparatively weak in competing for limited food resources, prone to starvation, and have a narrower range of food size that they consume (Thorp and Rogers 2015).
Distribution of zooplankton based on their relationships with environmental parameters is illustrated in Fig. 5. Most zooplankton were dependent upon DO (upper right quadrant), but showed lower sensitivity towards TSS, NO3–N, BOD, and NH4–N (lower left quadrant). Species such as M. leuckarti, Euchlanis dilatata, Filinia opoliensis, T. crassus, and T. similis showed higher response towards temperature, pH, conductivity, TDS, and NO2–N (lower right quadrant), but were less affected by COD, as observed in B. falcatus, B. patulus, A. brightwell, and P. vulgari (upper left quadrant).
Canonical correspondence analysis. Factorial diagram showing the abundance of zooplankton in the Air Itam Reservoir. Environmental parameters: temperature (temp), pH, dissolved oxygen (DO), total dissolved solids (TDS), conductivity (cond), total suspended solids (TSS), phosphate (PO 4), nitrate, nitrite, ammonia, biological oxygen demand (BOD), and chemical oxygen demand (COD). Species; 1 Pompholyx complanata, 2 Mesocyclops leuckarti, 3 Alona karua, 4 Euchlanis dilatata, 5 Filinia opoliensis, 6 Brachionus caudatus, 7 L. (Monostilla) bulla, 8 Keratella tropica, 9 Chydorus barroisi, 10 Diaphanosoma excisum, 11 Thermocyclops crassus, 12 Moina micrura, 13 Microcyclops varicans, 14 Ceriodaphnia cornuta, 15 Trichocerca similis, 16 Brachionus falcatus, 17 Brachionus patulus, 18 Diaphanosoma sarsi, 19 Testudinella patina, 20 Lecane luna, 21 Lecane sp., 22 Asplanchna brightwelli, 23 Polyarthra vulgaris, 24 Trichocerca sp., 25 Tropocyclops prasinus, 26 Harpacticoida
Copepoda, in contrast, had the lowest species richness (5 species) but the highest relative abundance of 74.77%. Copepoda constitutes the largest component and biomass in freshwater environment. Across the species recorded, T. crassus was only found at Stations B and C, which was situated at the centre of the reservoir and have a greater depth of 30 and 25 m, respectively, compared to Station C with 8–10 m depth. Mesocyclops leuckarti and T. crassus have shown negative correlation with dissolved oxygen and positive correlation with temperature (Table 6). This is probably related to the preference for deeper water and resistance to anoxia and hydrogen sulphide.
In this study, low abundance and diversity of Cladocera was observed. Among the abundant species, C. cornuta and Moina micrura showed significant positive correlations with temperature as reported by Sa-Ardrit and Frederick (2005). Cladocera were not recorded at Station D, and this is probably due to higher water velocity of the upstream river in this study, which is not favourable for most Cladocerans due to their weak swimming abilities (Sa-Ardrit and Frederick 2005). The cladoceran community is an informative indicator of the trophic state of water bodies (Haberman et al. 2007). In a comparative study carried out by Haberman et al. (2007), cladoceran abundance was reported to be higher in eutrophic lakes, whereas in the present study, abundance and diversity of Cladocera was low, thus indicating that the Air Itam Reservoir is relatively clean. Zooplankton are good indicators for aquatic environmental conditions (Overholt et al. 2016), because their abundance and distribution are influenced by various physico-biological interactions (Nogueira 2001). Thermocyclops sp. was among the dominant species recorded in eutrophic water samples by Sousa et al. (2008).
Species with higher adaptability towards a wide range of environmental conditions recorded in this study include Euchlanis dilatata, Moina micrura, Brachionus spp., and Mesocyclops leuckarti. Although Asplanchna brightwelli was reported to be an indicator species of eutrophic conditions (Attayde and Bozelli 1998) and can be found almost at all stations in this study, they were not among the dominant species. The water quality conditions of this tropical man-made lake have significant effects on the zooplankton assemblages; thus, it is important for zooplankton community structural analysis to be part of biomonitoring in lake management and protection.
Conclusion
The occurrence and abundance of zooplankton were associated with the quality of their environment. CCA analysis showed the correlation of dominant species to the environmental parameters measured, while cluster analysis differentiated lentic and lotic assemblages of zooplankton. The results of this study showed that the Air Itam Reservoir was in good condition and have significant effects on the zooplankton assemblages. This study showed that zooplankton community structure reflects the water conditions, thus playing an important part in biomonitoring of aquatic ecosystem health.
References
APHA (1998) Standard methods for the examination of water and wastewater. In: Greenberg AH, Clesceri LS, Eaton AD (eds) 20th edn. American Public Health Association, Washington
Armengol X, Miracle MR (2000) Diel vertical movements of zooplankton in lake La Criz (Cuenca Spain). J Plankton Res 22(9):1683–1703
Arora J, Mehra NK (2003) Seasonal dynamics of rotifers in relation to physical and chemical conditions of the river Yamuna (Delhi) India. Hydrobiologia 491:101–109
Attayde JL, Bozelli RL (1998) Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Can J Fisheries Aquat Sci 55:1789–1797
DeBoer JA, Webber CM, Dixon TA, Pope KL (2016) The influence of a severe reservoir drawdown on springtime zooplankton and larval fish assemblages in Red Willow Reservoir Nebraska. J Freshw Ecol 31(1):131–146
Deepthi S, Sadanand M, Yamakanamardi (2014) Water quality index and total abundance of zooplankton in Varuna, Madappa and Giribettethe lakes of Mysore, Karnataka State India. Int J Environ Sci 4(5):683–693
Department of Environment (DOE) (2010) Malaysia environmental quality report 2010. Department of Environment, Ministry of Natural Resources and Environment, p 78
Diéguez MC, Gilbert JJ (2002) Suppression of the rotifer Polyarthra remata by omnivorous copepod Tropocyclops extensus: predation or competition. J Plankton Res 24(4):359–369
Dorak ZO, Gaygusuz AS, Tarkan, Aydin H (2013) Diurnal vertical distribution of zooplankton in newly formed reservoir (Tahtali reservoir, Kocaeli): the role of abiotic factors and chlorophyll a. Turk J Zool 37:1–10
Dumont HJF (2003) Guides to the identification of the microinvertebrates of the continental waters of the world. Backhuys Publisher, Leiden
Fatema K, Wan Maznah WO, Isa MM (2015) Spatial variation of water quality parameters in a mangrove estuary. Int J Environ Sci Technol 12(6):2091–2102
Haberman J, Laugaste R, Nõges T (2007) The role of cladocerans reflecting the trophic status of two large and shallow Estonian lakes. Hydrobiologia 584:157–166
Idris BAG (1983) Freshwater zooplankton of Malaysia (Crustacean: Cladocera). Penerbit Universiti Pertanian Malaysia, Selangor, p 153
Idris BAG, Fernando CH (1981) Two new species of cladoceran crustaceans of the genera Macrothrix Baird and Alona Baird from Malaysia. Hydrobiologia 76:81–85
Khalifa N, El-Damhogy KA, Fishar MR, Nasef AM, Hegab MH (2015) Using zooplankton in some environmental biotic indices to assess water quality of Lake Nasser Egypt. Int J Fisheries Aquat Stud 2(4):281–289
Ludwig JA, Reynolds JF (1998) Statistical ecology. John Wiley and Sons, New York
Nogueira MG (2001) Zooplankton composition, dominance and abundance as indicators of environmental compartmentalization in Jurumirim Reservoir (Paranapanema River), São Paulo, Brazil. Hydrobiologia 455:1–18
Overholt EP, Kevin CR, Williamson CE, Fischer JM, Cabrol NA (2016) Behavioral responses of freshwater calanoid copepods to the presence of ultraviolet radiation: avoidance and attraction. J Plankton Res 38(1):16–26. doi:10.1093/plankt/fbv113
PBA (Perbadanan Bekalan Air Sdn. Bhd) (2007) Unpublished book of PBA Pulau Pinang Sdn. Bhd about history of water supply in Penang. PBA Group of Companies, Penang
Pennak RW (1978) Fresh water invertebrates of the United States, 2nd edn. Wiley, New York
Ramdani M, Elkhiati N, Flower RJ, Birks HH, Kraϊem MM, Fathi AA, Patrick ST (2001) Open water zooplankton communities in North African wetland lakes: the CASSARINA Project. Aquat Ecol 35:319–333
Rushforth SR, Brock JT (1991) Attached diatom communities from the lower Truckee river, summer and fall, 1986. Hydrobiologia 224:49–64
Sa-Ardrit P, Frederick B (2005) Cladocera diversity, abundance and habitat in a Western Thailand stream. Aquat Ecol 39(3):353–365
Smirnov NN (1996) Cladocera: the Chydorinae and Sayciinae (Chydoridae) of the world. SPB Academic Publishing, Amsterdam
Sousa W, Attayde JL, Rocha EDS, Eskinazi-Sant’anna EM (2008) The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. J Plankton Res 30(6):699–708
Thorp JH, Rogers DC (2015) Ecology and general biology (4th ed) 1 Elsevier, China
Wan Maznah WO, Makhlough A (2015) Water quality of tropical reservoir based on spatio-temporal variation in phytoplankton composition and physico-chemical analysis. Int J Environ Sci Technol 12(7):2221–2232
Wan Maznah WO, Mansor M (2002) Aquatic pollution assessment based on attached diatom communities in the Pinang River Basin, Malaysia. Hydrobiologia 487:229–241
Zati S, Zulkifli Y (2007) The status of eutrophication of lake in Malaysia. Colloquium on lakes and reservoir management: status and issues. Dewan Baiduri, Kementerian Sumber Asli dan Alam Sekitar, Putrajaya, Malaysia
Acknowledgements
The authors would like to thank staff and students of School of Biological Sciences, Universiti Sains Malaysia (USM) for their help in the field and laboratory analysis. Thanks are also due to Penang Water Resource Authority (Perbadanan Bekalan Air (PBA) Pulau Pinang) for their logistic support and for giving basic information on the Air Itam Reservoir. We are indebted to Prof. Peter Convey of British Antarctic Survey, UK, for his constructive criticisms on the manuscript. This research was funded by a Universiti Sains Malaysia (USM) Short-term Term Research Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Wan Maznah, W.O., Intan, S., Sharifah, R. et al. Lentic and lotic assemblages of zooplankton in a tropical reservoir, and their association with water quality conditions. Int. J. Environ. Sci. Technol. 15, 533–542 (2018). https://doi.org/10.1007/s13762-017-1412-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1412-1